Acquisition of Dual Ribozyme-Functions in Nonfunctional Short Hairpin RNAs through Kissing-Loop Interactions
Abstract
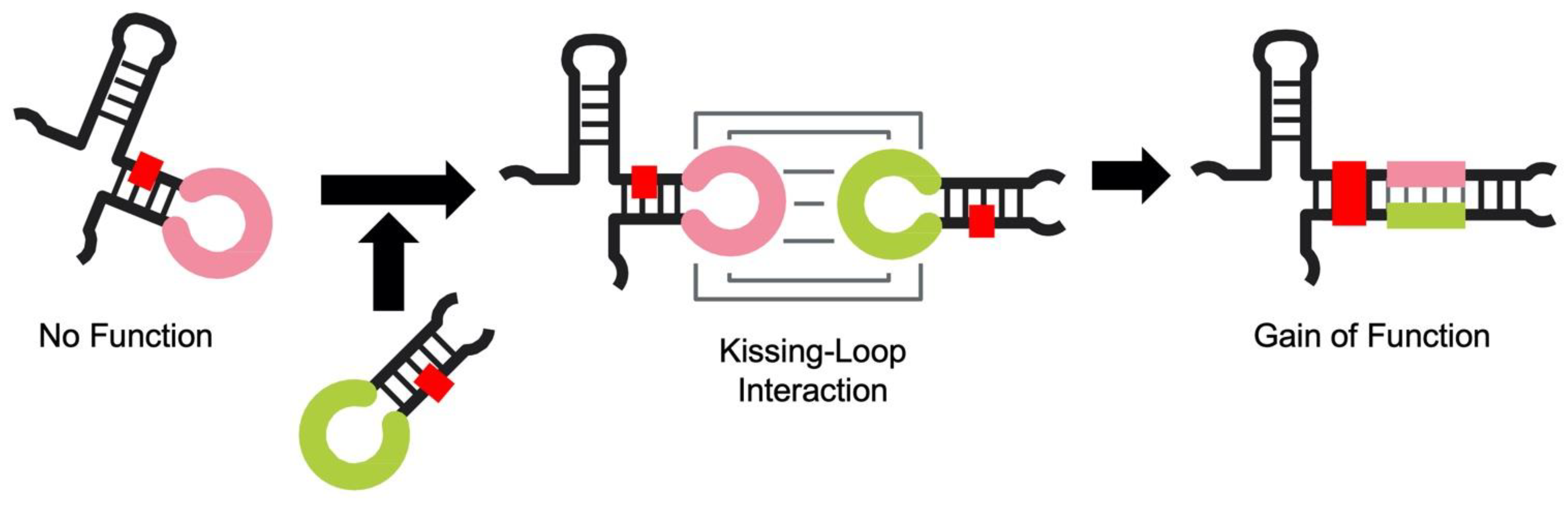
:1. Introduction
2. Materials and Methods
2.1. Preparation of RNAs
2.2. Electrophoretic Mobility Shift Assay (EMSA)
2.3. Analysis of Ligation and Cleavage Activity
3. Results

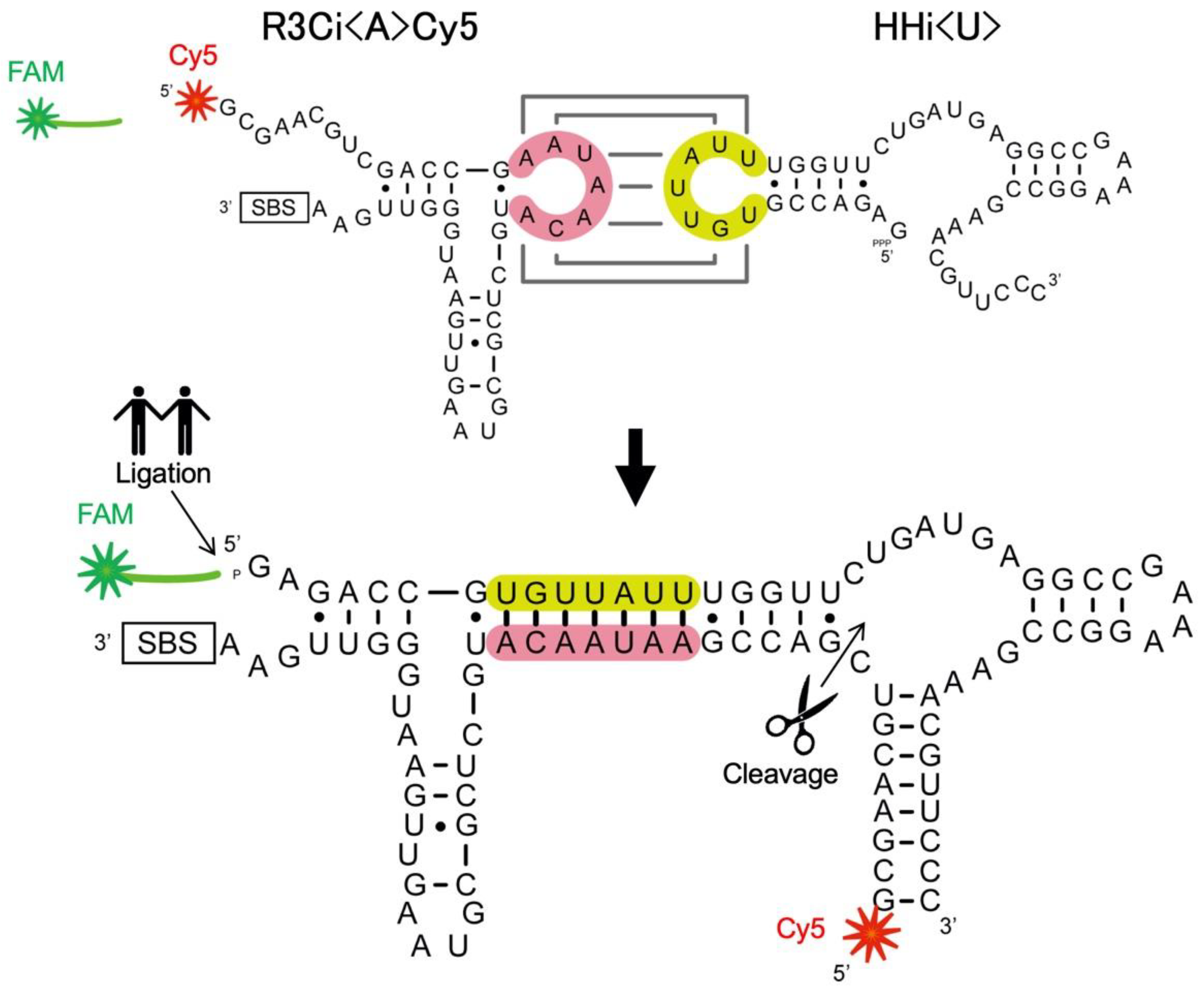
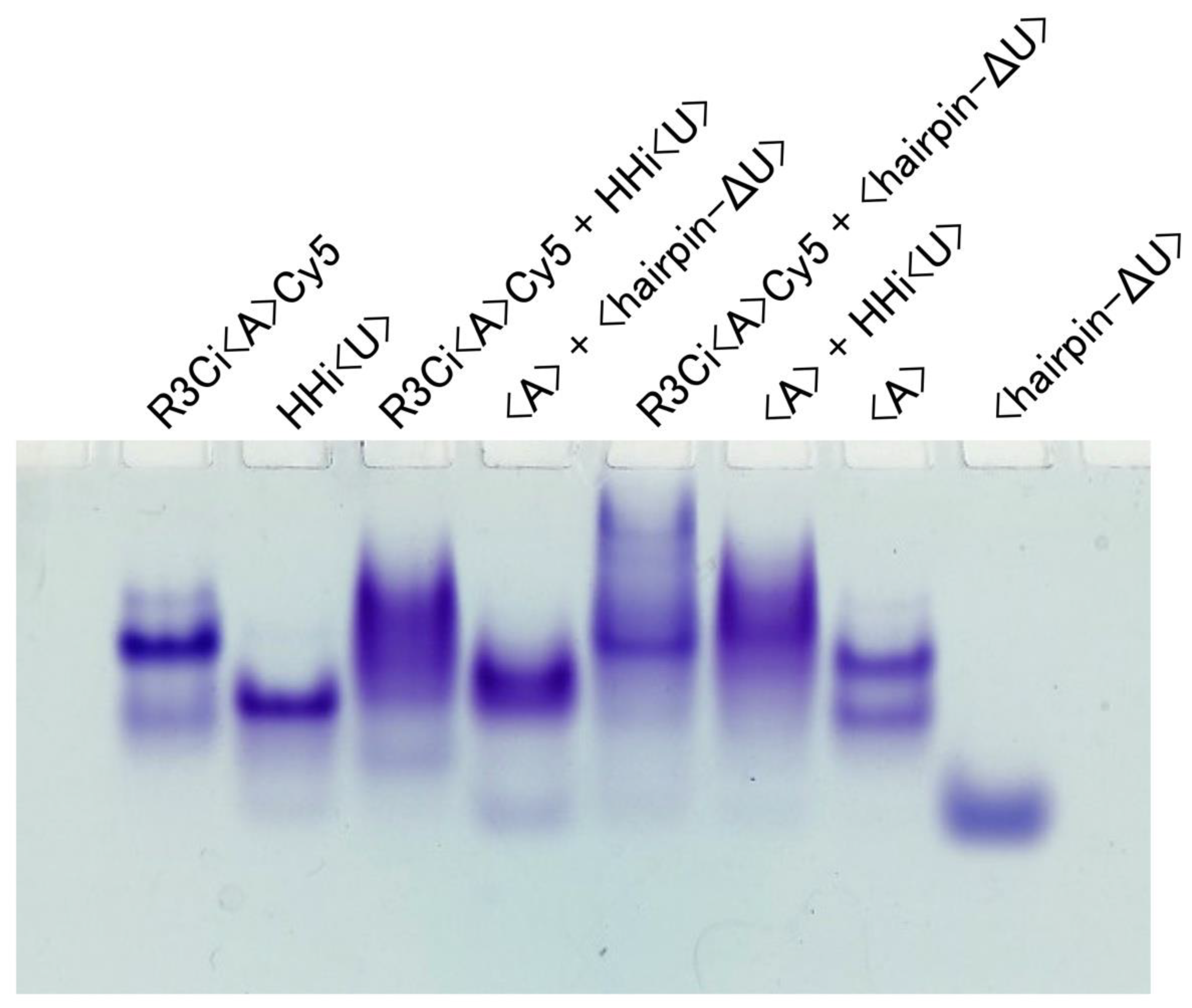
3.1. Electrophoretic Mobility Shift Assay (EMSA)
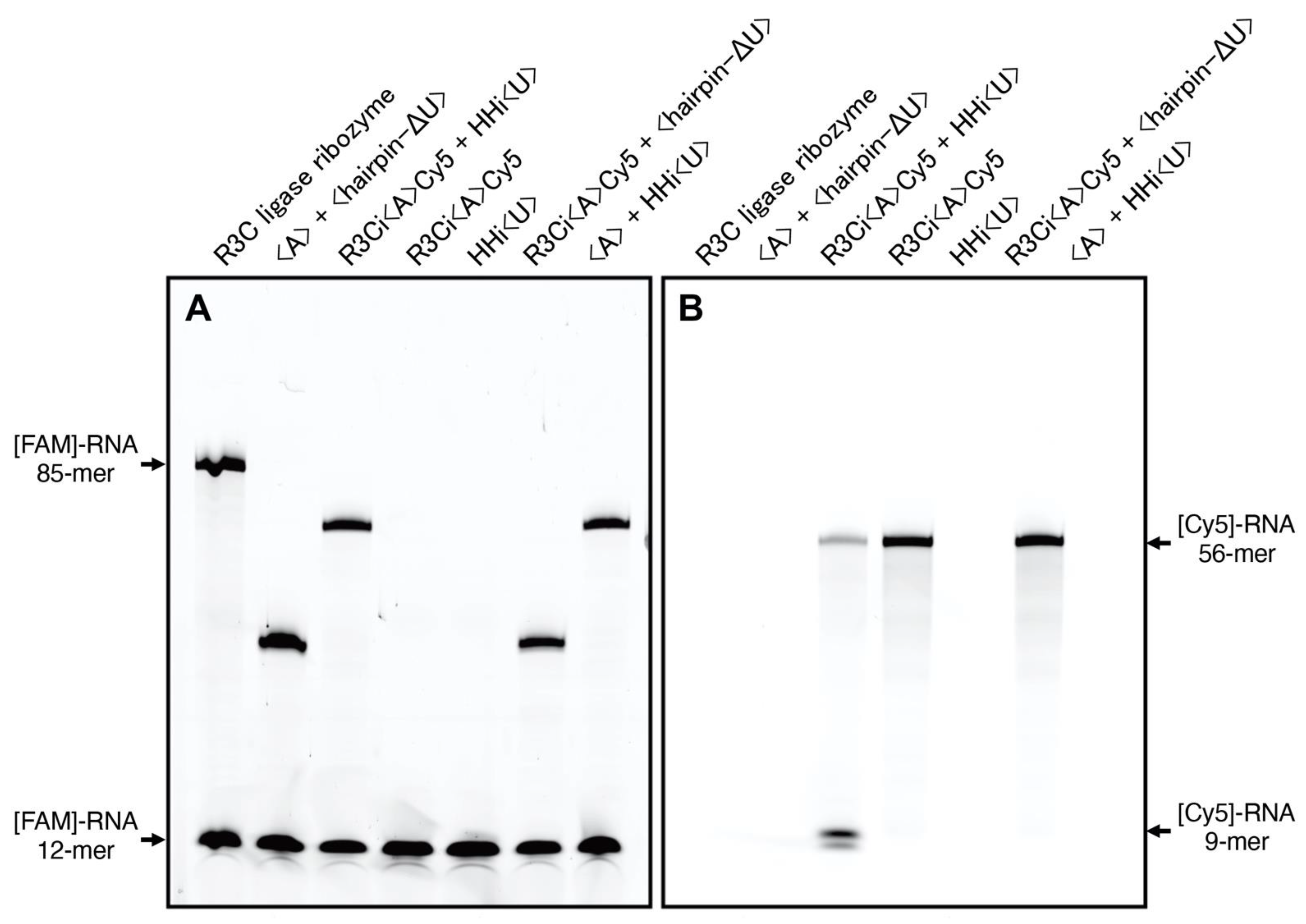
3.2. Ligation Activity towards 6-FAM Labeled Substrates
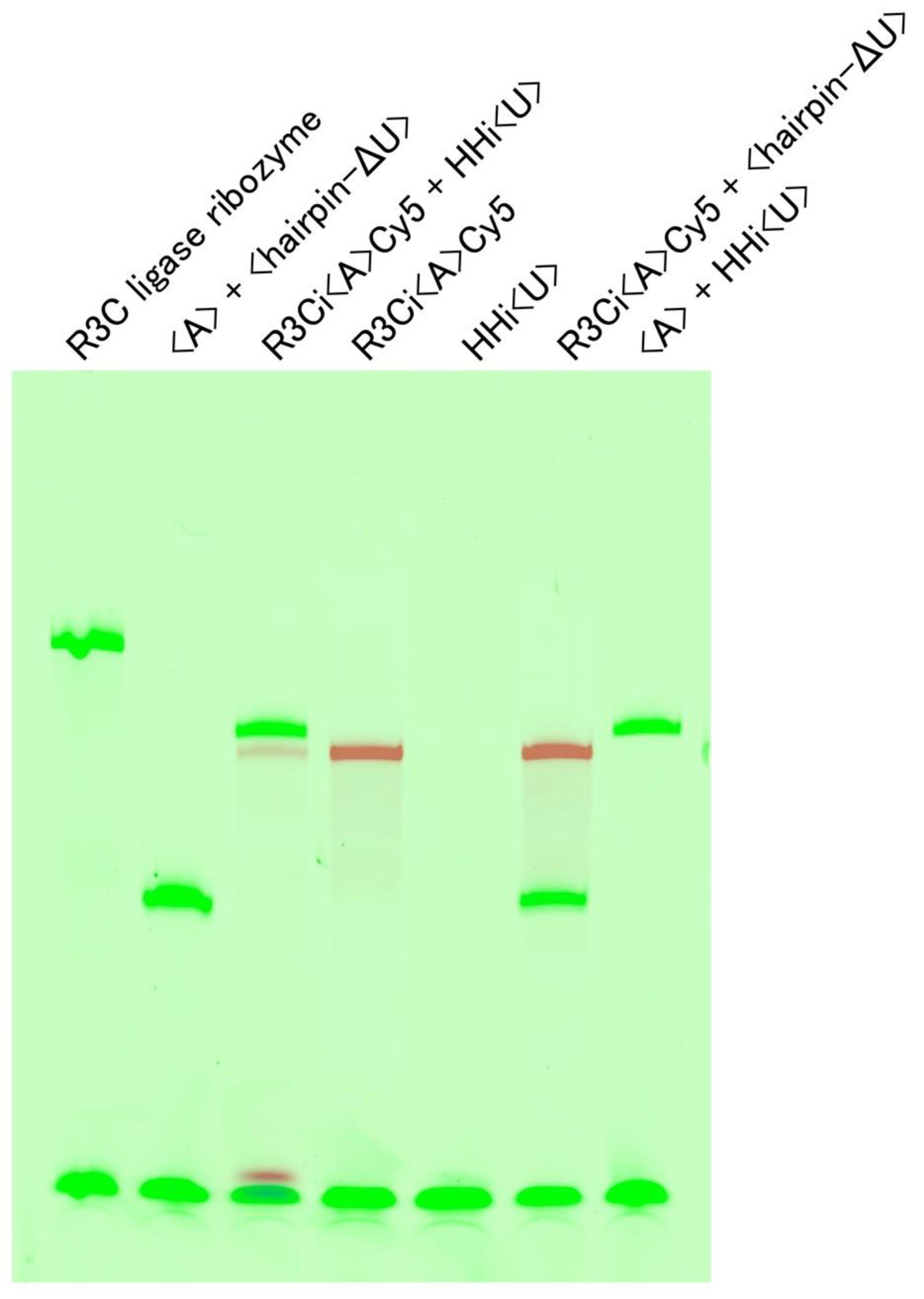
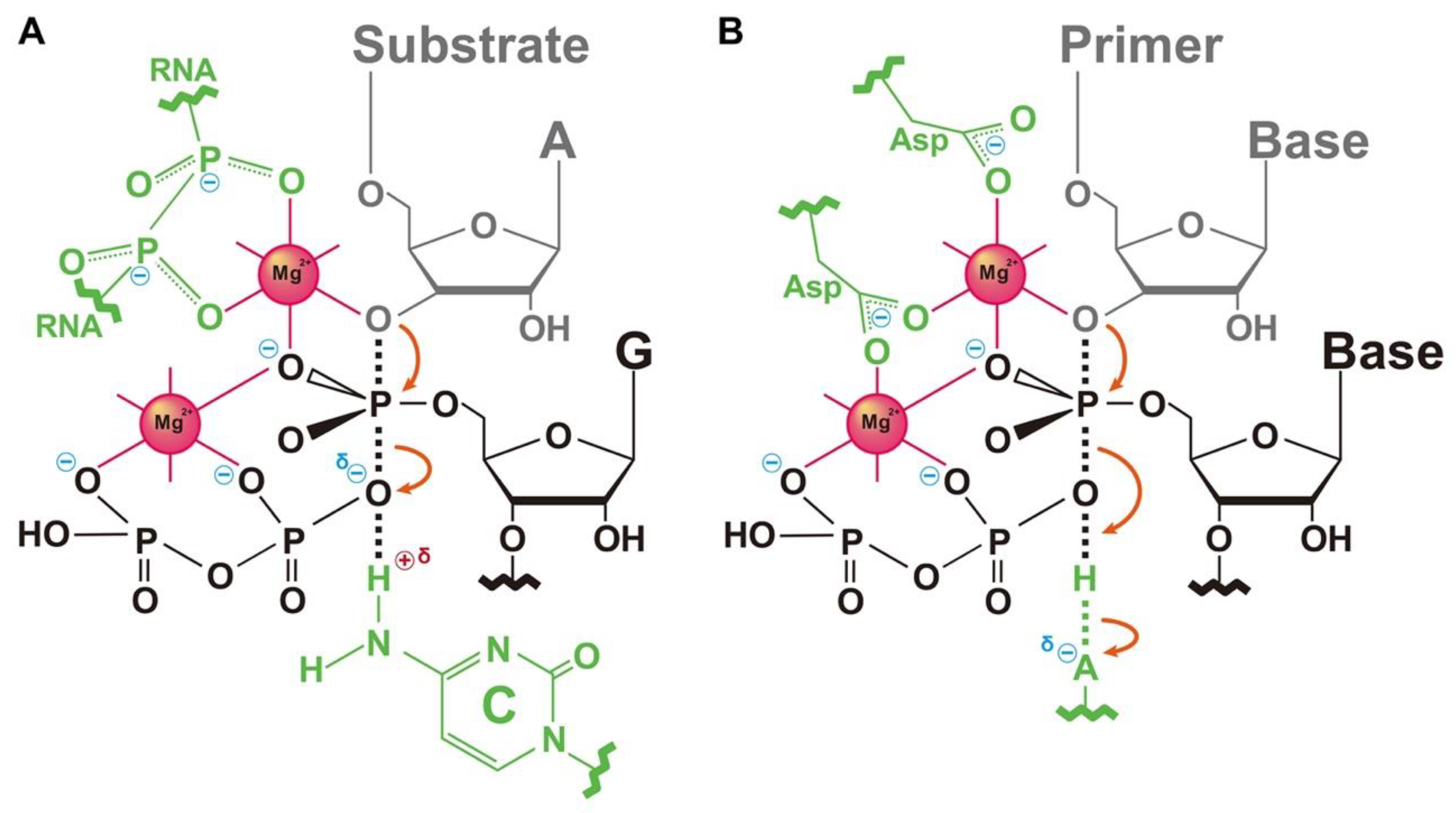
3.3. Cleavage of R3Ci<A>Cy5 by the Expected Hammerhead Ribozyme
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gilbert, W. Origin of life: The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Kruger, K.; Grabowski, P.J.; Zaug, A.J.; Sands, J.; Gottschling, D.E.; Cech, T.R. Self-splicing RNA: Autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell 1982, 31, 147–157. [Google Scholar] [CrossRef]
- Guerrier-Takada, C.; Gardiner, K.; Marsh, T.; Pace, N.; Altman, S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 1983, 35, 849–857. [Google Scholar] [CrossRef]
- Noller, H.F.; Hoffarth, V.; Zimniak, L. Unusual resistance of peptidyl transferase to protein extraction procedures. Science 1992, 256, 1416–1419. [Google Scholar] [CrossRef]
- Ban, N.; Nissen, P.; Hansen, J.; Moore, P.B.; Steitz, T.A. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 2000, 289, 905–920. [Google Scholar] [CrossRef]
- Nissen, P.; Hansen, J.; Ban, N.; Moore, P.B.; Steitz, T.A. The structural basis of ribosome activity in peptide bond synthesis. Science 2000, 289, 920–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Matsumura, S.; Ikawa, Y. Effects of molecular crowding on a bimolecular group I ribozyme and its derivative that self-assembles to form ribozyme oligomers. Biochem. Biophys. Res. Commun. 2018, 507, 136–141. [Google Scholar] [CrossRef]
- Rahman, M.S.; Gulshan, M.A.; Matsumura, S.; Ikawa, Y. Polyethylene glycol molecular crowders enhance the catalytic ability of bimolecular bacterial RNase P ribozymes. Nucleosides Nucleotides Nucleic Acids 2020, 39, 715–729. [Google Scholar] [CrossRef]
- Attwater, J.; Raguram, A.; Morgunov, A.S.; Gianni, E.; Holliger, P. Ribozyme-catalysed RNA synthesis using triplet building blocks. eLife 2018, 7, e35255. [Google Scholar] [CrossRef]
- Rogers, J.; Joyce, G.F. The effect of cytidine on the structure and function of an RNA ligase ribozyme. RNA 2001, 7, 395–404. [Google Scholar] [CrossRef]
- Kurihara, E.; Uchida, S.; Umehara, T.; Tamura, K. Development of a functionally minimized mutant of the R3C ligase ribozyme offers insight into the plausibility of the RNA world hypothesis. Biology 2014, 3, 452–465. [Google Scholar] [CrossRef] [Green Version]
- Tanizawa, K.; Uchida, S.; Kurihara, E.; Umehara, T.; Tamura, K. The kiss switch brings inactive R3C ligase ribozyme back to life. Biology 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamachi, K.; Mutsuro-Aoki, H.; Tanizawa, K.; Hirasawa, I.; Umehara, T.; Tamura, K. Effects of complementary loop composition in truncated R3C ligase ribozymes on kiss switch activation. Biosystems 2019, 177, 9–15. [Google Scholar] [CrossRef]
- Mutsuro-Aoki, H.; Hamachi, K.; Kurihara, R.; Tamura, K. Aminoacylation of short hairpin RNAs through kissing-loop interactions indicates evolutionary trend of RNA molecules. Biosystems 2020, 197, 104206. [Google Scholar] [CrossRef]
- Hou, Y.M.; Schimmel, P. A simple structural feature is a major determinant of the identity of a transfer RNA. Nature 1988, 333, 140–145. [Google Scholar] [CrossRef]
- McClain, W.H.; Foss, K. Changing the identity of a tRNA by introducing a G-U wobble pair near the 3′ acceptor end. Science 1988, 240, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.R.; Uhlenbeck, O.C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc. Natl. Acad. Sci. USA 1988, 85, 1033–1037. [Google Scholar] [CrossRef] [Green Version]
- Hamachi, K.; Hayashi, H.; Shimamura, M.; Yamaji, Y.; Kaneko, A.; Fujisawa, A.; Umehara, T.; Tamura, K. Glycols modulate terminator stem stability and ligand-dependency of a glycine riboswitch. Biosystems 2013, 113, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Buzayan, J.M.; Gerlach, W.L.; Bruening, G. Non-enzymatic cleavage and ligation of RNAs complementary to a plant virus satellite RNA. Nature 1986, 323, 349–353. [Google Scholar] [CrossRef]
- Forster, A.C.; Symons, R.H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell 1987, 49, 211–220. [Google Scholar] [CrossRef]
- Tamura, K. Origins and early evolution of the tRNA molecule. Life 2015, 5, 1687–1699. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Granja, J.R.; Martinez, J.A.; Severin, K.; Ghadiri, M.R. A self-replicating peptide. Nature 1996, 382, 525–528. [Google Scholar] [CrossRef]
- Tamura, K. Ribosome evolution: Emergence of peptide synthesis machinery. J. Biosci. 2011, 36, 921–928. [Google Scholar] [CrossRef]
- Agmon, I. The dimeric proto-ribosome: Structural details and possible implications on the origin of life. Int. J. Mol. Sci. 2009, 10, 2921–2934. [Google Scholar] [CrossRef] [Green Version]
- Agmon, I.; Bashan, A.; Zarivach, R.; Yonath, A. Symmetry at the active site of the ribosome: Structural and functional implications. Biol. Chem. 2005, 386, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, C.; Belousoff, M.; Wekselman, I.; Shapira, T.; Krupkin, M.; Zimmerman, E.; Bashan, A.; Yonath, A. The proto-ribosome: An ancient nano-machine for peptide bond formation. Isr. J. Chem. 2010, 50, 29–35. [Google Scholar] [CrossRef]
- Bose, T.; Fridkin, G.; Davidovich, C.; Krupkin, M.; Dinger, N.; Falkovich, A.H.; Peleg, Y.; Agmon, I.; Bashan, A.; Yonath, A. Origin of life: Protoribosome forms peptide bonds and links RNA and protein dominated worlds. Nucleic Acids Res. 2022, 50, 1815–1828. [Google Scholar] [CrossRef]
- Schmeing, T.M.; Seila, A.C.; Hansen, J.L.; Freeborn, B.; Soukup, J.K.; Scaringe, S.A.; Strobel, S.A.; Moore, P.B.; Steitz, T.A. A pre-ranslocational intermediate in protein synthesis observed in crystals of enzymatically active 50S subunits. Nat. Struct. Biol. 2002, 9, 225–230. [Google Scholar]
- Kawabata, M.; Kawashima, K.; Mutsuro-Aoki, H.; Ando, T.; Umehara, T.; Tamura, K. Peptide bond formation between aminoacyl-minihelices by a scaffold derived from the peptidyl transferase center. Life 2022, 12, 573. [Google Scholar] [CrossRef]
- Di Giulio, M. On the origin of protein synthesis: A speculative model based on hairpin RNA structures. J. Theor. Biol. 1994, 171, 303–308. [Google Scholar] [CrossRef]
- Di Giulio, M. Was it an ancient gene codifying for a hairpin RNA that, by means of direct duplication, gave rise to the primitive tRNA molecule? J. Theor. Biol. 1995, 177, 95–101. [Google Scholar] [CrossRef]
- Schimmel, P.; Giegé, R.; Moras, D.; Yokoyama, S. An operational RNA code for amino acids and possible relationship to genetic code. Proc. Natl. Acad. Sci. USA 1993, 90, 8763–8768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schimmel, P.; Ribas de Pouplana, L. Transfer RNA: From minihelix to genetic code. Cell 1995, 81, 983–986. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Schimmel, P. Chiral-selective aminoacylation of an RNA minihelix. Science 2004, 305, 1253. [Google Scholar] [CrossRef]
- Tamura, K.; Schimmel, P.R. Chiral-selective aminoacylation of an RNA minihelix: Mechanistic features and chiral suppression. Proc. Natl. Acad. Sci. USA 2006, 103, 13750–13752. [Google Scholar] [CrossRef] [Green Version]
- Ando, T.; Takahashi, S.; Tamura, K. Principles of chemical geometry underlying chiral selectivity in RNA minihelix aminoacylation. Nucleic Acids Res. 2018, 46, 11144–11152. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K. Perspectives on the origin of biological homochirality on Earth. J. Mol. Evol. 2019, 87, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Franch, T.; Petersen, M.; Wagner, E.G.; Jacobsen, J.P.; Gerdes, K. Antisense RNA regulation in prokaryotes: Rapid RNA/RNA interaction facilitated by a general U-turn loop structure. J. Mol. Biol. 1999, 294, 1115–1125. [Google Scholar] [CrossRef]
- Franch, T.; Gerdes, K. U-turns and regulatory RNAs. Curr. Opin. Microbiol. 2000, 3, 159–164. [Google Scholar] [CrossRef]
- Kolb, F.A.; Westhof, E.; Ehresmann, B.; Ehresmann, C.; Wagner, E.G.; Romby, P. Four-way junctions in antisense RNA–mRNA complexes involved in plasmid replication control: A common theme? J. Mol. Biol. 2001, 309, 605–614. [Google Scholar] [CrossRef]
- Andersen, A.A.; Collins, R.A. Rearrangement of a stable RNA secondary structure during VS ribozyme catalysis. Mol. Cell 2000, 5, 469–478. [Google Scholar] [CrossRef]
- DasGupta, S.; Suslov, N.B.; Piccirilli, J.A. Structural basis for substrate helix remodeling and cleavage loop activation in the Varkud satellite ribozyme. J. Am. Chem. Soc. 2017, 139, 9591–9597. [Google Scholar] [CrossRef]
- DasGupta, S.; Nykiel, K.; Piccirilli, J.A. The hammerhead self-cleaving motif as a precursor to complex endonucleolytic ribozymes. RNA 2021, 27, 1017–1024. [Google Scholar] [CrossRef]
- Skripkin, E.; Paillart, J.C.; Marquet, R.; Ehresmann, B.; Ehresmann, C. Identification of the primary site of the human immunodeficiency virus type 1 RNA dimerization in vitro. Proc. Natl. Acad. Sci. USA 1994, 91, 4945–4949. [Google Scholar] [CrossRef] [Green Version]
- Laughrea, M.; Jetté, L. A 19-nucleotide sequence upstream of the 5′ major splice donor is part of the dimerization domain of human immunodeficiency virus 1 genomic RNA. Biochemistry 1994, 33, 13464–13474. [Google Scholar] [CrossRef]
- Laughrea, M.; Jetté, L. Kissing-loop model of HIV-1 genome dimerization: HIV-1 RNAs can assume alternative dimeric forms, and all sequences upstream or downstream of hairpin 248-271 are dispensable for dimer formation. Biochemistry 1996, 35, 1589–1598. [Google Scholar] [CrossRef]
- Takahashi, K.I.; Baba, S.; Chattopadhyay, P.; Koyanagi, Y.; Yamamoto, N.; Takaku, H.; Kawai, G. Structural requirement for the two-step dimerization of human immunodeficiency virus type 1 genome. RNA 2000, 6, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Brunel, C.; Marquet, R.; Romby, P.; Ehresmann, C. RNA loop-loop interactions as dynamic functional motifs. Biochimie 2002, 84, 925–944. [Google Scholar] [CrossRef]
- Eigen, M.; Schuster, P. The hypercycle. A principle of natural self-organization. Part A: Emergence of the hypercycle. Naturwissenschaften 1977, 64, 541–565. [Google Scholar] [CrossRef]
- Ferris, J.P. Montmorillonite-catalysed formation of RNA oligomers: The possible role of catalysis in the origins of life. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1777–1786. [Google Scholar] [CrossRef] [Green Version]
- Robertson, M.P.; Hesselberth, J.R.; Ellington, A.D. Optimization and optimality of a short ribozyme ligase that joins non-Watson-Crick base pairings. RNA 2001, 7, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Yokobayashi, Y. Systematic minimization of RNA ligase ribozyme through large-scale design-synthesis-sequence cycles. Nucleic Acids Res. 2019, 47, 8950–8960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekland, E.H.; Szostak, J.W.; Bartel, D.P. Structurally complex and highly active RNA ligases derived from random RNA sequences. Science 1995, 269, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Joyce, G.F. A glimpse of biology’s first enzyme. Science 2007, 315, 1507–1508. [Google Scholar] [CrossRef] [Green Version]
- Shechner, D.M.; Grant, R.A.; Bagby, S.C.; Koldobskaya, Y.; Piccirilli, J.A.; Bartel, D.P. Crystal structure of the catalytic core of an RNA-polymerase ribozyme. Science 2009, 326, 1271–1275. [Google Scholar] [CrossRef] [Green Version]
- Sträter, N.; Lipscomb, W.N.; Klabunde, T.; Krebs, B. Two-metal ion catalysis in enzymatic acyl- and phosphoryl-transfer reactions. Angew. Chem. Int. Ed. Engl. 1996, 35, 2024–2055. [Google Scholar] [CrossRef]
- Castro, C.; Smidansky, E.D.; Arnold, J.J.; Maksimchuk, K.R.; Moustafa, I.; Uchida, A.; Götte, M.; Konigsberg, W.; Cameron, C.E. Nucleic acid polymerases use a general acid for nucleotidyl transfer. Nat. Struct. Mol. Biol. 2009, 16, 212–218. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutsuro-Aoki, H.; Tamura, K. Acquisition of Dual Ribozyme-Functions in Nonfunctional Short Hairpin RNAs through Kissing-Loop Interactions. Life 2022, 12, 1561. https://doi.org/10.3390/life12101561
Mutsuro-Aoki H, Tamura K. Acquisition of Dual Ribozyme-Functions in Nonfunctional Short Hairpin RNAs through Kissing-Loop Interactions. Life. 2022; 12(10):1561. https://doi.org/10.3390/life12101561
Chicago/Turabian StyleMutsuro-Aoki, Hiromi, and Koji Tamura. 2022. "Acquisition of Dual Ribozyme-Functions in Nonfunctional Short Hairpin RNAs through Kissing-Loop Interactions" Life 12, no. 10: 1561. https://doi.org/10.3390/life12101561
APA StyleMutsuro-Aoki, H., & Tamura, K. (2022). Acquisition of Dual Ribozyme-Functions in Nonfunctional Short Hairpin RNAs through Kissing-Loop Interactions. Life, 12(10), 1561. https://doi.org/10.3390/life12101561





