Higher Expressions of SHH and AR Are Associated with a Positive Receptor Status and Have Impact on Survival in a Cohort of Croatian Breast Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Archival Tumors Tissue Samples and Patients Data Collection
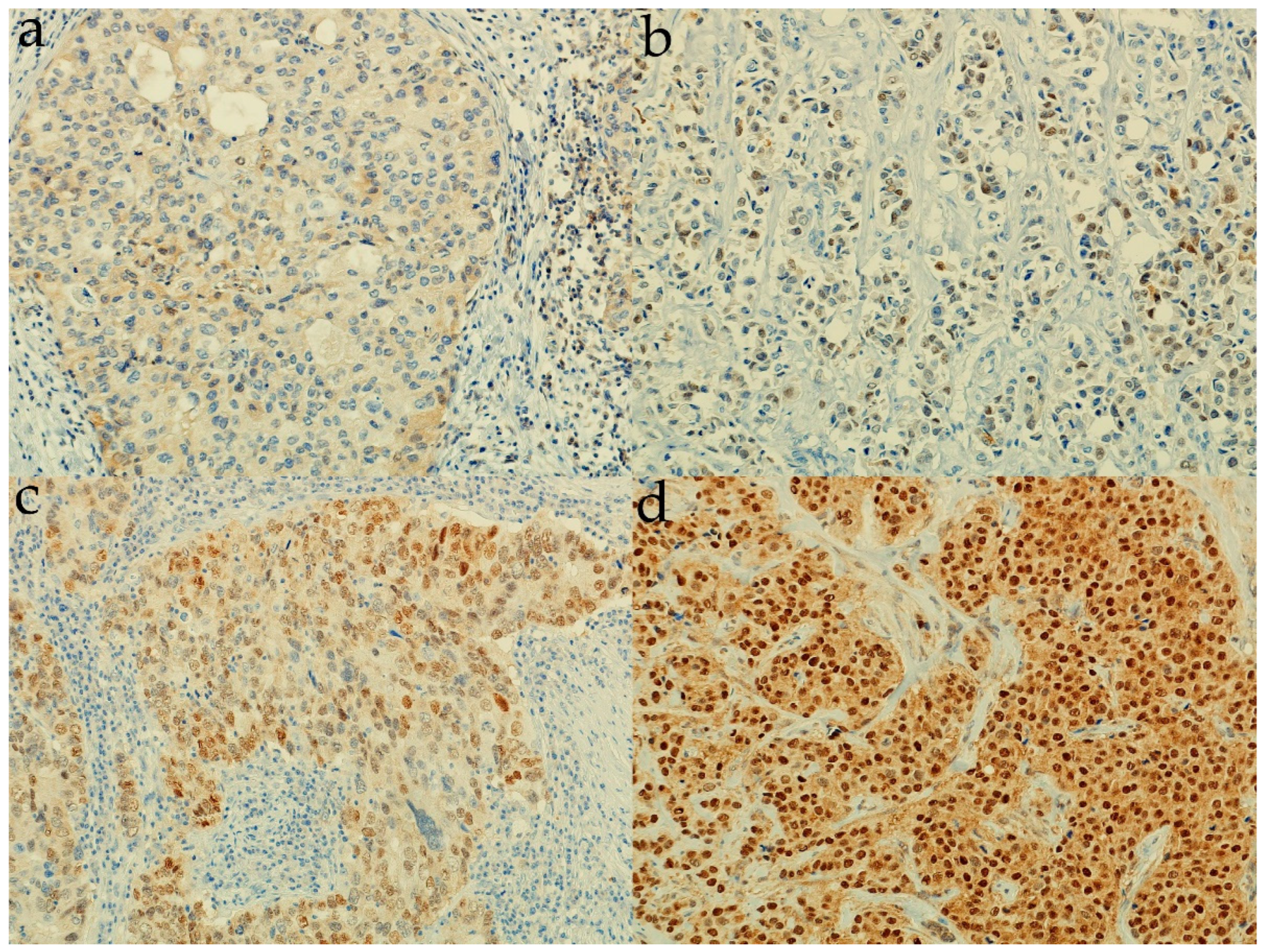
2.2. Determination of Androgen Receptor Protein Expression
2.3. Determination of Sonic Hedgehog Protein Expression
2.4. Statistical Analysis
3. Results
3.1. Association of Clinicopathological Characteristics with Molecular Subtype and Receptor Status in a Cohort of Breast Cancer Patients from Croatia
3.2. Expression of Sonic Hedgehog Protein and Androgen Receptor and Its Association with a Molecular Subtype and Receptor Status of Breast Cancer Patients from Croatia
3.3. Impact of Clinicopathological Characteristics and Sonic Hedgehog Protein and Androgen Receptor Expressions on Survival of Croatian Patients with Breast Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, B.O.; Yip, C.H.; Smith, R.A.; Shyyan, R.; Sener, S.F.; Eniu, A.; Carlson, R.W.; Azavedo, E.; Harford, J. Guideline implementation for breast healthcare in low-income and middle-income countries: Overview of the Breast Health Global Initiative Global Summit 2007. Cancer 2008, 113, 2221–2243. [Google Scholar] [CrossRef]
- W.H.O. The Global Cancer Observatory. 2018 Statistics. Available online: http://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (accessed on 10 September 2022).
- Incidencija i Mortalitet od Raka u EU-27 Zemljama za 2020. Godinu. Available online: https://www.hzjz.hr/sluzba-epidemiologija-prevencija-nezaraznih-bolesti/incidencija-i-mortalitet-od-raka-u-eu-27-zemljama-za-2020-godinu/ (accessed on 10 September 2022).
- Prat, A.; Pineda, E.; Adamo, B.; Galvan, P.; Fernandez, A.; Gaba, L.; Diez, M.; Viladot, M.; Arance, A.; Munoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24, S26–S35. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Han, J.; Liang, X.; Sun, S.; Jiang, Y.; Xia, B.; Niu, M.; Li, D.; Zhang, J.; Wang, S.; et al. Androgen Receptor Expression and Bicalutamide Antagonize Androgen Receptor Inhibit beta-Catenin Transcription Complex in Estrogen Receptor-Negative Breast Cancer. Cell Physiol. Biochem. 2017, 43, 2212–2225. [Google Scholar] [CrossRef]
- Murria, R.; Palanca, S.; de Juan, I.; Alenda, C.; Egoavil, C.; Segui, F.J.; Garcia-Casado, Z.; Juan, M.J.; Sanchez, A.B.; Segura, A.; et al. Immunohistochemical, genetic and epigenetic profiles of hereditary and triple negative breast cancers. Relevance in personalized medicine. Am. J. Cancer Res. 2015, 5, 2330–2343. [Google Scholar]
- Hickey, T.E.; Robinson, J.L.; Carroll, J.S.; Tilley, W.D. Minireview: The androgen receptor in breast tissues: Growth inhibitor, tumor suppressor, oncogene? Mol. Endocrinol. 2012, 26, 1252–1267. [Google Scholar] [CrossRef]
- Salvi, S.; Bonafe, M.; Bravaccini, S. Androgen receptor in breast cancer: A wolf in sheep’s clothing? A lesson from prostate cancer. Semin. Cancer Biol. 2020, 60, 132–137. [Google Scholar] [CrossRef]
- Sabol, M.; Trnski, D.; Musani, V.; Ozretic, P.; Levanat, S. Role of GLI Transcription Factors in Pathogenesis and Their Potential as New Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 2562. [Google Scholar] [CrossRef] [Green Version]
- Porter, J.A.; Young, K.E.; Beachy, P.A. Cholesterol modification of hedgehog signaling proteins in animal development. Science 1996, 274, 255–259. [Google Scholar] [CrossRef]
- Katoh, Y.; Katoh, M. Hedgehog target genes: Mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr. Mol. Med. 2009, 9, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Pietrobono, S.; Gagliardi, S.; Stecca, B. Non-canonical Hedgehog Signaling Pathway in Cancer: Activation of GLI Transcription Factors Beyond Smoothened. Front. Genet. 2019, 10, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riobo-Del Galdo, N.A.; Lara Montero, A.; Wertheimer, E.V. Role of Hedgehog Signaling in Breast Cancer: Pathogenesis and Therapeutics. Cells 2019, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Souzaki, M.; Kubo, M.; Kai, M.; Kameda, C.; Tanaka, H.; Taguchi, T.; Tanaka, M.; Onishi, H.; Katano, M. Hedgehog signaling pathway mediates the progression of non-invasive breast cancer to invasive breast cancer. Cancer Sci. 2011, 102, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.S.; Uddin, M.; Rahman, M.Z.; Nayeem, M.J.; Alam, S.S.; Khatun, Z.; Wahiduzzaman, M.; Sultana, A.; Rahman, M.L.; Ali, M.Y.; et al. Overexpression of sonic hedgehog in the triple negative breast cancer: Clinicopathological characteristics of high burden breast cancer patients from Bangladesh. Sci. Rep. 2016, 6, 18830. [Google Scholar] [CrossRef] [Green Version]
- Benvenuto, M.; Masuelli, L.; De Smaele, E.; Fantini, M.; Mattera, R.; Cucchi, D.; Bonanno, E.; Di Stefano, E.; Frajese, G.V.; Orlandi, A.; et al. In vitro and in vivo inhibition of breast cancer cell growth by targeting the Hedgehog/GLI pathway with SMO (GDC-0449) or GLI (GANT-61) inhibitors. Oncotarget 2016, 7, 9250–9270. [Google Scholar] [CrossRef] [Green Version]
- Kurebayashi, J.; Kanomata, N.; Yamashita, T.; Shimo, T.; Moriya, T. Antitumor and anticancer stem cell activities of eribulin mesylate and antiestrogens in breast cancer cells. Breast Cancer 2016, 23, 425–436. [Google Scholar] [CrossRef]
- O’Brien, C.S.; Farnie, G.; Howell, S.J.; Clarke, R.B. Breast cancer stem cells and their role in resistance to endocrine therapy. Horm. Cancer 2011, 2, 91–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeng, K.S.; Sheen, I.S.; Jeng, W.J.; Yu, M.C.; Hsiau, H.I.; Chang, F.Y. High expression of Sonic Hedgehog signaling pathway genes indicates a risk of recurrence of breast carcinoma. OncoTargets Ther. 2013, 7, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, W.; Wang, L.H.; Wen, Y.Y.; Song, M.; Li, B.L.; Chen, X.L.; Xu, M.; An, S.X.; Zhao, J.; Lu, Y.Y.; et al. Expression and regulation mechanisms of Sonic Hedgehog in breast cancer. Cancer Sci. 2010, 101, 927–933. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, S.A.; Machalek, D.A.; Shearer, R.F.; Millar, E.K.; Nair, R.; Schofield, P.; McLeod, D.; Cooper, C.L.; McNeil, C.M.; McFarland, A.; et al. Hedgehog overexpression is associated with stromal interactions and predicts for poor outcome in breast cancer. Cancer Res. 2011, 71, 4002–4014. [Google Scholar] [CrossRef] [Green Version]
- Matevossian, A.; Resh, M.D. Hedgehog Acyltransferase as a target in estrogen receptor positive, HER2 amplified, and tamoxifen resistant breast cancer cells. Mol. Cancer 2015, 14, 72. [Google Scholar] [CrossRef] [Green Version]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Charafe-Jauffret, E.; Ginestier, C.; Iovino, F.; Tarpin, C.; Diebel, M.; Esterni, B.; Houvenaeghel, G.; Extra, J.M.; Bertucci, F.; Jacquemier, J.; et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin. Cancer Res. 2010, 16, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Geradts, J.; Dewhirst, M.W.; Lo, H.W. Upregulation of VEGF-A and CD24 gene expression by the tGLI1 transcription factor contributes to the aggressive behavior of breast cancer cells. Oncogene 2012, 31, 104–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, L.G.; Pannell, L.K.; Singh, S.; Samant, R.S.; Shevde, L.A. Increased vascularity and spontaneous metastasis of breast cancer by hedgehog signaling mediated upregulation of cyr61. Oncogene 2012, 31, 3370–3380. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.W.; Nguyen, M.P.; Padalecki, S.S.; Grubbs, B.G.; Merkel, A.R.; Oyajobi, B.O.; Matrisian, L.M.; Mundy, G.R.; Sterling, J.A. TGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical Hedgehog signaling. Cancer Res. 2011, 71, 822–831. [Google Scholar] [CrossRef] [Green Version]
- Sterling, J.A.; Oyajobi, B.O.; Grubbs, B.; Padalecki, S.S.; Munoz, S.A.; Gupta, A.; Story, B.; Zhao, M.; Mundy, G.R. The hedgehog signaling molecule Gli2 induces parathyroid hormone-related peptide expression and osteolysis in metastatic human breast cancer cells. Cancer Res. 2006, 66, 7548–7553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trnski, D.; Sabol, M.; Tomic, S.; Stefanac, I.; Mrcela, M.; Musani, V.; Rincic, N.; Kurtovic, M.; Petric, T.; Levanat, S.; et al. SHH-N non-canonically sustains androgen receptor activity in androgen-independent prostate cancer cells. Sci. Rep. 2021, 11, 14880. [Google Scholar] [CrossRef]
- Sabol, M.; Trnski, D.; Uzarevic, Z.; Ozretic, P.; Musani, V.; Rafaj, M.; Cindric, M.; Levanat, S. Combination of cyclopamine and tamoxifen promotes survival and migration of mcf-7 breast cancer cells--interaction of hedgehog-gli and estrogen receptor signaling pathways. PLoS ONE 2014, 9, e114510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef]
- Luque-Bolivar, A.; Perez-Mora, E.; Villegas, V.E.; Rondon-Lagos, M. Resistance and Overcoming Resistance in Breast Cancer. Breast Cancer 2020, 12, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Maric, P.; Ozretic, P.; Levanat, S.; Oreskovic, S.; Antunac, K.; Beketic-Oreskovic, L. Tumor markers in breast cancer—Evaluation of their clinical usefulness. Coll. Antropol. 2011, 35, 241–247. [Google Scholar]
- Cochrane, C.R.; Szczepny, A.; Watkins, D.N.; Cain, J.E. Hedgehog Signaling in the Maintenance of Cancer Stem Cells. Cancers 2015, 7, 1554–1585. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.K.; Khan, J.S.; Shah, S.T.A.; Wang, F.; Ye, L.; Jiang, W.G.; Malik, M.F.A. Involvement of hedgehog pathway in early onset, aggressive molecular subtypes and metastatic potential of breast cancer. Cell Commun. Signal. 2018, 16, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, K.; Nakamura, M.; Nakashima, H.; Akiyoshi, T.; Kubo, M.; Sato, N.; Kuroki, S.; Nomura, M.; Tanaka, M.; Katano, M. Novel link between estrogen receptor alpha and hedgehog pathway in breast cancer. Anticancer Res. 2008, 28, 731–740. [Google Scholar] [PubMed]
- Im, S.; Choi, H.J.; Yoo, C.; Jung, J.H.; Jeon, Y.W.; Suh, Y.J.; Kang, C.S. Hedgehog related protein expression in breast cancer: Gli-2 is associated with poor overall survival. Korean J. Pathol. 2013, 47, 116–123. [Google Scholar] [CrossRef]
- Kebenko, M.; Drenckhan, A.; Gros, S.J.; Jucker, M.; Grabinski, N.; Ewald, F.; Grottke, A.; Schultze, A.; Izbicki, J.R.; Bokemeyer, C.; et al. ErbB2 signaling activates the Hedgehog pathway via PI3K-Akt in human esophageal adenocarcinoma: Identification of novel targets for concerted therapy concepts. Cell. Signal. 2015, 27, 373–381. [Google Scholar] [CrossRef]
- Kasperczyk, H.; Baumann, B.; Debatin, K.M.; FuMa, S. Characterization of sonic hedgehog as a novel NF-kappaB target gene that promotes NF-kappaB-mediated apoptosis resistance and tumor growth in vivo. FASEB J. 2009, 23, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Nakamura, M.; Yamaguchi, H.; Yamanaka, N.; Akiyoshi, T.; Koga, K.; Yamaguchi, K.; Tsuneyoshi, M.; Tanaka, M.; Katano, M. Nuclear factor-kappaB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer Res. 2006, 66, 7041–7049. [Google Scholar] [CrossRef] [Green Version]
- Shostak, K.; Chariot, A. NF-kappaB, stem cells and breast cancer: The links get stronger. Breast Cancer Res. 2011, 13, 214. [Google Scholar] [CrossRef]
- Wang, L.H.; Choi, Y.L.; Hua, X.Y.; Shin, Y.K.; Song, Y.J.; Youn, S.J.; Yun, H.Y.; Park, S.M.; Kim, W.J.; Kim, H.J.; et al. Increased expression of sonic hedgehog and altered methylation of its promoter region in gastric cancer and its related lesions. Mod. Pathol. 2006, 19, 675–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, J.G.; O’Shaughnessy, J.A. The hedgehog pathway in triple-negative breast cancer. Cancer Med. 2016, 5, 2989–3006. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.S.; Uddin, M.; Chowdhury, A.A.; Nayeem, M.J.; Raihan, Z.; Rashid, M.I.; Azad, A.K.; Rahman, M.L.; Barua, D.; Sultana, A.; et al. Serum sonic hedgehog (SHH) and interleukin-(IL-6) as dual prognostic biomarkers in progressive metastatic breast cancer. Sci. Rep. 2017, 7, 1796. [Google Scholar] [CrossRef] [PubMed]
- Bieche, I.; Lerebours, F.; Tozlu, S.; Espie, M.; Marty, M.; Lidereau, R. Molecular profiling of inflammatory breast cancer: Identification of a poor-prognosis gene expression signature. Clin. Cancer Res. 2004, 10, 6789–6795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Z.H.; Wang, H.C.; Zhao, D.M.; Ji, X.X.; Song, M.; Yang, X.J.; Cui, W. Cooperatively transcriptional and epigenetic regulation of sonic hedgehog overexpression drives malignant potential of breast cancer. Cancer Sci. 2015, 106, 1084–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten Haaf, A.; Bektas, N.; von Serenyi, S.; Losen, I.; Arweiler, E.C.; Hartmann, A.; Knuchel, R.; Dahl, E. Expression of the glioma-associated oncogene homolog (GLI) 1 in human breast cancer is associated with unfavourable overall survival. BMC Cancer 2009, 9, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaglio, D.; Infante, P.; Di Marcotullio, L.; Botta, B.; Mori, M. Hedgehog signaling pathway inhibitors: An updated patent review (2015-present). Expert Opin. Ther. Pat. 2020, 30, 235–250. [Google Scholar] [CrossRef]
- Maun, H.R.; Wen, X.; Lingel, A.; de Sauvage, F.J.; Lazarus, R.A.; Scales, S.J.; Hymowitz, S.G. Hedgehog pathway antagonist 5E1 binds hedgehog at the pseudo-active site. J. Biol. Chem. 2010, 285, 26570–26580. [Google Scholar] [CrossRef] [Green Version]
- Katano, M. Hedgehog signaling pathway as a therapeutic target in breast cancer. Cancer Lett. 2005, 227, 99–104. [Google Scholar] [CrossRef]
- Das, S.; Tucker, J.A.; Khullar, S.; Samant, R.S.; Shevde, L.A. Hedgehog signaling in tumor cells facilitates osteoblast-enhanced osteolytic metastases. PLoS ONE 2012, 7, e34374. [Google Scholar] [CrossRef]
- Stanton, B.Z.; Peng, L.F.; Maloof, N.; Nakai, K.; Wang, X.; Duffner, J.L.; Taveras, K.M.; Hyman, J.M.; Lee, S.W.; Koehler, A.N.; et al. A small molecule that binds Hedgehog and blocks its signaling in human cells. Nat. Chem. Biol. 2009, 5, 154–156. [Google Scholar] [CrossRef] [Green Version]
- Yun, T.; Wang, J.; Yang, J.; Huang, W.; Lai, L.; Tan, W.; Liu, Y. Discovery of Small Molecule Inhibitors Targeting the Sonic Hedgehog. Front. Chem. 2020, 8, 498. [Google Scholar] [CrossRef]
- Owens, A.E.; de Paola, I.; Hansen, W.A.; Liu, Y.W.; Khare, S.D.; Fasan, R. Design and Evolution of a Macrocyclic Peptide Inhibitor of the Sonic Hedgehog/Patched Interaction. J. Am. Chem. Soc. 2017, 139, 12559–12568. [Google Scholar] [CrossRef]
- Petrova, E.; Rios-Esteves, J.; Ouerfelli, O.; Glickman, J.F.; Resh, M.D. Inhibitors of Hedgehog acyltransferase block Sonic Hedgehog signaling. Nat. Chem. Biol. 2013, 9, 247–249. [Google Scholar] [CrossRef] [Green Version]
- Levanat, S.; Sabol, M.; Musani, V.; Ozretic, P.; Trnski, D. Hedgehog Signaling Pathway as Genetic and Epigenetic Target in Ovarian Tumors. Curr. Pharm. Des. 2017, 23, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.K.; Ke, Y.; Wang, F.; Kayani, M.A.; Malik, M.F.A. Influence of SHH/GLI1 axis on EMT mediated migration and invasion of breast cancer cells. Sci. Rep. 2019, 9, 6620. [Google Scholar] [CrossRef] [Green Version]
- Bhateja, P.; Cherian, M.; Majumder, S.; Ramaswamy, B. The Hedgehog Signaling Pathway: A Viable Target in Breast Cancer? Cancers 2019, 11, 1126. [Google Scholar] [CrossRef] [Green Version]
- Collins, L.C.; Cole, K.S.; Marotti, J.D.; Hu, R.; Schnitt, S.J.; Tamimi, R.M. Androgen receptor expression in breast cancer in relation to molecular phenotype: Results from the Nurses’ Health Study. Mod. Pathol. 2011, 24, 924–931. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Yang, Y.; Xu, K.; Li, L.; Huang, J.; Qiu, F. Androgen Receptor in Breast Cancer: From Bench to Bedside. Front. Endocrinol. 2020, 11, 573. [Google Scholar] [CrossRef]
- Kensler, K.H.; Poole, E.M.; Heng, Y.J.; Collins, L.C.; Glass, B.; Beck, A.H.; Hazra, A.; Rosner, B.A.; Eliassen, A.H.; Hankinson, S.E.; et al. Androgen Receptor Expression and Breast Cancer Survival: Results From the Nurses’ Health Studies. J. Natl. Cancer Inst. 2019, 111, 700–708. [Google Scholar] [CrossRef]
- Kensler, K.H.; Regan, M.M.; Heng, Y.J.; Baker, G.M.; Pyle, M.E.; Schnitt, S.J.; Hazra, A.; Kammler, R.; Thurlimann, B.; Colleoni, M.; et al. Prognostic and predictive value of androgen receptor expression in postmenopausal women with estrogen receptor-positive breast cancer: Results from the Breast International Group Trial 1-98. Breast Cancer Res. 2019, 21, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Wu, J.; Ling, R.; Li, N. Quadruple negative breast cancer. Breast Cancer 2020, 27, 527–533. [Google Scholar] [CrossRef]
- Bozovic-Spasojevic, I.; Zardavas, D.; Brohee, S.; Ameye, L.; Fumagalli, D.; Ades, F.; de Azambuja, E.; Bareche, Y.; Piccart, M.; Paesmans, M.; et al. The Prognostic Role of Androgen Receptor in Patients with Early-Stage Breast Cancer: A Meta-analysis of Clinical and Gene Expression Data. Clin. Cancer Res. 2017, 23, 2702–2712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattarai, S.; Klimov, S.; Mittal, K.; Krishnamurti, U.; Li, X.B.; Oprea-Ilies, G.; Wetherilt, C.S.; Riaz, A.; Aleskandarany, M.A.; Green, A.R.; et al. Prognostic Role of Androgen Receptor in Triple Negative Breast Cancer: A Multi-Institutional Study. Cancers 2019, 11, 995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, Y.; Lin, F.; Shi, X.; Xiang, L.; Li, L. Shh Overexpression Is Correlated with GRP78 and AR Expression in Primary Prostate Cancer: Clinicopathological Features and Outcomes in a Chinese Cohort. Cancer Manag. Res. 2020, 12, 1569–1578. [Google Scholar] [CrossRef]



| Characteristic | n Out of 185 (%) |
|---|---|
| Age * (years) | 60 (28–87) |
| Histotype | |
| Invasive ductal carcinoma | 160 (86.5) |
| Invasive ductal carcinoma and ductal carcinoma in situ | 25 (13.5) |
| Tumor size (T) | |
| T1 (≤2 cm) | 105 (56.8) |
| T2 (2.1–5 cm) | 71 (38.4) |
| T3 (>5.1 cm) | 9 (4.9) |
| Nodal involvement (N) | |
| N0 (0) | 113 (61.4) |
| N1 (1–3) | 46 (25.0) |
| N2 (4–9) | 14 (7.6) |
| N3 (≥10) | 11 (6.0) |
| Distant metastasis (M) | |
| M0 | 183 (98.9) |
| M1 | 2 (1.1) |
| Ki-67 | |
| <20% | 53 (28.6) |
| ≥20% | 132 (71.4) |
| Lymphovascular invasion | |
| Absent | 116 (62.7) |
| Present | 69 (37.3) |
| Molecular subtype | |
| Luminal A | 48 (25.9) |
| Luminal B | 74 (40.0) |
| HER2-enriched | 16 (8.6) |
| Triple-negative | 47 (25.4) |
| Survival | |
| Alive | 140 (75.7) |
| Deceased | 45 (24.3) |
| Recurrence | |
| Absent | 177 (95.7) |
| Present | 8 (4.3) |
| Metastasis | |
| Absent | 166 (89.7) |
| Present | 19 (10.3) |
| Characteristic | SHH | AR | ||||
|---|---|---|---|---|---|---|
| ‘Low’ | ‘High’ | p-Value | ‘Low’ | ‘High’ | p-Value | |
| Age | ||||||
| ≤50 years | 20 (43.5) | 26 (56.5) | 0.070 | 27 (58.7) | 19 (41.3) | 0.017 |
| >50 years | 40 (29.0) | 98 (71.0) | 53 (38.4) | 85 (61.6) | ||
| Histotype | ||||||
| IDC | 81 (50.6) | 79 (49.4) | 0.084 | 58 (36.2) | 102 (63.7) | 0.017 |
| IDC + DCIS | 8 (32.0) | 17 (68.0) | 3 (12.0) | 22 (88.0) | ||
| Tumor size | ||||||
| T1 | 43 (41.0) | 62 (59.0) | 0.026 | 35 (33.3) | 70 (66.7) | 0.002 |
| T2 + T3 | 46 (57.5) | 34 (42.5) | 45 (56.2) | 35 (43.7) | ||
| Nodal involvement | ||||||
| 0–3 | 124 (78.0) | 35 (22.0) | 0.052 | 17 (10.7) | 142 (89.3) | 0.087 |
| ≥4 | 15 (60.0) | 10 (40.0) | 0 (0.0) | 25 (100.0) | ||
| Ki-67 | ||||||
| <20% | 23 (43.4) | 30 (56.6) | 0.418 | 8 (15.1) | 45 (84.9) | 0.001 |
| ≥20% | 66 (50.0) | 66 (50.0) | 53 (40.2) | 79 (59.8) | ||
| LVI | ||||||
| Absent | 91 (78.4) | 25 (21.6) | 0.256 | 42 (36.2) | 74 (63.8) | 0.226 |
| Present | 49 (71.0) | 20 (29.0) | 19 (27.5) | 50 (72.5) | ||
| Molecular subtype * | ||||||
| Luminal A | 22 (45.8) | 26 (54.2) | 0.037 | 16 (33.3) | 32 (66.7) | <0.0001 |
| Luminal B | 37 (50.0) | 37 (50.0) | 33 (44.6) | 41 (55.4) | ||
| HER2-E | 5 (31.2) | 11 (68.8) | 12 (75.0) | 4 (25.0) | ||
| Triple-negative | 32 (68.1) | 15 (31.9) | 40 (85.1) | 7 (14.9) | ||
| Receptor status | ||||||
| ER-negative | 31 (48.4) | 33 (51.6) | 0.041 | 43 (67.2) | 21 (32.8) | <0.0001 |
| ER-positive | 40 (33.1) | 81 (66.9) | 10 (8.3) | 111 (91.7) | ||
| PR-negative | 31 (39.2) | 48 (60.8) | 0.089 | 44 (55.7) | 35 (44.3) | <0.0001 |
| PR-positive | 29 (27.4) | 77 (72.6) | 9 (8.5) | 97 (91.5) | ||
| HER2-negative | 80 (56.3) | 62 (43.7) | 0.028 | 32 (22.5) | 110 (77.5) | 0.008 |
| HER2-positive | 16 (37.2) | 27 (62.8) | 2 (4.7) | 41 (95.3) | ||
| Survival | ||||||
| Alive | 101 (72.1) | 39 (27.9) | 0.209 | 73 (52.1) | 67 (47.9) | 0.239 |
| Deceased | 28 (62.2) | 17 (37.8) | 28 (62.2) | 17 (37.8) | ||
| Recurrence | ||||||
| Absent | 165 (93.2) | 12 (6.8) | 0.057 | 33 (18.6) | 144 (81.4) | 0.003 |
| Present | 6 (75.0) | 2 (25.0) | 5 (62.5) | 3 (37.5) | ||
| Metastasis | ||||||
| Absent | 77 (46.4) | 89 (53.6) | 0.167 | 67 (40.4) | 99 (59.6) | 0.020 |
| Present | 12 (63.2) | 7 (36.8) | 13 (68.4) | 6 (31.6) | ||
| Characteristic | Category | ρ | p-Value |
|---|---|---|---|
| All patients | 0.07 | 0.336 | |
| Age | ≤50 years | 0.14 | 0.356 |
| >50 years | 0.02 | 0.787 | |
| Histotype | IDC | 0.10 | 0.218 |
| IDC + DCIS | −0.13 | 0.533 | |
| Tumor size | <2 cm | −0.03 | 0.749 |
| ≥2 cm | 0.17 | 0.134 | |
| Nodal involvement | 0–3 | 0.06 | 0.474 |
| ≥4 | 0.13 | 0.534 | |
| Ki-67 | <20% | 0.18 | 0.205 |
| ≥20% | 0.04 | 0.615 | |
| Lymphovascular invasion | Absent | 0.03 | 0.765 |
| Present | 0.14 | 0.243 | |
| Molecular subtype | Luminal A | 0.14 | 0.337 |
| Luminal B | −0.06 | 0.597 | |
| HER2-E | 0.11 | 0.680 | |
| TNBC | −0.03 | 0.846 | |
| Receptor status | ER-negative | 0.10 | 0.437 |
| ER-positive | 0.02 | 0.847 | |
| PR-negative | 0.10 | 0.398 | |
| PR-positive | 0.04 | 0.717 | |
| HER2-negative | 0.10 | 0.247 | |
| HER2-positive | −0.06 | 0.721 | |
| Survival | Alive | 0.08 | 0.341 |
| Deceased | 0.05 | 0.765 | |
| Recurrence | Absent | 0.09 | 0.210 |
| Present | −0.55 | 0.157 | |
| Metastasis | Absent | 0.09 | 0.246 |
| Present | 0.03 | 0.900 |
| Characteristic | Molecular Subtype | Receptor Status | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | LumA | LumB | HER2-E | TNBC | ER- | ER+ | PR- | PR+ | HER2- | HER2+ | ||
| Age ≤50 vs. >50 years | OS | 2.5 (1.29–4.84) | 3.7 (1.32–10.23) | 3.2 (1.25–8.42) | 2.6 (1.07–6.49) | N.A. | ||||||
| RFS | N.A. | |||||||||||
| T <2 vs. ≥2 cm | OS | 2.7 (1.49–4.91) | 3.1 (1.12–8.38) | 2.6 (1.10–6.27) | 2.9 (1.29–6.44) | 3.6 (1.78–7.15) | ||||||
| MFS | 5.0 (1.98–12.41) | 3.6 (1.08–12.11) | 4.3 (1.42–12.77) | 4.3 (1.52–12.37) | 5.7 (1.95–16.52) | |||||||
| N 0–3 vs. ≥4 | OS | 4.5 (1.78–11.16) | 7.0 (1.48–32.96) | 7.0 (1.26–39.06) | 4.4 (1.20–16.02) | 4.2 (1.16–14.97) | 5.1 (1.61–16.41) | 14.7 (3.83–56.50) | ||||
| MFS | 19.9 (4.88–81.40) | 11.2 (1.29–96.24) | 19.0 (2.64–137.16) | 9.6 (1.89–48.20) | 47.2 (3.77–591.45) | 10.20 (2.28–45.47) | 24.9 (1.39–445.77) | 133.9 (18.92–947.40) | ||||
| Ki-67 <20% vs. ≥20% | MFS | 3.3 (1.25–8.81) | 3.9 (1.35–10.99) | |||||||||
| LVI no vs. yes | OS | 4.0 (1.07–15.11) | ||||||||||
| SHH ‘low’ vs. ‘high’ | OS | 6.7 (1.06–42.82) | 2.7 (1.03–6.96) | |||||||||
| RFS | 30.8 (1.63–580.74) | 12.8 (1.40–116.76) | 29.23 (1.56–547.50) | 56.3 (2.46–1288.75) | ||||||||
| MFS | 8.2 (1.08–62.79) | 0.19 (0.038–0.958) | 5.4 (1.05–28.23) | |||||||||
| AR ‘low’ vs. ‘high’ | OS | 4.1 (1.48–11.23) | 3.1 (1.17–8.20) | 2.8 (1.05–7.47) | N.A. | |||||||
| RFS | 0.05 (0.009–0.323) | N.A. | 0.16 (0.032–0.834) | N.A. | 0.10 (0.016–0.639) | |||||||
| MFS | 0.33 (0.133–0.824) | N.A. | N.A. | N.A. | N.A. | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budimir, I.; Tomasović-Lončarić, Č.; Kralik, K.; Čonkaš, J.; Eljuga, D.; Žic, R.; Gorjanc, B.; Tucaković, H.; Caktaš, D.; Jaman, J.; et al. Higher Expressions of SHH and AR Are Associated with a Positive Receptor Status and Have Impact on Survival in a Cohort of Croatian Breast Cancer Patients. Life 2022, 12, 1559. https://doi.org/10.3390/life12101559
Budimir I, Tomasović-Lončarić Č, Kralik K, Čonkaš J, Eljuga D, Žic R, Gorjanc B, Tucaković H, Caktaš D, Jaman J, et al. Higher Expressions of SHH and AR Are Associated with a Positive Receptor Status and Have Impact on Survival in a Cohort of Croatian Breast Cancer Patients. Life. 2022; 12(10):1559. https://doi.org/10.3390/life12101559
Chicago/Turabian StyleBudimir, Ivan, Čedna Tomasović-Lončarić, Kristina Kralik, Josipa Čonkaš, Domagoj Eljuga, Rado Žic, Božo Gorjanc, Hrvoje Tucaković, Doroteja Caktaš, Josip Jaman, and et al. 2022. "Higher Expressions of SHH and AR Are Associated with a Positive Receptor Status and Have Impact on Survival in a Cohort of Croatian Breast Cancer Patients" Life 12, no. 10: 1559. https://doi.org/10.3390/life12101559
APA StyleBudimir, I., Tomasović-Lončarić, Č., Kralik, K., Čonkaš, J., Eljuga, D., Žic, R., Gorjanc, B., Tucaković, H., Caktaš, D., Jaman, J., Lisek, V., Vlajčić, Z., Martić, K., & Ozretić, P. (2022). Higher Expressions of SHH and AR Are Associated with a Positive Receptor Status and Have Impact on Survival in a Cohort of Croatian Breast Cancer Patients. Life, 12(10), 1559. https://doi.org/10.3390/life12101559







