Study of the Polysaccharide Production by the Microalga Vischeria punctata in Relation to Cultivation Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Cultivation
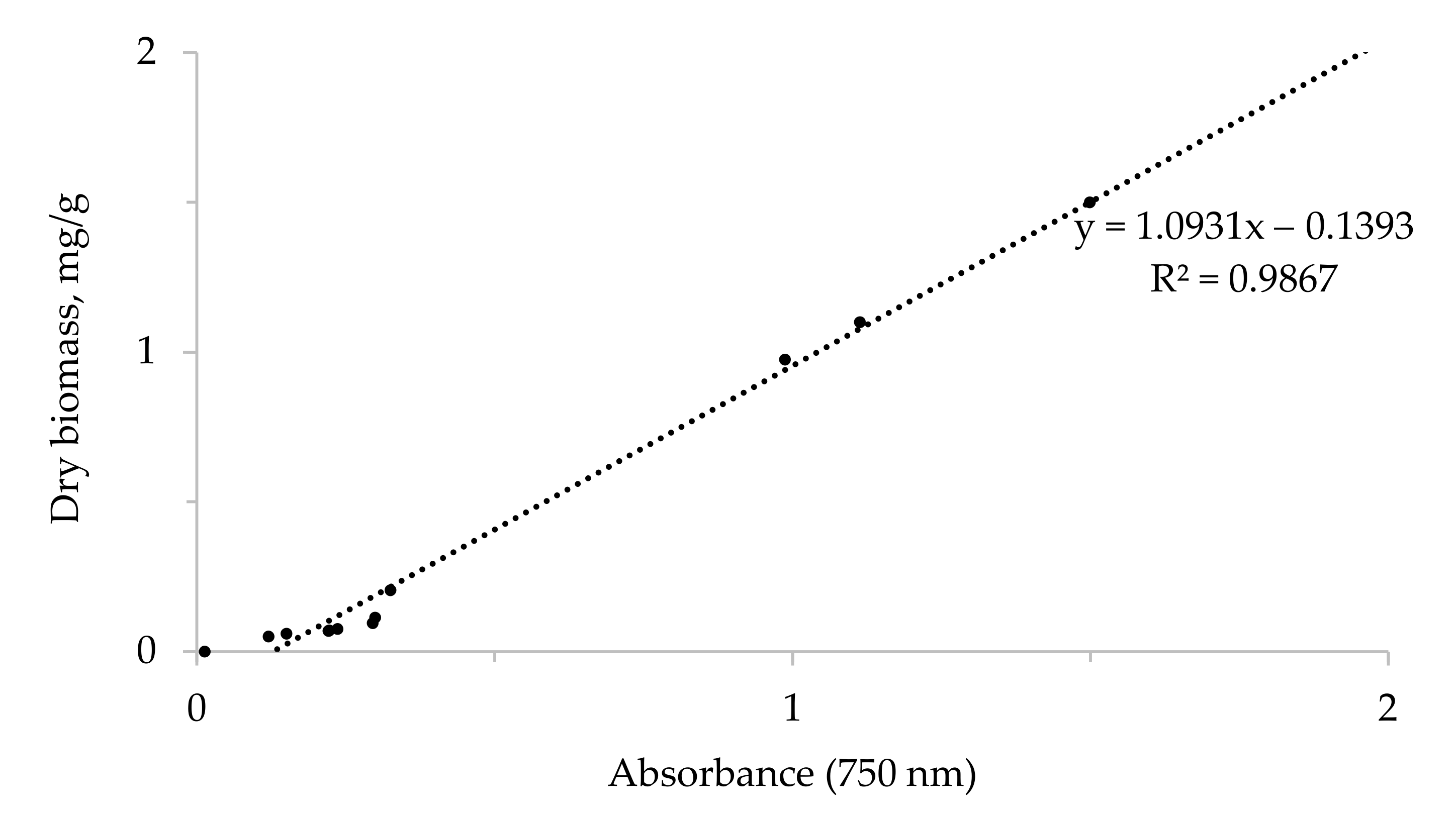
2.2. Assessment of Cell and Biomass Growth
2.3. Quantification of Polysaccharides
2.4. Alcohol Precipitation of Exopolysaccharides
2.5. Ultrasonic Extraction of Bound Polysaccharides
2.6. Extraction of Bound Polysaccharides by Heat Treatment with the NaOH Addition
2.7. Quantification of Uronic Acids
2.8. Quantification of Neutral Sugars
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreira, J.B.; Vaz, B.D.S.; Cardias, B.B.; Cruz, C.G.; Almeida, A.C.A.D.; Costa, J.A.V.; Morais, M.G.D. Microalgae Polysaccharides: An Alternative Source for Food Production and Sustainable Agriculture. Polysaccharides 2022, 3, 441–457. [Google Scholar] [CrossRef]
- Song, H.; He, M.; Gu, C.; Wei, D.; Liang, Y.; Yan, J.; Wang, C. Extraction Optimization, Purification, Antioxidant Activity, and Preliminary Structural Characterization of Crude Polysaccharide from an Arctic Chlorella sp. Polymers 2018, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, J.B.; Santos, T.D.; Duarte, J.H.; Bezerra, P.Q.M.; de Morais, M.G.; Costa, J.A.V. Role of microalgae in circular bioeconomy: From waste treatment to biofuel production. Clean Technol. Environ. Policy 2021, 23, 1–11. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. Novel Technologies for Seaweed Polysaccharides Extraction and Their Use in Food with Therapeutically Applications—A Review. Foods 2022, 11, 2654. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, Z.; Ma, S.; Rasool, A.; Wang, L.; Zhang, J. Optimization of ultrasonic-assisted extraction, refinement and characterization of water-soluble polysaccharide from Dictyosphaerium sp. and evaluation of antioxidant activity in vitro. J. Food Meas. Charact. 2020, 14, 963–977. [Google Scholar] [CrossRef]
- Kryvenda, A.; Rybalka, N.; Wolf, M.; Friedl, T. Species distinctions among closely related strains of Eustigmatophyceae (Stramenopiles) emphasizing ITS2 sequence-structure data: Eustigmatos and Vischeria. Eur. J. Phycol. 2018, 53, 471–491. [Google Scholar] [CrossRef]
- Stoykova, P.; Stoyneva-Gärtner, M.; Uzunov, B.; Gärtner, G.; Atanassov, I.; Draganova, P.; Borisova, C. Morphological characterization and phylogenetic analysis of aeroterrestrial Vischeria/Eustigmatos strains with industrial potential. Biotechnol. Biotechnol. Equip. 2019, 33, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Stoyneva-Gärtner, M.; Uzunov, B.; Gärtner, G.; Borisova, C.; Draganova, P.; Radkova, M.; Stoykova, P.; Atanassov, I. Current bioeconomical interest in stramenopilic Eustigmatophyceae: A review. Biotechnol. Biotechnol. Equip. 2019, 33, 302–314. [Google Scholar] [CrossRef] [Green Version]
- Remias, D.; Nicoletti, C.; Krennhuber, K.; Möderndorfer, B.; Nedbalová, L.; Procházková, L. Growth, fatty, and amino acid profiles of the soil alga Vischeria sp. E71.10 (Eustigmatophyceae) under different cultivation conditions. Folia Microbiol. 2020, 65, 1017–1023. [Google Scholar] [CrossRef]
- Wang, F.; Gao, B.; Huang, L.; Su, M.; Dai, C.; Zhang, C. Evaluation of oleaginous eustigmatophycean microalgae as potential biorefinery feedstock for the production of palmitoleic acid and biodiesel. Bioresour. Technol. 2018, 270, 30–37. [Google Scholar] [CrossRef]
- Sinetova, M.A.; Sidorov, R.A.; Starikov, A.Y.; Voronkov, A.S.; Medvedeva, A.S.; Krivova, Z.V.; Los, D.A. Assessment of the Biotechnological Potential of Cyanobacterial and Microalgal Strains from IPPAS Culture Collection. Appl. Biochem. Microbiol. 2020, 56, 794–808. [Google Scholar] [CrossRef]
- She, Y.; Gao, X.; Jing, X.; Wang, J.; Dong, Y.; Cui, J.; Xue, H.; Li, Z.; Zhu, D. Effects of nitrogen source and NaCl stress on oil production in Vischeria sp. WL1 (Eustigmatophyceae) isolated from dryland biological soil crusts in China. J. Appl. Phycol. 2022, 34, 1281–1291. [Google Scholar] [CrossRef]
- Schnepf, E.; Niemann, A.W. Pseudostaurastrum limneticum, a eustigmatophycean alga with astigmatic zoospores: Morphogenesis, fine structure, pigment composition and taxonomy. Arch. Protistenkd. 1996, 146, 237–249. [Google Scholar] [CrossRef]
- Cui, F.-J.; Qian, L.-S.; Sun, W.-J.; Zhang, J.-S.; Yang, Y.; Li, N.; Zhuang, H.-N.; Wu, D. Ultrasound-Assisted Extraction of Polysaccharides from Volvariella volvacea: Process Optimization and Structural Characterization. Molecules 2018, 23, 1706. [Google Scholar] [CrossRef] [Green Version]
- Tao, T.L.; Cui, F.J.; Chen, X.-X.; Sun, W.-J.; Huang, D.-M.; Zhang, J.; Yang, Y.; Wu, D.; Liu, W.-M. Improved mycelia and polysaccharide production of Grifola frondosa by controlling morphology with microparticle Talc. Microb. Cell Factories 2018, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gaignard, C.; Gargouch, N.; Dubessay, P.; Delattre, C.; Pierre, G.; Laroche, C.; Fendri, I.; Abdelkafi, S.; Michaud, P. New horizons in culture and valorization of red microalgae. Biotechnol. Adv. 2019, 37, 193–222. [Google Scholar] [CrossRef]
- Mugnai, B.; Rossi, F.; Felde, V.J.M.N.L.; Colesie, C.; Budel, B.; Peth, S.; Kaplan, A.; De Philippis, R. The potential of the cyanobacterium Leptolyngbya ohadii as inoculum for stabilizing bare sandy substrates. Soil Biol. Biochem 2018, 127, 318–328. [Google Scholar] [CrossRef]
- Lukyanov, V.A.; Stifeev, A.I.; Gorbunova, S.Y. Science-based cultivation of microalgae. Bulletin of the Kursk State Agricultureal Academy 2013, 9, 55–57. (In Russian) [Google Scholar]
- Zhou, Q.; Feng, F.; Yang, Y.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Characterization of a dextran produced by Leuconostoc Pseudomesenteroides XG5 from homemade wine. Int. J. Biol. Macromol. 2018, 107, 2234–2241. [Google Scholar] [CrossRef]
- Watanabe, M.M. Freshwater culture media. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: London, UK, 2005; pp. 13–21. [Google Scholar]
- Prát, S. Algarum, Hepaticarum, Muscurumque in culturis collectio. Preslia 1948, 12–13, 1–12. [Google Scholar]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Mu, P.; Plummer, D.T. Introduction to Practical Biochemistry; Tata McGraw-Hill Education: New York, NY, USA, 2001. [Google Scholar]
- Guo, Q.; Ai, L.; Cui, S.W. Polysaccharide Extraction and Fractionation. In Methodology for Structural Analysis of Polysaccharides; Springer: Cham, Switzerland, 2018; pp. 9–17. [Google Scholar]
- Gerde, J.A.; Montalbo-Lomboy, M.; Yao, L.; Grewell, D.; Wang, T. Evaluation of microalgae cell disruption by ultrasonic treatment. Bioresour. Technol. 2012, 125, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.A.; Buchanan-Smith, J.G. A colorimetric method for the quantitation of uronic acids and a specific assay for galacturonic acid. Anal. Biochem. 1992, 201, 190–196. [Google Scholar] [CrossRef]
- Monsigny, M.; Petit, C.; Roche, A.C. Colorimetric determination of neutral sugars by a resorcinol sulfuric acid micromethod. Anal. Biochem. 1988, 175, 525–530. [Google Scholar] [CrossRef]
- Attanzio, A.; Ippolito, M.; Girasolo, M.A.; Saiano, F.; Rotondo, A.; Rubino, S. Anti-cancer activity of di- and tri-organotin(IV) compounds with D-(+)-galacturonic acid on human tumor cells. J. Inorg. Biochem. 2018, 188, 102–112. [Google Scholar] [CrossRef]
- Agustini, N.W.S.; Kusmiati. Potency of endo-exopolysaccharide from Porphyridium cruentum (SF Gray) Nägeli as antioxidant (DPPH) and biological toxicity (BSLT). KnE Life Sci. 2017, 2017, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.A.V.; Lucas, B.F.; Alvarenga, A.G.P.; Moreira, J.B.; de Morais, M.G. Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications. Polysaccharides 2021, 2, 759–772. [Google Scholar] [CrossRef]
- Laroche, C. Exopolysaccharides from Microalgae and Cyanobacteria: Diversity of Strains, Production Strategies, and Applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef]
- Zhang, S.J.; Santschi, P.H. Application of cross-flow ultrafiltration for isolating exopolymeric substances from a marine diatom (Amphora sp.). Limnol. Oceanogr. Meth. 2009, 7, 419–429. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, X.; Wu, D.; Ouyang, Y.; Gao, L.; Chen, Z.; El-Seedi, H.R.; Wang, M.-f.; Chen, X.; Zhao, C. Characterization of the structure and analysis of the anti-oxidant effect of microalga Spirulina platensis polysaccharide on Caenorhabditis elegans mediated by modulating microRNAs and gut microbiota. Int. J. Biol. Macromol. 2020, 163, 2295–2305. [Google Scholar] [CrossRef]
- Wan, X.-Z.; Ai, C.; Chen, Y.-H.; Gao, X.-X.; Zhong, R.-T.; Liu, B.; Chen, X.-H.; Zhao, C. Physicochemical characterization of a polysaccharide from green microalga Chlorella pyrenoidosa and its hypolipidemic activity via gut microbiota regulation in rats. J. Agric. Food Chem. 2020, 68, 1186–1197. [Google Scholar] [CrossRef]
- Pińkowska, H.; Krzywonos, M.; Wolak, P.; Złocińska, A. Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste. Green Process. Synth. 2019, 8, 683–690. [Google Scholar] [CrossRef]
- Rachidi, F.; Benhima, R.; Kasmi, Y.; Sbabou, L.; Arroussi, H.E. Evaluation of microalgae polysaccharides as biostimulants of tomato plant defense using metabolomics and biochemical approaches. Sci. Rep. 2021, 11, 930. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Liu, H.; Su, J.; Lan, C.Q.; Zhong, M.; Hu, X. Production, isolation and bioactive estimation of extracellular polysaccharides of green microalga Neochloris Oleoabundans. Algal Res. 2020, 48, 101883. [Google Scholar] [CrossRef]
- Cruz, D.; Vasconcelos, V.; Pierre, G.; Michaud, P.; Delattre, C. Exopolysaccharides from Cyanobacteria: Strategies for Bioprocess Development. Appl. Sci. 2020, 10, 3763. [Google Scholar] [CrossRef]
- Frazzini, S.; Scaglia, E.; Dell’Anno, M.; Reggi, S.; Panseri, S.; Giromini, C.; Lanzoni, D.; Sgoifo Rossi, C.A.; Rossi, L. Antioxidant and antimicrobial activity of algal and cyanobacterial extracts: An in vitro study. Antioxidants 2022, 11, 992. [Google Scholar] [CrossRef]
- Mota, R.; Guimarães, R.; Büttel, Z.; Rossi, F.; Colica, G.; Silva, C.J.; Santos, C.; Gales, L.; Zille, A.; De Philippis, R. Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110. Carbohydr. Polym. 2013, 92, 1408–1415. [Google Scholar] [CrossRef]
- Costa, B.; Mota, R.; Parreira, P.; Tamagnini, P.; Martins, C.M.; Costa, F. Broad-Spectrum Anti-Adhesive Coating Based on an Extracellular Polymer from a Marine Cyanobacterium. Mar. Drug. 2019, 17, 243. [Google Scholar] [CrossRef] [Green Version]
- Berta, N.; Estevinho, R.M.; José, P.L.; Tamagnini, P.; Gales, L.; Rocha, F. Application of a cyanobacterial extracellular polymeric substance in the microencapsulation of vitamin B12. Powder Technol. 2019, 343, 644–665. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Khangembam, R.; Shamjetshabam, M.; Sharma, A.S.; Oinam, G.; Brand, J.J. Characterization and optimization of bioflocculant exopolysaccharide production by Cyanobacteria Nostoc sp. BTA97 and Anabaena sp. BTA990 in culture conditions. Appl. Biochem. Biotechnol. 2015, 176, 1950–1963. [Google Scholar] [CrossRef]
- Han, P.; Shen, S.; Wang, H.-Y.; Yao, S.; Tan, Z.; Zhong, C.; Jia, S. Applying the strategy of light environment control to improve the biomass and polysaccharide production of Nostoc flagelliforme. J. Appl. Phycol. 2017, 29, 55–65. [Google Scholar] [CrossRef]
- Borah, D.; Nainamalai, S.; Gopalakrishnan, S.; Rout, J.; Alharbi, N.S.; Alharbi, S.A.; Nooruddin, T. Biolubricant potential of exopolysaccharides from the cyanobacterium Cyanothece epiphytica. Appl. Microbiol. Biotechnol. 2018, 102, 3635–3647. [Google Scholar] [CrossRef]
- Han, P.; Yao, S.; Guo, R.; Yan, R.; Wu, Y.; Shen, S.; Jia, S. Influence of culture conditions on extracellular polysaccharide production and the activities of enzymes involved in the polysaccharide synthesis of Nostoc flagelliforme. RSC Adv. 2017, 7, 45075–45084. [Google Scholar] [CrossRef] [Green Version]
- Phlips, E.J.; Zeman, C.; Hansen, P. Growth, photosynthesis, nitrogen fixation and carbohydrate production by a unicellular cyanobacterium, Synechococcus sp. (Cyanophyta). J. Appl. Phycol. 1989, 1, 137–145. [Google Scholar] [CrossRef]




| Component, g L−1 | Medium | ||
|---|---|---|---|
| BBM 3N | Prat | PratM * | |
| NaNO3 | 0.75 | - | - |
| KNO3 | - | 0.1 | 0.001 |
| KH2PO4 | 0.175 | - | - |
| K2HPO4 | 0.075 | 0.01 | 0.001 |
| MgSO4∙7H2O | 0.075 | 0.01 | 0.001 |
| CaCl2∙2H2O | 0.025 | - | - |
| NaCl | 0.025 | - | - |
| FeSO4∙7H2O | 0.005 | 0.005 | 0.005 |
| H3BO3 | 2.86 | - | - |
| MnCl2∙4H2O | 1.81 | - | - |
| ZnSO4∙7H2O | 0.222 | - | - |
| MoO3 | 0.018 | - | - |
| NH4VO3 | 0.023 | - | - |
| Solvent | Solvent Purity, % | Extraction Module, (Sample: Alcohol) | Precipitation Temperature, °C | Yield, mg/g d.w. |
|---|---|---|---|---|
| H-242 | ||||
| Ethanol | 96 | 1:1 | 20 | 104.5 ± 3.1a |
| 10 | 179.1 ± 5.3b | |||
| 0 | 179.1 ± 5.3b | |||
| −10 | 253.7 ± 7.6c | |||
| −20 | 179.1 ± 5.3a | |||
| Ethanol | 96 | 1:2 | 20 | 806.0 ± 24.1a |
| 10 | 194.0 ± 5.8b | |||
| 0 | 253.7 ± 7.6c | |||
| −10 | 626.9 ± 18.7d | |||
| −20 | 507.5 ± 15.2e | |||
| Ethanol | 96 | 1:3 | 20 | 925.4 ± 27.7a |
| 10 | 343.3 ± 10.9b | |||
| 0 | 432.8 ± 12.9c | |||
| −10 | 44.8 ± 1.3d | |||
| −20 | 74.6 ± 2.2e | |||
| Isopropanol | 99 | 1:1 | 20 | 328.4 ± 9.8a |
| 10 | 358.2 ± 10.7a | |||
| 0 | 432.8 ± 12.9b | |||
| −10 | 59.7 ± 1.8c | |||
| −20 | 74.6 ± 2.2d | |||
| Isopropanol | 99 | 1:2 | 30 | 1059.7 ± 31.7a |
| 20 | 1611.9 ± 48.3b | |||
| 10 | 507.5 ± 15.2c | |||
| 0 | 806.0 ± 24.2d | |||
| −10 | 716.4 ± 21.4d | |||
| −20 | 1597.0 ± 47.9b | |||
| −30 | 716.4 ± 21.4d | |||
| Isopropanol | 99 | 1:3 | 20 | 850.7 ± 25.5a |
| 10 | 955.2 ± 28.6a | |||
| 0 | 1134.3 ± 34.0b | |||
| −10 | 641.8 ± 19.2c | |||
| −20 | 1597.0 ± 47.9d | |||
| −30 | 1194.0 ± 35.8b | |||
| Butanol | 99 | 1:1 | 20 | 477.6 ± 14.3a |
| 10 | 955.2 ± 28.6b | |||
| 0 | 373.1 ± 11.2c | |||
| −10 | 985.1 ± 29.5b | |||
| −20 | 343.3 ± 10.3c | |||
| Butanol | 99 | 1:2 | 20 | 820.9 ± 24.6a |
| 10 | 1597.0 ± 47.9b | |||
| 0 | 820.9 ± 24.6a | |||
| −10 | 985.1 ± 29.5c | |||
| −20 | 1611.9 ± 48.3b | |||
| −30 | 1044.8 ± 31.3c | |||
| Butanol | 99 | 1:3 | 30 | 820.9 ± 24.6a |
| 20 | 940.3 ± 28.2b | |||
| 10 | 179.1 ± 5.3c | |||
| 0 | 223.9 ± 6.7c | |||
| −10 | 597.0 ± 17.9d | |||
| −20 | 223.9 ± 6.7c |
| pH | T, °C | Extraction time, min | ||||
|---|---|---|---|---|---|---|
| 25 | 45 | 65 | 85 | 100 | ||
| 9 | 60 | 66.8 ± 2.0a/a | 57.9 ± 1.7a/a | 81.7 ± 2.4a/b | 78.7 ± 2.3a/b | 74.3 ± 2.2a/b |
| 120 | 89.1 ± 2.6b/a | 78.7 ± 2.3b/b | 80.2 ± 2.4a/ab | 86.2 ± 2.5a/a | 86.2 ± 2.6b/a | |
| 180 | 77.3 ± 2.3c/a | 75.8 ± 2.2b/a | 107.0 ± 3.2b/b | 101.1 ± 3.1b/b | 112.9 ± 3.4c/b | |
| 240 | 63.9 ± 1.9a/a | 50.5 ± 1.5a/b | 54.9 ± 1.6c/b | 56.5 ± 1.7c/b | 63.9 ± 1.9a/a | |
| 10 | 60 | 41.6 ± 1.2a/a | 54.9 ± 1.5a/b | 47.5 ± 1.4a/a | 69.8 ± 2.1a/c | 83.2 ± 2.5a/d |
| 120 | 65.4 ± 1.9b/a | 72.8 ± 2.1b/a | 95.1 ± 2.8b/b | 83.2 ± 2.5b/c | 95.1 ± 2.8b/b | |
| 180 | 71.3 ± 2.1b/a | 69.8 ± 2.1bc/a | 80.2 ± 2.4b/b | 84.7 ± 2.5b/b | 89.2 ± 2.6ab/b | |
| 240 | 56.5 ± 1.7c/a | 65.4 ± 2.0c/b | 59.4 ± 1.8c/ab | 74.3 ± 2.2a/c | 60.9 ± 1.8c/ab | |
| 11 | 60 | 57.9 ± 1.7a/a | 43.1 ± 1.3a/b | 89.2 ± 2.6a/c | 60.9 ± 1.8a/ | 112.9 ± 3.4a/ |
| 120 | 87.7 ± 2.6b/ab | 84.7 ± 2.5b/a | 92.1 ± 2.7a/b | 65.4 ± 1.9a/c | 102.5 ± 3.0a/b | |
| 180 | 72.8 ± 2.2c/a | 65.4 ± 1.9c/b | 77.3 ± 2.3b/a | 86.2 ± 2.6b/c | 132.2 ± 3.9b/d | |
| 240 | 49.0 ± 1.4d/a | 57.9 ± 1.7d/b | 66.9 ± 2.0c/c | 74.3 ± 2.2c/c | 83.2 ± 2.4c/d | |
| Indicator | Endopolysaccharides | Exopolysaccharides | ||||
|---|---|---|---|---|---|---|
| BBM 3N | Prat | PratM | BBM 3N | Prat | PratM | |
| Neutral sugars, mg/g PS | 440.6 ± 13.2a | 104.0 ± 3.1b | 272.3 ± 8.1c | 247.5 ± 7.4a | 500.0 ± 15.0b | 802.0 ± 24.0c |
| Uronic acids, mg/g PS | 95.0 ± 2.8a | 103.7 ± 3.1a | 95.4 ± 2.7a | 96.0 ± 2.8a | 96.8 ± 2.8a | 92.4 ± 2.7a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babich, O.; Budenkova, E.; Kashirskikh, E.; Dolganyuk, V.; Ivanova, S.; Prosekov, A.; Anokhova, V.; Andreeva, A.; Sukhikh, S. Study of the Polysaccharide Production by the Microalga Vischeria punctata in Relation to Cultivation Conditions. Life 2022, 12, 1614. https://doi.org/10.3390/life12101614
Babich O, Budenkova E, Kashirskikh E, Dolganyuk V, Ivanova S, Prosekov A, Anokhova V, Andreeva A, Sukhikh S. Study of the Polysaccharide Production by the Microalga Vischeria punctata in Relation to Cultivation Conditions. Life. 2022; 12(10):1614. https://doi.org/10.3390/life12101614
Chicago/Turabian StyleBabich, Olga, Ekaterina Budenkova, Egor Kashirskikh, Vyacheslav Dolganyuk, Svetlana Ivanova, Alexander Prosekov, Veronika Anokhova, Anna Andreeva, and Stanislav Sukhikh. 2022. "Study of the Polysaccharide Production by the Microalga Vischeria punctata in Relation to Cultivation Conditions" Life 12, no. 10: 1614. https://doi.org/10.3390/life12101614
APA StyleBabich, O., Budenkova, E., Kashirskikh, E., Dolganyuk, V., Ivanova, S., Prosekov, A., Anokhova, V., Andreeva, A., & Sukhikh, S. (2022). Study of the Polysaccharide Production by the Microalga Vischeria punctata in Relation to Cultivation Conditions. Life, 12(10), 1614. https://doi.org/10.3390/life12101614











