Human Retrotransposons and Effective Computational Detection Methods for Next-Generation Sequencing Data
Abstract
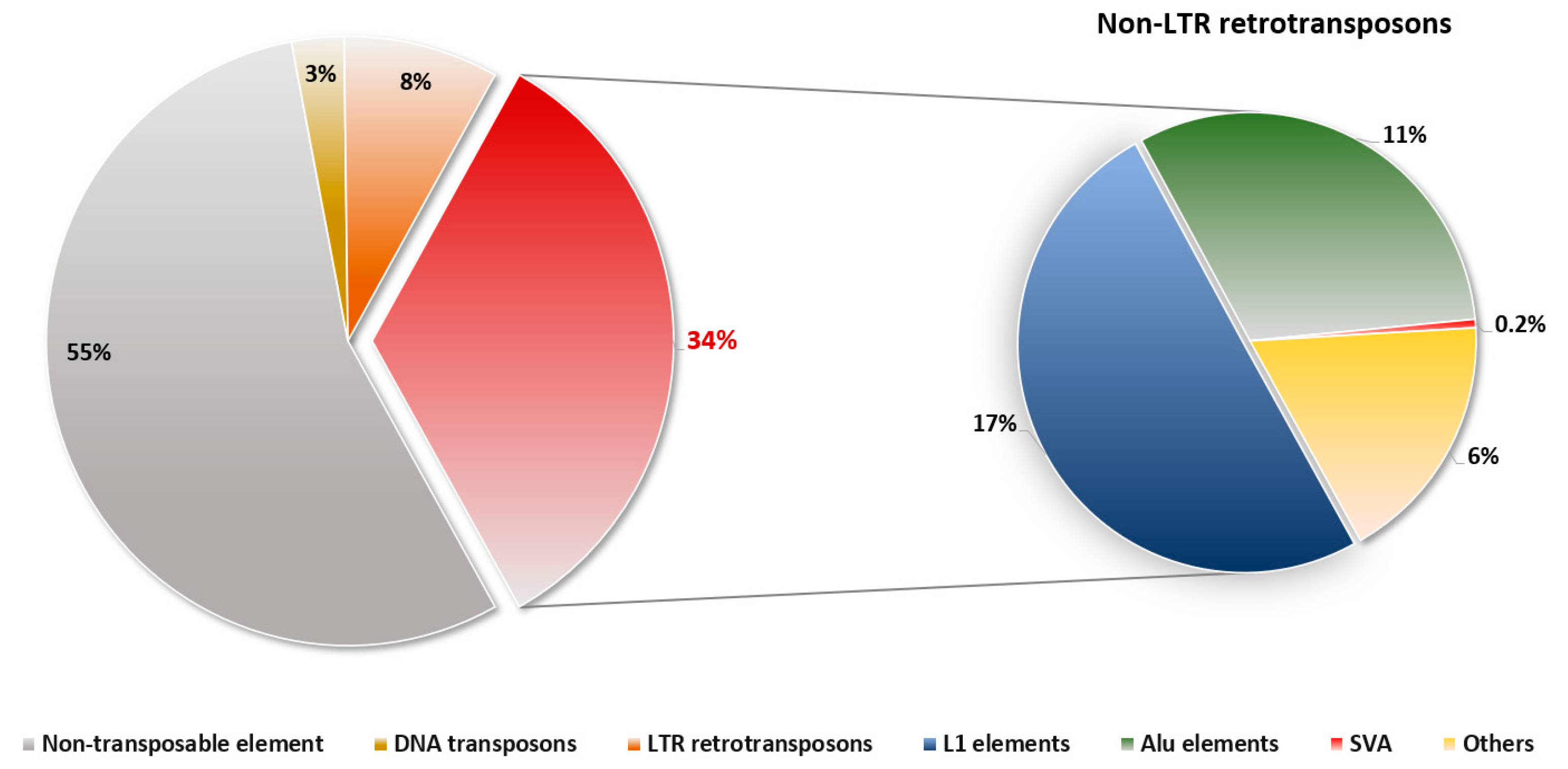
:1. Introduction
2. Non-LTR Retrotransposons in Humans
2.1. LINE-1 (L1) Elements
2.2. Alu Elements
2.3. SINE-VNTR-Alu (SVA) Elements
3. Representative Next-Generation Sequencing (NGS) Platforms
3.1. Illumina
3.2. MGI
3.3. PacBio and Nanopore
4. Computational Methods to Detect Retrotransposons in Humans Based on NGS
4.1. Short-Read Sequencing Data
4.1.1. RetroSeq
4.1.2. Alu-Detect
4.1.3. Tangram
4.1.4. Mobile Element Locator Tool (MELT)
4.1.5. IMGEins
4.2. Alignment-Free Raw Reads
AluMine
4.3. Long-Read Sequencing Data
PremAsking Long Reads for Mobile Element InseRtion (PALMER)
4.4. Hybrid Sequencing Data
x-Transposable Element Analyzer (xTea)
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cordaux, R.; Batzer, M.A. The impact of retrotransposons on human genome evolution. Nat. Rev. Genet. 2009, 10, 691–703. [Google Scholar] [CrossRef] [Green Version]
- McClintock, B. Controlling elements and the gene. Cold Spring Harb. Symp. Quant. Biol. 1956, 21, 197–216. [Google Scholar] [CrossRef]
- de Koning, A.P.J.; Gu, W.J.; Castoe, T.A.; Batzer, M.A.; Pollock, D.D. Repetitive Elements May Comprise Over Two-Thirds of the Human Genome. PLoS Genet. 2011, 7, e1002384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pheasant, M.; Mattick, J.S. Raising the estimate of functional human sequences. Genome Res. 2007, 17, 1245–1253. [Google Scholar] [CrossRef] [Green Version]
- Kazazian, H.H. Mobile elements: Drivers of genome evolution. Science 2004, 303, 1626–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batzer, M.A.; Deininger, P.L. Alu repeats and human genomic diversity. Nat. Rev. Genet. 2002, 3, 370–379. [Google Scholar] [CrossRef]
- Bourque, G.; Burns, K.H.; Gehring, M.; Gorbunova, V.; Seluanov, A.; Hammell, M.; Imbeault, M.; Izsvák, Z.; Levin, H.L.; Macfarlan, T.S.; et al. Ten things you should know about transposable elements. Genome Biol. 2018, 19, 199. [Google Scholar] [CrossRef] [PubMed]
- Callinan, P.A.; Batzer, M.A. Retrotransposable elements and human disease. Genome Dyn. 2006, 1, 104–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodier, J.L.; Kazazian, H.H. Retrotransposons revisited: The restraint and rehabilitation of parasites. Cell 2008, 135, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Pace, J.K.; Feschotte, C. The evolutionary history of human DNA transposons: Evidence for intense activity in the primate lineage. Genome Res. 2007, 17, 422–432. [Google Scholar] [CrossRef]
- Goodier, J.L. Restricting retrotransposons: A review. Mob. DNA 2016, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babushok, D.V.; Kazazian, H.H., Jr. Progress in understanding the biology of the human mutagen LINE-1. Hum. Mutat. 2007, 28, 527–539. [Google Scholar] [CrossRef]
- Beck, C.R.; Garcia-Perez, J.L.; Badge, R.M.; Moran, J.V. LINE-1 elements in structural variation and disease. Annu. Rev. Genom. Hum. Genet. 2011, 12, 187–215. [Google Scholar] [CrossRef] [Green Version]
- Benachenhou, F.; Jern, P.; Oja, M.; Sperber, G.; Blikstad, V.; Somervuo, P.; Kaski, S.; Blomberg, J. Evolutionary conservation of orthoretroviral long terminal repeats (LTRs) and ab initio detection of single LTRs in genomic data. PLoS ONE 2009, 4, e5179. [Google Scholar] [CrossRef] [PubMed]
- Durnaoglu, S.; Lee, S.K.; Ahnn, J. Human Endogenous Retroviruses as Gene Expression Regulators: Insights from Animal Models into Human Diseases. Mol. Cells 2021, 44, 861–878. [Google Scholar] [CrossRef] [PubMed]
- Alcazer, V.; Bonaventura, P.; Depil, S. Human Endogenous Retroviruses (HERVs): Shaping the Innate Immune Response in Cancers. Cancers 2020, 12, 610. [Google Scholar] [CrossRef] [Green Version]
- Deininger, P.L.; Batzer, M.A. Alu repeats and human disease. Mol. Genet. Metab. 1999, 67, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez, N.A.; Macia, A.; Muotri, A.R. LINE-1 retrotransposons in healthy and diseased human brain. Dev. Neurobiol. 2018, 78, 434–455. [Google Scholar] [CrossRef]
- Burwinkel, B.; Kilimann, M.W. Unequal homologous recombination between LINE-1 elements as a mutational mechanism in human genetic disease. J. Mol. Biol. 1998, 277, 513–517. [Google Scholar] [CrossRef]
- Taskesen, M.; Collin, G.B.; Evsikov, A.V.; Guzel, A.; Ozgul, R.K.; Marshall, J.D.; Naggert, J.K. Novel Alu retrotransposon insertion leading to Alstrom syndrome. Hum. Genet. 2012, 131, 407–413. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, X.X.; Zhang, H.; Xiao, N.; Zhu, C.D.; He, Q.; Guo, W.W.; Cai, Z.M.; Shen, H.B.; Wang, Y.P. AluYb8 Insertion in the MUTYH Gene and Risk of Early-onset Breast and Gastric Cancers in the Chinese Population. Asian Pac. J. Cancer Prev. 2011, 12, 1451–1455. [Google Scholar] [PubMed]
- Venet, T.; Masson, E.; Talbotec, C.; Billiemaz, K.; Touraine, R.; Gay, C.; Destombe, S.; Cooper, D.N.; Patural, H.; Chen, J.M.; et al. Severe infantile isolated exocrine pancreatic insufficiency caused by the complete functional loss of the SPINK1 gene. Hum. Mutat. 2017, 38, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Terry, D.M.; Devine, S.E. Aberrantly High Levels of Somatic LINE-1 Expression and Retrotransposition in Human Neurological Disorders. Front. Genet. 2020, 10, 1244. [Google Scholar] [CrossRef] [Green Version]
- Kazazian, H.H., Jr.; Wong, C.; Youssoufian, H.; Scott, A.F.; Phillips, D.G.; Antonarakis, S.E. Haemophilia A resulting from de novo insertion of L1 sequences represents a novel mechanism for mutation in man. Nature 1988, 332, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, V.; Lopez-Diaz, C.; Di Pietro, A.; Ma, L.J.; Ayhan, D.H. TEfinder: A Bioinformatics Pipeline for Detecting New Transposable Element Insertion Events in Next-Generation Sequencing Data. Genes 2021, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Zytnicki, M.; Akhunov, E.; Quesneville, H. Tedna: A transposable element de novo assembler. Bioinformatics 2014, 30, 2656–2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, P.; Neumann, P.; Pech, J.; Steinhaisl, J.; Macas, J. RepeatExplorer: A Galaxy-based web server for genome-wide characterization of eukaryotic repetitive elements from next-generation sequence reads. Bioinformatics 2013, 29, 792–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Wang, H. LTR_FINDER: An efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res. 2007, 35, W265–W268. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, E.M.; McDonald, J.F. LTR_STRUC: A novel search and identification program for LTR retrotransposons. Bioinformatics 2003, 19, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Kalyanaraman, A.; Aluru, S. Efficient algorithms and software for detection of full-length LTR retrotransposons. J. Bioinform. Comput. Biol. 2006, 4, 197–216. [Google Scholar] [CrossRef]
- Jeong, H.H.; Yalamanchili, H.K.; Guo, C.W.; Shulman, J.M.; Liu, Z.D. An ultra-fast and scalable quantification pipeline for transposable elements from next generation sequencing data. Pac. Symp. Biocomput. 2018, 23, 168–179. [Google Scholar]
- McCoy, R.C.; Taylor, R.W.; Blauwkamp, T.A.; Kelley, J.L.; Kertesz, M.; Pushkarev, D.; Petrov, D.A.; Fiston-Lavier, A.S. Illumina TruSeq synthetic long-reads empower de novo assembly and resolve complex, highly-repetitive transposable elements. PLoS ONE 2014, 9, e106689. [Google Scholar] [CrossRef] [Green Version]
- Mir, A.A.; Philippe, C.; Cristofari, G. euL1db: The European database of L1HS retrotransposon insertions in humans. Nucleic Acids Res. 2015, 43, D43–D47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khazina, E.; Weichenrieder, O. Human LINE-1 retrotransposition requires a metastable coiled coil and a positively charged N-terminus in L1ORF1p. Elife 2018, 7, e34960. [Google Scholar] [CrossRef] [PubMed]
- Pinter, T.B.J.; Ruckthong, L.; Stuckey, J.A.; Deb, A.; Penner-Hahn, J.E.; Pecoraro, V.L. Open Reading Frame 1 Protein of the Human Long Interspersed Nuclear Element 1 Retrotransposon Binds Multiple Equivalents of Lead. J. Am. Chem. Soc. 2021, 143, 15271–15278. [Google Scholar] [CrossRef]
- Ruckthong, L.; Zastrow, M.L.; Stuckey, J.A.; Pecoraro, V.L. A Crystallographic Examination of Predisposition versus Preorganization in de Novo Designed Metalloproteins. J. Am. Chem. Soc. 2016, 138, 11979–11988. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Gilbert, N.; Ooi, S.L.; Lawler, J.F.; Ostertag, E.M.; Kazazian, H.H.; Boeke, J.D.; Moran, J.V. Human L1 retrotransposition: Cis preference versus trans complementation. Mol. Cell. Biol. 2001, 21, 1429–1439. [Google Scholar] [CrossRef] [Green Version]
- Sultana, T.; van Essen, D.; Siol, O.; Bailly-Bechet, M.; Philippe, C.; El Aabidine, A.Z.; Pioger, L.; Nigumann, P.; Saccani, S.; Andrau, J.C.; et al. The Landscape of L1 Retrotransposons in the Human Genome Is Shaped by Pre-insertion Sequence Biases and Post-insertion Selection. Mol. Cell 2019, 74, 555–570.e7. [Google Scholar] [CrossRef]
- Shin, W.; Mun, S.; Kim, J.; Lee, W.; Park, D.G.; Choi, S.; Lee, T.Y.; Cha, S.; Han, K. Novel Discovery of LINE-1 in a Korean Individual by a Target Enrichment Method. Mol. Cells 2019, 42, 87–95. [Google Scholar] [CrossRef]
- Khan, H.; Smit, A.; Boissinot, S. Molecular evolution and tempo of amplification of human LINE-1 retrotransposons since the origin of primates. Genome Res. 2006, 16, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Sudmant, P.H.; Rausch, T.; Gardner, E.J.; Handsaker, R.E.; Abyzov, A.; Huddleston, J.; Zhang, Y.; Ye, K.; Jun, G.; Fritz, M.H.Y.; et al. An integrated map of structural variation in 2504 human genomes. Nature 2015, 526, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Szak, S.T.; Pickeral, O.K.; Makalowski, W.; Boguski, M.S.; Landsman, D.; Boeke, J.D. Molecular archeology of L1 insertions in the human genome. Genome Biol. 2002, 3, research0052. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Lee, J.; Meyer, T.J.; Remedios, P.; Goodwin, L.; Batzer, M.A. L1 recombination-associated deletions generate human genomic variation. Proc. Natl. Acad. Sci. USA 2008, 105, 19366–19371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovchinnikov, I.; Troxel, A.B.; Swergold, G.D. Genomic characterization of recent human LINE-1 insertions: Evidence supporting random insertion. Genome Res. 2001, 11, 2050–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [Green Version]
- Scott, E.C.; Devine, S.E. The Role of Somatic L1 Retrotransposition in Human Cancers. Viruses 2017, 9, 131. [Google Scholar] [CrossRef]
- Burns, K.H. Transposable elements in cancer. Nat. Rev. Cancer 2017, 17, 415–424. [Google Scholar] [CrossRef]
- Faulkner, G.J.; Garcia-Perez, J.L. L1 Mosaicism in Mammals: Extent, Effects, and Evolution. Trends Genet. 2017, 33, 802–816. [Google Scholar] [CrossRef] [Green Version]
- Ullu, E.; Tschudi, C. Alu sequences are processed 7SL RNA genes. Nature 1984, 312, 171–172. [Google Scholar] [CrossRef]
- Kriegs, J.O.; Churakov, G.; Jurka, J.; Brosius, J.; Schmitz, J. Evolutionary history of 7SL RNA-derived SINEs in Supraprimates. Trends Genet. 2007, 23, 158–161. [Google Scholar] [CrossRef]
- Li, T.H.; Schmid, C.W. Alu’s dimeric consensus sequence destabilizes its transcripts. Gene 2004, 324, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.Y.; Hsu, K.; Maraia, R.J. Monomeric scAlu and nascent dimeric Alu RNAs induced by adenovirus are assembled into SRP9/14-containing RNPs in HeLa cells. Nucleic Acids Res. 1996, 24, 4165–4170. [Google Scholar] [CrossRef] [PubMed]
- Sarrowa, J.; Chang, D.Y.; Maraia, R.J. The decline in human Alu retroposition was accompanied by an asymmetric decrease in SRP9/14 binding to dimeric Alu RNA and increased expression of small cytoplasmic Alu RNA. Mol. Cell. Biol. 1997, 17, 1144–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weichenrieder, O.; Wild, K.; Strub, K.; Cusack, S. Structure and assembly of the Alu domain of the mammalian signal recognition particle. Nature 2000, 408, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, E.A.; Keller, H.; Mills, R.E.; Schmidt, S.; Moran, J.V.; Weichenrieder, O.; Devine, S.E. Active Alu retrotransposons in the human genome. Genome Res. 2008, 18, 1875–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comeaux, M.S.; Roy-Engel, A.M.; Hedges, D.J.; Deininger, P.L. Diverse cis factors controlling Alu retrotransposition: What causes Alu elements to die? Genome Res. 2009, 19, 545–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, A.M. SINEs and LINEs: The art of biting the hand that feeds you. Curr. Opin. Cell Biol. 2002, 14, 343–350. [Google Scholar] [CrossRef]
- Ostertag, E.M.; Goodier, J.L.; Zhang, Y.; Kazazian, H.H. SVA elements are nonautonomous retrotransposons that cause disease in humans. Am. J. Hum. Genet. 2003, 73, 1444–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroutter, E.N.; Belancio, V.P.; Wagstaff, B.J.; Roy-Engel, A.M. The RNA polymerase dictates ORF1 requirement and timing of LINE and SINE retrotransposition. PLoS Genet. 2009, 5, e1000458. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Cho, C.S.; Han, K.; Lee, J. Structural Variation of Alu Element and Human Disease. Genom. Inform. 2016, 14, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batzer, M.A.; Deininger, P.L. A human-specific subfamily of Alu sequences. Genomics 1991, 9, 481–487. [Google Scholar] [CrossRef]
- Baker, J.N.; Walker, J.A.; Vanchiere, J.A.; Phillippe, K.R.; Romain, C.P.S.; Gonzalez-Quiroga, P.; Denham, M.W.; Mierl, J.R.; Konkel, M.K.; Batzer, M.A. Evolution of Alu Subfamily Structure in the Saimiri Lineage of New World Monkeys. Genome Biol. Evol. 2017, 9, 2365–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLain, A.T.; Carman, G.W.; Fullerton, M.L.; Beckstrom, T.O.; Gensler, W.; Meyer, T.J.; Faulk, C.; Batzer, M.A. Analysis of western lowland gorilla (Gorilla gorilla gorilla) specific Alu repeats. Mob. DNA 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steely, C.J.; Baker, J.N.; Walker, J.A.; Loupe, C.D., 3rd; Baboon Genome Analysis Consortium; Batzer, M.A. Analysis of lineage-specific Alu subfamilies in the genome of the olive baboon, Papio anubis. Mob. DNA 2018, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Konkel, M.K.; Walker, J.A.; Hotard, A.B.; Ranck, M.C.; Fontenot, C.C.; Storer, J.; Stewart, C.; Marth, G.T.; Batzer, M.A.; Consortium, G. Sequence Analysis and Characterization of Active Human Alu Subfamilies Based on the 1000 Genomes Pilot Project. Genome Biol. Evol. 2015, 7, 2608–2622. [Google Scholar] [CrossRef] [Green Version]
- Apoil, P.A.; Kuhlein, E.; Robert, A.; Rubie, H.; Blancher, A. HIGM syndrome caused by insertion of an AluYb8 element in exon 1 of the CD40LG gene. Immunogenetics 2007, 59, 17–23. [Google Scholar] [CrossRef]
- Ade, C.; Roy-Engel, A.M.; Deininger, P.L. Alu elements: An intrinsic source of human genome instability. Curr. Opin. Virol. 2013, 3, 639–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teugels, E.; De Brakeleer, S.; Goelen, G.; Lissens, W.; Sermijn, E.; De Greve, J. De Novo Alu Element Insertions Targeted to a Sequence Common to the BRCA1 and BRCA2 Genes. Hum. Mutat. 2005, 26, 284. [Google Scholar] [CrossRef] [PubMed]
- Belancio, V.P.; Hedges, D.J.; Deininger, P. Mammalian non-LTR retrotransposons: For better or worse, in sickness and in health. Genome Res. 2008, 18, 343–358. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.; Kural, D.; Stromberg, M.P.; Walker, J.A.; Konkel, M.K.; Stutz, A.M.; Urban, A.E.; Grubert, F.; Lam, H.Y.; Lee, W.P.; et al. A comprehensive map of mobile element insertion polymorphisms in humans. PLoS Genet. 2011, 7, e1002236. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.; Loh, J.W.; Xing, J.C. Identification of polymorphic SVA retrotransposons using a mobile element scanning method for SVA (ME-Scan-SVA). Mob. DNA 2016, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xing, J.; Grover, D.; Hedges, D.J.; Han, K.; Walker, J.A.; Batzer, M.A. SVA elements: A hominid-specific retroposon family. J. Mol. Biol. 2005, 354, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Raiz, J.; Damert, A.; Chira, S.; Held, U.; Klawitter, S.; Hamdorf, M.; Lower, J.; Stratling, W.H.; Lower, R.; Schumann, G.G. The non-autonomous retrotransposon SVA is trans-mobilized by the human LINE-1 protein machinery. Nucleic Acids Res. 2012, 40, 1666–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancks, D.C.; Kazazian, H.H. SVA retrotransposons: Evolution and genetic instability. Semin. Cancer Biol. 2010, 20, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Murata, M.; Takagi, Y.; Kozuka, T.; Nakata, Y.; Hasebe, R.; Takagi, A.; Kitazawa, J.; Shima, M.; Kojima, T. SVA retrotransposition in exon 6 of the coagulation factor IX gene causing severe hemophilia B. Int. J. Hematol. 2015, 102, 134–139. [Google Scholar] [CrossRef]
- Chesnokova, E.; Beletskiy, A.; Kolosov, P. The Role of Transposable Elements of the Human Genome in Neuronal Function and Pathology. Int. J. Mol. Sci. 2022, 23, 5847. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Choi, Y.; Eo, J.; Noh, Y.N.; Gim, J.A.; Jung, Y.D.; Lee, J.R.; Kim, H.S. Structure and Expression Analyses of SVA Elements in Relation to Functional Genes. Genom. Inform. 2013, 11, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.Y.; Chiu, Y.C.; Wang, L.B.; Kuo, Y.L.; Chuang, E.Y.; Lai, L.C.; Tsai, M.H. Common applications of next-generation sequencing technologies in genomic research. Transl. Cancer Res. 2013, 2, 33–45. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Steinberg, K.M.; Larson, D.E.; Wilson, R.K.; Mardis, E.R. The next-generation sequencing revolution and its impact on genomics. Cell 2013, 155, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, C.D.; Di Giacomo, G.; Mesoraca, A.; D’Emidio, L.; Iaconianni, P.; Minutolo, E.; Lippa, A.; Giorlandino, C. Next generation sequencing in the identification of a rare genetic disease from preconceptional couple screening to preimplantation genetic diagnosis. J. Prenat. Med. 2014, 8, 17–24. [Google Scholar] [CrossRef]
- Metzker, M.L. Sequencing technologies—the next generation. Nat. Rev. Genet. 2010, 11, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Rattenberry, E.; Vialard, L.; Yeung, A.; Bair, H.; McKay, K.; Jafri, M.; Canham, N.; Cole, T.R.; Denes, J.; Hodgson, S.V.; et al. A comprehensive next generation sequencing-based genetic testing strategy to improve diagnosis of inherited pheochromocytoma and paraganglioma. J. Clin. Endocrinol. Metab. 2013, 98, E1248–E1256. [Google Scholar] [CrossRef] [PubMed]
- Hartman, P.; Beckman, K.; Silverstein, K.; Yohe, S.; Schomaker, M.; Henzler, C.; Onsongo, G.; Lam, H.C.; Munro, S.; Daniel, J.; et al. Next generation sequencing for clinical diagnostics: Five year experience of an academic laboratory. Mol. Genet. Metab. Rep. 2019, 19, 100464. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Esposito, M.V.; Telese, A.; Precone, V.; Starnone, F.; Nunziato, M.; Cantiello, P.; Iorio, M.; Evangelista, E.; D’Aiuto, M.; et al. The molecular analysis of BRCA1 and BRCA2: Next-generation sequencing supersedes conventional approaches. Clin. Chim. Acta 2015, 446, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, R.A.; Belmont, J.W.; Hardenbol, P.; Willis, T.D.; Yu, F.L.; Yang, H.M.; Ch’ang, L.Y.; Huang, W.; Liu, B.; Shen, Y.; et al. The International HapMap Project. Nature 2003, 426, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.M.; Ju, X.C.; Yi, X.; Zhu, Q.; Qu, N.; Liu, T.F.; Chen, Y.; Jiang, H.; Yang, G.H.; Zhen, R.; et al. Identification of Sequence Variants in Genetic Disease-Causing Genes Using Targeted Next-Generation Sequencing. PLoS ONE 2011, 6, e29500. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.F.; Wan, Z.Z.; Coarfa, C.; Drabek, R.; Chen, L.; Ostrowski, E.A.; Liu, Y.; Weinstock, G.M.; Wheeler, D.A.; Gibbs, R.A.; et al. A SNP discovery method to assess variant allele probability from next-generation resequencing data. Genome Res. 2010, 20, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Xing, J.C.; Zhang, Y.H.; Han, K.; Salem, A.H.; Sen, S.K.; Huff, C.D.; Zhou, Q.; Kirkness, E.F.; Levy, S.; Batzer, M.A.; et al. Mobile elements create structural variation: Analysis of a complete human genome. Genome Res. 2009, 19, 1516–1526. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Grzeda, K.R.; Stewart, C.; Grubert, F.; Urban, A.E.; Snyder, M.P.; Marth, G.T. Copy Number Variation detection from 1000 Genomes Project exon capture sequencing data. BMC Bioinform. 2012, 13, 305. [Google Scholar] [CrossRef] [Green Version]
- Voelkerding, K.V.; Dames, S.A.; Durtschi, J.D. Next-generation sequencing: From basic research to diagnostics. Clin. Chem. 2009, 55, 641–658. [Google Scholar] [CrossRef] [Green Version]
- Heather, J.M.; Chain, B. The sequence of sequencers: The history of sequencing DNA. Genomics 2016, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Slatko, B.E.; Gardner, A.F.; Ausubel, F.M. Overview of Next-Generation Sequencing Technologies. Curr. Protoc. Mol. Biol. 2018, 122, e59. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, F.; Mastroiaco, V.; Di Marco, A.; Compagnoni, C.; Capece, D.; Zazzeroni, F.; Capalbo, C.; Alesse, E.; Tessitore, A. Next-generation sequencing: Recent applications to the analysis of colorectal cancer. J. Transl. Med. 2017, 15, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Xu, F.; Wu, J.; Schubert, J.; Li, M.M. Application of Next Generation Sequencing in Laboratory Medicine. Ann. Lab. Med. 2021, 41, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.R.; Cowley, M.J.; Davis, R.L. Next-Generation Sequencing and Emerging Technologies. Semin. Thromb. Hemost. 2019, 45, 661–673. [Google Scholar] [CrossRef]
- Korostin, D.; Kulemin, N.; Naumov, V.; Belova, V.; Kwon, D.; Gorbachev, A. Comparative analysis of novel MGISEQ-2000 sequencing platform vs Illumina HiSeq 2500 for whole-genome sequencing. PLoS ONE 2020, 15, e0230301. [Google Scholar] [CrossRef]
- Drmanac, R.; Sparks, A.B.; Callow, M.J.; Halpern, A.L.; Burns, N.L.; Kermani, B.G.; Carnevali, P.; Nazarenko, I.; Nilsen, G.B.; Yeung, G.; et al. Human Genome Sequencing Using Unchained Base Reads on Self-Assembling DNA Nanoarrays. Science 2010, 327, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.A.; Park, J.L.; Park, S.J.; Kim, J.H.; Goh, S.H.; Han, J.Y.; Kim, S.Y. Comparison between MGI and Illumina sequencing platforms for whole genome sequencing. Genes Genom. 2021, 43, 713–724. [Google Scholar] [CrossRef]
- Lang, J.; Zhu, R.; Sun, X.; Zhu, S.; Li, T.; Shi, X.; Sun, Y.; Yang, Z.; Wang, W.; Bing, P.; et al. Evaluation of the MGISEQ-2000 Sequencing Platform for Illumina Target Capture Sequencing Libraries. Front. Genet. 2021, 12, 730519. [Google Scholar] [CrossRef]
- Wang, O.; Chin, R.; Cheng, X.; Wu, M.K.Y.; Mao, Q.; Tang, J.; Sun, Y.; Anderson, E.; Lam, H.K.; Chen, D.; et al. Efficient and unique cobarcoding of second-generation sequencing reads from long DNA molecules enabling cost-effective and accurate sequencing, haplotyping, and de novo assembly. Genome Res. 2019, 29, 798–808. [Google Scholar] [CrossRef] [Green Version]
- Rhoads, A.; Au, K.F. PacBio Sequencing and Its Applications. Genom. Proteom. Bioinform. 2015, 13, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulou, K.; Boti, M.A.; Adamopoulos, P.G.; Skourou, P.C.; Scorilas, A. Third-Generation Sequencing: The Spearhead towards the Radical Transformation of Modern Genomics. Life 2022, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, S.L.; Su, S.; Dong, X.Y.; Zappia, L.; Ritchie, M.E.; Gouil, Q. Opportunities and challenges in long-read sequencing data analysis. Genome Biol. 2020, 21, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schadt, E.E.; Turner, S.; Kasarskis, A. A window into third-generation sequencing. Hum. Mol. Genet. 2010, 19, R227–R240. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wadden, J.; Erb-Downward, J.R.; Ranjan, P.; Zhou, W.; McDonald, T.L.; Mills, R.E.; Boyle, A.P.; Dickson, R.P.; Blaauw, D.; et al. SquiggleNet: Real-time, direct classification of nanopore signals. Genome Biol. 2021, 22, 298. [Google Scholar] [CrossRef]
- Bleidorn, C. Third generation sequencing: Technology and its potential impact on evolutionary biodiversity research. Syst. Biodivers. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Miller, D.E.; Sulovari, A.; Wang, T.Y.; Loucks, H.; Hoekzema, K.; Munson, K.M.; Lewis, A.P.; Fuerte, E.P.A.; Paschal, C.R.; Walsh, T.; et al. Targeted long-read sequencing identifies missing disease-causing variation. Am. J. Hum. Genet. 2021, 108, 1436–1449. [Google Scholar] [CrossRef]
- Mitsuhashi, S.; Matsumoto, N. Long-read sequencing for rare human genetic diseases. J. Hum. Genet. 2020, 65, 11–19. [Google Scholar] [CrossRef]
- Goncalves, A.; Oliveira, J.; Coelho, T.; Taipa, R.; Melo-Pires, M.; Sousa, M.; Santos, R. Exonization of an Intronic LINE-1 Element Causing Becker Muscular Dystrophy as a Novel Mutational Mechanism in Dystrophin Gene. Genes 2017, 8, 253. [Google Scholar] [CrossRef]
- Eid, J.; Fehr, A.; Gray, J.; Luong, K.; Lyle, J.; Otto, G.; Peluso, P.; Rank, D.; Baybayan, P.; Bettman, B.; et al. Real-time DNA sequencing from single polymerase molecules. Science 2009, 323, 133–138. [Google Scholar] [CrossRef]
- Ambardar, S.; Gupta, R.; Trakroo, D.; Lal, R.; Vakhlu, J. High Throughput Sequencing: An Overview of Sequencing Chemistry. Indian J. Microbiol. 2016, 56, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Ardui, S.; Ameur, A.; Vermeesch, J.R.; Hestand, M.S. Single molecule real-time (SMRT) sequencing comes of age: Applications and utilities for medical diagnostics. Nucleic Acids Res. 2018, 46, 2159–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhao, Y.; Bollas, A.; Wang, Y.; Au, K.F. Nanopore sequencing technology, bioinformatics and applications. Nat. Biotechnol. 2021, 39, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, E.L.; Jaszczyszyn, Y.; Naquin, D.; Thermes, C. The Third Revolution in Sequencing Technology. Trends Genet. 2018, 34, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Holt, K.E. Performance of neural network basecalling tools for Oxford Nanopore sequencing. Genome Biol. 2019, 20, 129. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Z.; Akdemir, A.; Tremmel, G.; Imoto, S.; Miyano, S.; Shibuya, T.; Yamaguchi, R. Nanopore basecalling from a perspective of instance segmentation. BMC Bioinform. 2020, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Hormozdiari, F.; Hajirasouliha, I.; Dao, P.; Hach, F.; Yorukoglu, D.; Alkan, C.; Eichler, E.E.; Sahinalp, S.C. Next-generation VariationHunter: Combinatorial algorithms for transposon insertion discovery. Bioinformatics 2010, 26, i350-357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlan, A.R.; Boland, M.J.; Leibowitz, M.L.; Shumilina, S.; Pehrson, S.M.; Baldwin, K.K.; Hall, I.M. Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming. Cell Stem Cell 2011, 9, 366–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keane, T.M.; Wong, K.; Adams, D.J. RetroSeq: Transposable element discovery from next-generation sequencing data. Bioinformatics 2013, 29, 389–390. [Google Scholar] [CrossRef] [Green Version]
- Slater, G.S.; Birney, E. Automated generation of heuristics for biological sequence comparison. BMC Bioinform. 2005, 6, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helman, E.; Lawrence, M.; Stewart, C.; Getz, G.; Meyerson, M. Identification of somatic retrotransposon insertions across cancer types using RetroSeq. Cancer Res. 2012, 72, Am2012–Am5060. [Google Scholar] [CrossRef]
- Wang, J.X.; Song, L.; Grover, D.; Azrak, S.; Batzer, M.A.; Liang, P. DbRIP: A highly integrated database of retrotransposon insertion polymorphisms in humans. Hum. Mutat. 2006, 27, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, M.; Mustafa, H.; Brudno, M. Detecting Alu insertions from high-throughput sequencing data. Nucleic Acids Res. 2013, 41, e169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, R.E.; Bennett, E.A.; Iskow, R.C.; Devine, S.E. Which transposable elements are active in the human genome? Trends Genet. 2007, 23, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lee, W.P.; Ward, A.; Walker, J.A.; Konkel, M.K.; Batzer, M.A.; Marth, G.T. Tangram: A comprehensive toolbox for mobile element insertion detection. BMC Genom. 2014, 15, 795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.P.; Wu, J.; Marth, G.T. Toolbox for mobile-element insertion detection on cancer genomes. Cancer Inform. 2015, 14, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Dewannieux, M.; Esnault, C.; Heidmann, T. LINE-mediated retrotransposition of marked Alu sequences. Nat. Genet. 2003, 35, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zingler, N.; Willhoeft, U.; Brose, H.P.; Schoder, V.; Jahns, T.; Hanschmann, K.M.O.; Morrish, T.A.; Lower, J.; Schumann, G.G. Analysis of 5’ junctions of human LINE-1 and Alu retrotransposons suggests an alternative model for 5′-end attachment requiring microhomology-mediated end-joining. Genome Res. 2005, 15, 780–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostertag, E.M.; Kazazian, H.H. Twin priming: A proposed mechanism for the creation of inversions in L1 retrotransposition. Genome Res. 2001, 11, 2059–2065. [Google Scholar] [CrossRef] [Green Version]
- Moran, J.V.; DeBerardinis, R.J.; Kazazian, H.H., Jr. Exon shuffling by L1 retrotransposition. Science 1999, 283, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Halabian, R.; Makalowski, W. A Map of 3 ’ DNA Transduction Variants Mediated by Non-LTR Retroelements on 3202 Human Genomes. Biology 2022, 11, 1032. [Google Scholar] [CrossRef]
- Gardner, E.J.; Lam, V.K.; Harris, D.N.; Chuang, N.T.; Scott, E.C.; Pittard, W.S.; Mills, R.E.; Devine, S.E.; Consortium, G.P. The Mobile Element Locator Tool (MELT): Population-scale mobile element discovery and biology. Genome Res. 2017, 27, 1916–1929. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.; Lee, K.W.; Islam, M.N.; Yim, H.S.; Park, H.; Rho, M. iMGEins: Detecting novel mobile genetic elements inserted in individual genomes. BMC Genom. 2018, 19, 944. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.B.; Liu, B.H.; Xie, Y.L.; Li, Z.Y.; Huang, W.H.; Yuan, J.Y.; He, G.Z.; Chen, Y.X.; Pan, Q.; Liu, Y.J.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler (vol 1, 18, 2012). GigaScience 2015, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Puurand, T.; Kukuskina, V.; Pajuste, F.D.; Remm, M. AluMine: Alignment-free method for the discovery of polymorphic Alu element insertions. Mob. DNA 2019, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Mitt, M.; Kals, M.; Parn, K.; Gabriel, S.B.; Lander, E.S.; Palotie, A.; Ripatti, S.; Morris, A.P.; Metspalu, A.; Esko, T.; et al. Improved imputation accuracy of rare and low-frequency variants using population-specific high-coverage WGS-based imputation reference panel. Eur. J. Hum. Genet. 2017, 25, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.C.; Emery, S.B.; Flasch, D.A.; Wang, Y.F.; Kwan, K.Y.; Kidd, J.M.; Moran, J.V.; Mills, R.E. Identification and characterization of occult human-specific LINE-1 insertions using long-read sequencing technology. Nucleic Acids Res. 2020, 48, 1146–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churakov, G.; Grundmann, N.; Kuritzin, A.; Brosius, J.; Makalowski, W.; Schmitz, J. A novel web-based TinT application and the chronology of the Primate Alu retroposon activity. BMC Evol. Biol. 2010, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.D.; Kojima, K.K.; Kohany, O. Repbase Update, a database of repetitive elements in eukaryotic genomes. Mob. DNA 2015, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Beck, C.R.; Collier, P.; Macfarlane, C.; Malig, M.; Kidd, J.M.; Eichler, E.E.; Badge, R.M.; Moran, J.V. LINE-1 Retrotransposition Activity in Human Genomes. Cell 2010, 141, 1159–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, I.; Rubin, A.; Swergold, G.D. Tracing the LINEs of human evolution. Proc. Natl. Acad. Sci. USA 2002, 99, 10522–10527. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.; Borges-Monroy, R.; Viswanadham, V.V.; Lee, S.; Li, H.; Lee, E.A.; Park, P.J. Comprehensive identification of transposable element insertions using multiple sequencing technologies. Nat. Commun. 2021, 12, 3836. [Google Scholar] [CrossRef]
- Zook, J.M.; Hansen, N.F.; Olson, N.D.; Chapman, L.; Mullikin, J.C.; Xiao, C.N.; Sherry, S.; Koren, S.; Phillippy, A.M.; Boutros, P.C.; et al. A robust benchmark for detection of germline large deletions and insertions. Nat. Biotechnol. 2020, 38, 1347–1355. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdottir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tubio, J.M.C.; Li, Y.L.; Ju, Y.S.; Martincorena, I.; Cooke, S.L.; Tojo, M.; Gundem, G.; Pipinikas, C.P.; Zamora, J.; Raine, K.; et al. Extensive transduction of nonrepetitive DNA mediated by L1 retrotransposition in cancer genomes. Science 2014, 345, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaisson, M.J.P.; Sanders, A.D.; Zhao, X.F.; Malhotra, A.; Porubsky, D.; Rausch, T.; Gardner, E.J.; Rodriguez, O.L.; Guo, L.; Collins, R.L.; et al. Multi-platform discovery of haplotype-resolved structural variation in human genomes. Nat. Commun. 2019, 10, 1784. [Google Scholar] [CrossRef] [Green Version]
- Audano, P.A.; Sulovari, A.; Graves-Lindsay, T.A.; Cantsilieris, S.; Sorensen, M.; Welch, A.E.; Dougherty, M.L.; Nelson, B.J.; Shah, A.; Dutcher, S.K.; et al. Characterizing the Major Structural Variant Alleles of the Human Genome. Cell 2019, 176, 663–675.e19. [Google Scholar] [CrossRef] [Green Version]
- Shafin, K.; Pesout, T.; Lorig-Roach, R.; Haukness, M.; Olsen, H.E.; Bosworth, C.; Armstrong, J.; Tigyi, K.; Maurer, N.; Koren, S.; et al. Nanopore sequencing and the Shasta toolkit enable efficient de novo assembly of eleven human genomes. Nat. Biotechnol. 2020, 38, 1044–1053. [Google Scholar] [CrossRef]
- Leo, L.; Marchetti, M.; Giunta, S.; Fanti, L. Epigenetics as an Evolutionary Tool for Centromere Flexibility. Genes 2020, 11, 809. [Google Scholar] [CrossRef]


| Instrument | Run Time | Maximum Read Length | Maximum Reads | Output(Gb) | Key Applications | * Accuracy (>Q30) | |
|---|---|---|---|---|---|---|---|
| Illumina | iSeq | 9.5~19 h | 2 × 150 bp | ~4 million | 1.2 | microbe WGS, targeted gene sequencing | >80% of bases |
| MiniSeq | 4~24 h | 2 × 150 bp | ~25 million | ~7.5 | microbe WGS, targeted gene sequencing, targeted gene expression profiling | >80% of bases | |
| MiSeq | 4~55 h | 2 × 300 bp | ~25 million | ~15 | microbe WGS, targeted gene sequencing, 16S metagenome sequencing | >75% of bases | |
| NextSeq 500 | 12~30 h | 2 × 150 bp | ~400 million | ~120 | microbe WGS, targeted gene sequencing, transcriptome sequencing | >75% of bases | |
| NovaSeq | ~44 h | 2 × 250 bp | ~20 million | ~6000 | large WGS (human, animal, plant), single-cell profiling, transcriptome sequencing | ≥75% of bases | |
| MGI | MGISEQ-2000 | 12~78 h | 2 × 200 bp | ~1800 million | ~1080 | WGS, WES, targeted sequencing | ≥75% of bases |
| DNBSEQ-T7 | 24~30 h | 2 × 150 bp | ~5000 million | ~6000 | WGS, WES, transcriptome sequencing, targeted panel projects | >85% of bases | |
| DNBSEQ-G400 | 17~30 h | 2 × 200 bp | ~1800 million | ~720 | WGS, WES, transcriptome sequencing, microbial detection | >75% of bases | |
| DNBSEQ-G50 | 9~40 h | 2 × 150 bp | ~500 million | ~150 | microbe WGS, targeted DNA/RNA panels, forensic testing | >80% of bases | |
| Instrument | Run Time | Read Length | Output | Application Features | Error Rate | |
|---|---|---|---|---|---|---|
| PacBio | RS II | ~4 h per SMRT cell | ~15 kb | ~10 Gb | WGS, targeted sequencing, metagenomics | 13~15% |
| Sequel | ~20 h per SMRT cell | |||||
| Sequel II | ~30 h per SMRT cell | ~500 Gb | ||||
| Sequel IIe | ||||||
| Oxford Nanopore | MinION | ~72 h | >4 Mb | ~50 Gb | WGS, WES, whole-transcriptome sequencing, metagenomics | 5~13% |
| GridION | ~250 Gb | whole-transcriptome sequencing, metagenomics | ||||
| PromethION | ~14 Tb | population-scale genome sequencing, whole-transcriptome sequencing | ||||
| Name of Method | Detection Use and Target | Sequencing Type | Data Type | Sensitivity | Availability*/-(Accessed on 7 July 2022) | Ref |
|---|---|---|---|---|---|---|
| (PCR-Based) | ||||||
| RetroSeq | Non-reference TE insertions, genotype | WGS | Short read | >90% | https://github.com/tk2/RetroSeq | [119] |
| alu-detect | Non-reference Alu insertions | WGS, WES | >97% | http://compbio.cs.toronto.edu/alu-detect/ | [123] | |
| Tangram | Non-reference TE insertions, genotype | WGS | >94% | https://github.com/jiantao/Tangram | [125] | |
| MELT | Population analysis of reference/non-reference TE insertions, genotype | WGS | >99% | http://melt.igs.umaryland.edu | [132] | |
| iMGEins | Non-reference TE insertions in individual genomes | WGS | >96% | https://github.com/DMnBI/iMGEins | [133] | |
| AluMine | Non-reference Alu insertions, missed Alu elements in reference, genotype | WGS | Raw short-read data | >98% | https://github.com/bioinfo-ut/AluMine | [135] |
| PALMER | Non-reference TE insertions, genotype | WGS | Long read | N/A | https://github.com/mills-lab/PALMER | [137] |
| xTea | Comprehensive analysis of non-reference and somatic TE insertions, genotype | WGS | Short or Long | >90% | https://github.com/parklab/xTea | [143] |
| (Hybrid) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Min, J.W.; Mun, S.; Han, K. Human Retrotransposons and Effective Computational Detection Methods for Next-Generation Sequencing Data. Life 2022, 12, 1583. https://doi.org/10.3390/life12101583
Lee H, Min JW, Mun S, Han K. Human Retrotransposons and Effective Computational Detection Methods for Next-Generation Sequencing Data. Life. 2022; 12(10):1583. https://doi.org/10.3390/life12101583
Chicago/Turabian StyleLee, Haeun, Jun Won Min, Seyoung Mun, and Kyudong Han. 2022. "Human Retrotransposons and Effective Computational Detection Methods for Next-Generation Sequencing Data" Life 12, no. 10: 1583. https://doi.org/10.3390/life12101583
APA StyleLee, H., Min, J. W., Mun, S., & Han, K. (2022). Human Retrotransposons and Effective Computational Detection Methods for Next-Generation Sequencing Data. Life, 12(10), 1583. https://doi.org/10.3390/life12101583






