Coronary Computed Tomography Angiography for the Assessment of Sirolimus-Eluting Resorbable Magnesium Scaffold
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Device
2.3. PCI Procedure and RMS Implantation
2.4. Coronary Computed Tomography Angiography (CCTA)
2.5. CCTA FFR Assessment
2.6. Follow-Up and Definitions
2.7. Statistical Analysis
3. Results
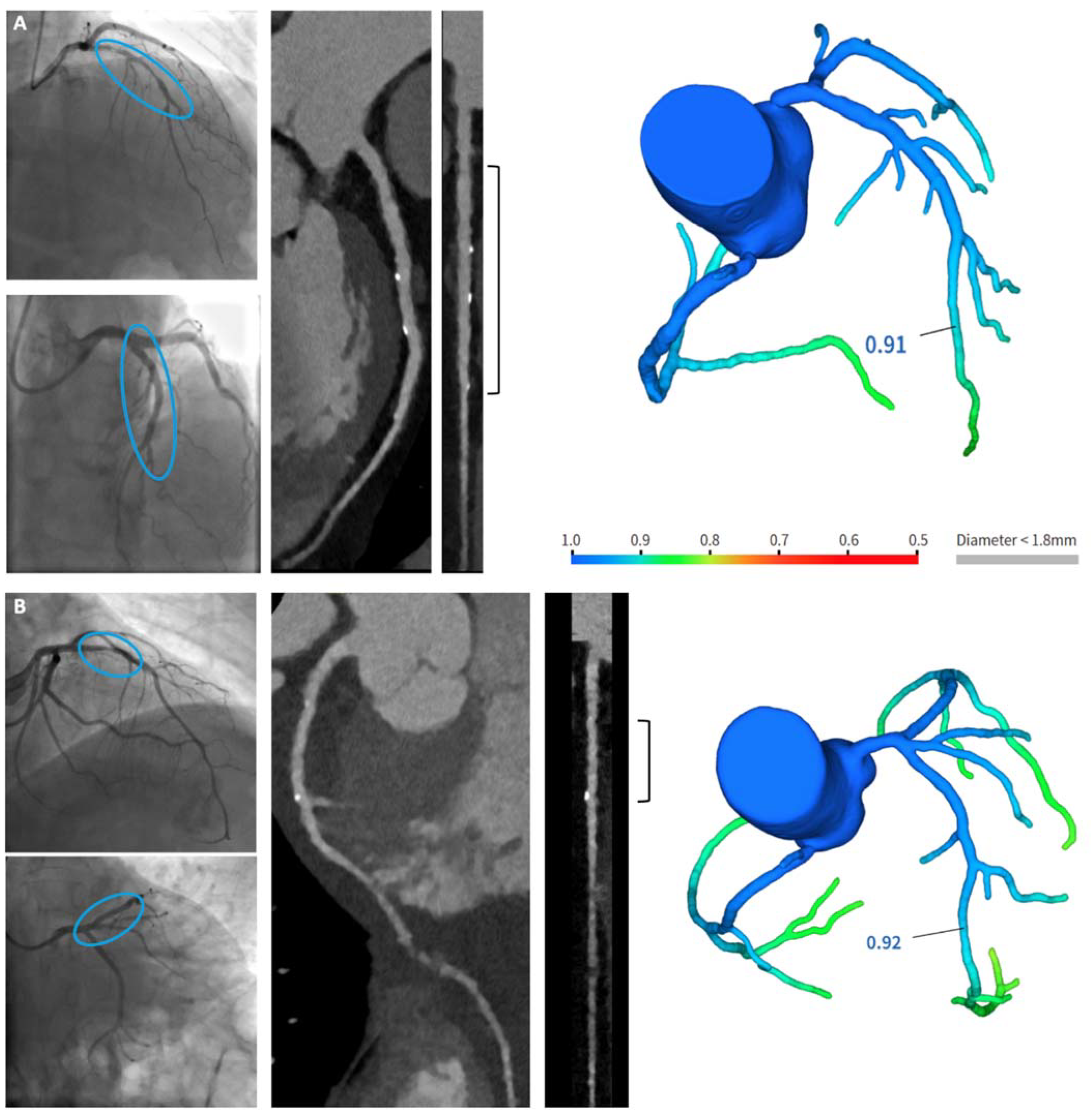
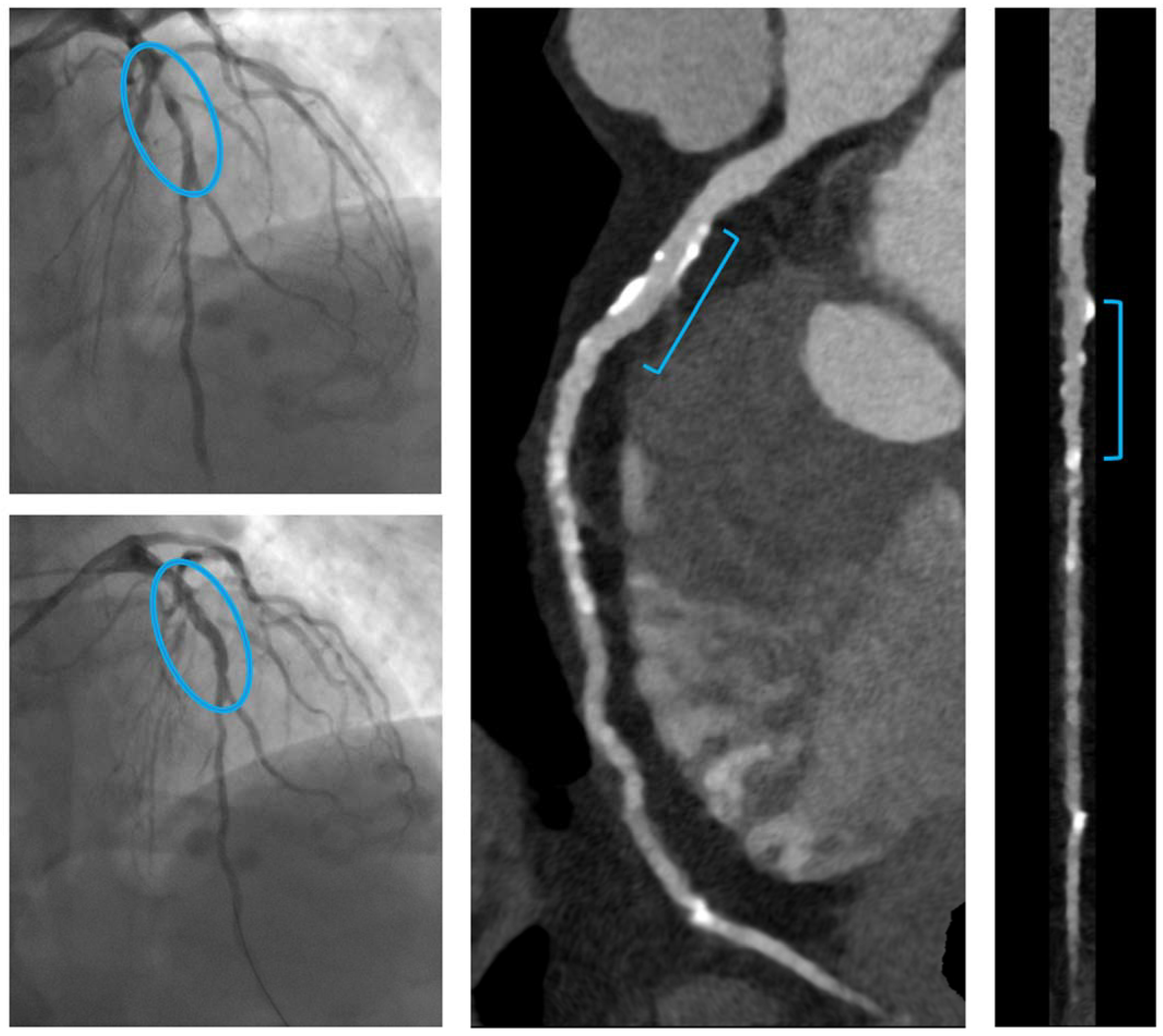
3.1. CCTA Findings
- A.
- A patient with long coronary stenosis on the LAD, treated with three RMS (Magmaris® 3.5 × 20 mm, 3.5 × 15 mm, 3 × 25 mm, respectively).
- B.
- A patient with significant stenosis at the bifurcation LAD-first diagonal branch, treated with one RMS on the LAD and balloon on the first diagonal branch (Magmaris® 3 × 25 mm).
- C.
- A patient with a focal culprit plaque on the LAD, treated by one RMS (Magmaris® 2.5 × 25 mm).
3.2. Noninvasive CT-FFR Findings
3.3. Year Clinical Outcome
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.J.; Birnbaum, Y. Noninvasive coronary angiography using multislice computerized tomography. J. Cardiovasc. Med. 2007, 8, 17–20. [Google Scholar]
- Onuma, Y.; Collet, C.; van Geuns, R.J.; de Bruyne, B.; Christiansen, E.; Koolen, J.; Smits, P.; Chevalier, B.; McClean, D.; Dudek, D.; et al. Long-term serial non-invasive multislice computed tomography angiography with functional evaluation after coronary implantation of bioresorbable everolimus-eluting scaffold: The ABSORB cohort B MSCT substury. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Sotomi, Y.; Cavalcante, R.; Asano, T.; Miyazaki, Y.; Tenekecioglu, E.; Kistlaar, P.; Zeng, Y.; Suwanasson, P.; de Winter, R.J.; et al. Accuracy of coronary computed tomography angiography for bioresorbabke scaffold luminal investigation: A comparison with optical coherence tomography. Int J Cardiovasc Imaging 2017, 33, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Onuma, Y.; Dudek, D.; Thuesen, L.; Webster, M.; Nieman, K.; Garcia-Garcia, H.M.; Ormiston, J.A.; Serruys, P.W. Five-year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus-eluting scaffold in patients with de novo coronary artery disease: The ABSORB cohort A trial. JACC Cardiovasc. Interv. 2013, 6, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Chebalier, B.; Cequier, A.; Fajadet, J.; Dominici, M.; Helqvist, S.; Van Boven, A.J.; Dudek, D.; McClean, D.; Almeida, M.; et al. Diagnostic Accuracy of Coronary CT Angiography for the Evaluation of Bioresorbable Vascular Scaffolds. JACC Cardiovasc. Imaging 2018, 11, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, X.; Sun, S.; Wu, D.; Bai, J.; Yin, Y.; Liu, X.; Zhang, H.; de Albuquerque, V.H.C. Learning physical properties in complex visual scenes: An intelligent machine for perceiving blood flow dynamics from static CT angiography imaging. Neural Netw. 2020, 123, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, R.; Pompei, G.; Tonet, E.; Vitali, F.; Guardigli, G.; Campo, G.; Pavasini, R. Safety and efficacy of new-generation coronary bioresorbable scaffolds: A systematic review. Minerva Cardiol. Angiol. 2021. (Online ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Puchner, S.B.; Lu, M.T.; Mayrhofer, T.; Liu, T.; Pursnani, A.; Ghoshhajra, B.B.; Truong, Q.A.; Wiviott, S.D.; Fleg, J.L.; Hoffmann, U.; et al. High-risk coronary plaque at coronary CT angiography is associated with nonalcoholic fatty liver disease, independent of coronary plaque and stenosis burden: Results from the ROMICAT II trial. Radiology 2015, 274, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, E.; McFadden, E.; Vranckx, P.; Garcia-Garcia, H.M.; Seltzer, J.H.; Held, C.; de Vries, T.; Menon, V.; Brown, K.J.; Soliman, O.I.I.; et al. Critical Appraisal of Contemporary Clinical Endpoint Definitions in Coronary Intervention Trials: A Guidance Document. JACC Cardiovasc. Interv. 2019, 12, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Haude, M.; Ince, H.; Kische, S.; Toelg, R.; Van Mieghem, N.M.; Verheye, S.; von Birgelen, C.; Christiansen, E.H.; Barbato, E.; Garcia-Garcia, H.M.; et al. Sustained Safety and Performance of the Second-Generation Sirolimus-Eluting Absorbable Metal Scaffold: Pooled Outcomes of the BIOSOLVE-II and -III Trials at 3 Years. Cardiovasc. Revasc. Med. 2020, 21, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Haude, M.; Toelg, R.; Lemos, P.A.; Christiansen, E.H.; Abizaid, A.; von Birgelen, C.; Neumann, F.J.; Wijns, W.; Ince, H.; Kaiser, C.; et al. Sustained Safety and Performance of the Second-Generation Sirolimus-Eluting Absorbable Metal Scaffold: Long-Term Data of the BIOSOLVE-II and -III Trials at 5 Years. Cardiovasc. Revasc. Med. 2022, 38, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Verheye, S.; Wlodarczak, A.; Montorsi, P.; Torzewski, J.; Bennett, J.; Haude, M.; Starmer, G.; Buck, T.; Wiemer, M.; Nuruddin, A.A.B.; et al. BIOSOLVE-IV-registry: Safety and performance of the Magmaris scaffold: 12-month outcomes of the first cohort of 1075 patients. Catheter. Cardiovasc. Interv. 2021, 98, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paz, L.; Brugaletta, S.; Gomez-Lara, J.; Alfonso, F.; Cequier, A.; Romani, S.; Bordes, P.; Serra, A.; Inigues, A.; Salinas, P.; et al. Magnesium-based resorbable scaffold vs permanent metallic sirolimus-eluting stent in patients with ST-segment elevation myocardial infarction: 3-year results of the MAGSTEMI randomized controlled trial. Eurointervention 2022, 18, e389–e396. [Google Scholar] [CrossRef] [PubMed]
- Salinas, P.; Pozo-Osinalde, E.; Cerrato, E.; Garcia-Blas, S.; Vaudano, G.P.; Parrilla, C.; Sanchis, J.; Varbella, F.; Escaned, J. Cardiac Computed Tomography Angiography Follow-Ip of Resorbable Magnesium Scaffolds. Cardiovasc. Revasc. Med. 2021, 29, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Montarello, N.J.; Singh, K.; Sinhal, A.; Wong, D.T.L.; Alcock, R.; Rajendran, S.; Dautov, R.; Barlis, P.; Patel, S.; Nidorf, S.M.; et al. Assessing the Impact of Colchicine on Coronary Plaque Phenotype After Myocardial Infarction with Optical Coherence Tomography: Rationale and Design of the COCOMO-ACS Study. Cardiovasc. Drugs Ther. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Frangogiannis, N.G. Anti-inflammatory therapies in myocardial infarction: Failures, hopes and challenges. Br. J. Pharmacol. 2018, 175, 1377–1400. [Google Scholar] [CrossRef] [PubMed]


| Total | |
|---|---|
| Patients (n = 26) | |
| Age (years) | 59 ± 7 |
| Male sex (%) | 22 (85) |
| CV risk factors (%) | |
| Diabetes | 1 (3.8) |
| Hypertension | 13 (50) |
| Hyperlipidemia | 14 (54) |
| Current smoker | 11 (42) |
| Medical history (%) | |
| MI and/or coronary revascularization | 0 (0) |
| PAD | 1 (4) |
| CKD | 3 (11) |
| Clinical presentation | |
| STEMI (%) | 8 (31) |
| NSTEMI (%) | 15 (58) |
| CCS (%) | 3 (11) |
| Medical therapy (%) | |
| Aspirin | 26 (100) |
| P2Y12 inhibitor | 26 (100) |
| ACE inhibitor/A2R blocker | 23 (88) |
| High-potency statin | 24 (92) |
| Ezetimibe | 18 (69) |
| PCSK9 inhibitor | 4 (15) |
| Vessels (n = 29) | |
| Target vessel (%) | |
| -left anterior descending | 19 (66) |
| -left circumflex | 5 (17) |
| -right coronary | 5 (17) |
| AHA/ACC classification (%) | |
| -A, B1 | 6 (21) |
| -B2 | 15 (52) |
| -C | 8 (27) |
| Reference vessel diameter, mm | 2.7 (2.5–3.3) |
| Minimal lumen diameter, mm | 0.6 (0.3–0.8) |
| Diameter stenosis, % | 77 (66–95) |
| Lesion length, mm | 18 (15–21) |
| Predilatation (%) | 28 (100) |
| Largest predilatation balloon, mm | 2.5 (2.5–3) |
| RMS diameter, mm | 3 (3–3.5) |
| Total RMS length, mm | 25 (20–25) |
| Overlapping RMS (%) | 6 (21) |
| Postdilatation (%) | 28 (100) |
| Largest postdilatation balloon, mm | 3.5 (3.5–3.75) |
| Intracoronary imaging, no. (%) | 20 (69) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonet, E.; Cossu, A.; Pompei, G.; Ruggiero, R.; Caglioni, S.; Mele, D.; Boccadoro, A.; Micillo, M.; Cocco, M.; De Raffele, M.; et al. Coronary Computed Tomography Angiography for the Assessment of Sirolimus-Eluting Resorbable Magnesium Scaffold. Life 2022, 12, 1612. https://doi.org/10.3390/life12101612
Tonet E, Cossu A, Pompei G, Ruggiero R, Caglioni S, Mele D, Boccadoro A, Micillo M, Cocco M, De Raffele M, et al. Coronary Computed Tomography Angiography for the Assessment of Sirolimus-Eluting Resorbable Magnesium Scaffold. Life. 2022; 12(10):1612. https://doi.org/10.3390/life12101612
Chicago/Turabian StyleTonet, Elisabetta, Alberto Cossu, Graziella Pompei, Rossella Ruggiero, Serena Caglioni, Daniela Mele, Alberto Boccadoro, Marco Micillo, Marta Cocco, Martina De Raffele, and et al. 2022. "Coronary Computed Tomography Angiography for the Assessment of Sirolimus-Eluting Resorbable Magnesium Scaffold" Life 12, no. 10: 1612. https://doi.org/10.3390/life12101612
APA StyleTonet, E., Cossu, A., Pompei, G., Ruggiero, R., Caglioni, S., Mele, D., Boccadoro, A., Micillo, M., Cocco, M., De Raffele, M., Giganti, M., Biscaglia, S., Sgura, F., Preti, G., Yin, Y., Wang, Y., Quadri, G., Cerrato, E., & Campo, G. (2022). Coronary Computed Tomography Angiography for the Assessment of Sirolimus-Eluting Resorbable Magnesium Scaffold. Life, 12(10), 1612. https://doi.org/10.3390/life12101612








