On the Role of 40K in the Origin of Terrestrial Life
Abstract
:1. Introduction
2. Sources of Natural Radiation in the Early Archean: The Case for K
2.1. Abundance and Distribution of Natural Radiation Sources on the Primitive Earth
2.2. Potential Role of the Stable Potassium Isotope in Prebiotic Chemistry
2.3. Compatibility of Decay Products with Protobiological Molecular Structures
2.4. Continuity with Present-Day Terrestrial Life
2.5. Enhancement of the Radiation Activity in the Early Archean
2.5.1. Short-Lived Radionuclides of Astrophysical Origin
2.5.2. Radiation Sources Generated by Galactic Cosmic Rays
2.6. Summary
3. Radiochemical Impact of K in the Primitive Earth
3.1. Radiation Dose of Particles
3.2. Indirect Effects on Prebiotic Molecules Dissolved in Diluted Solutions
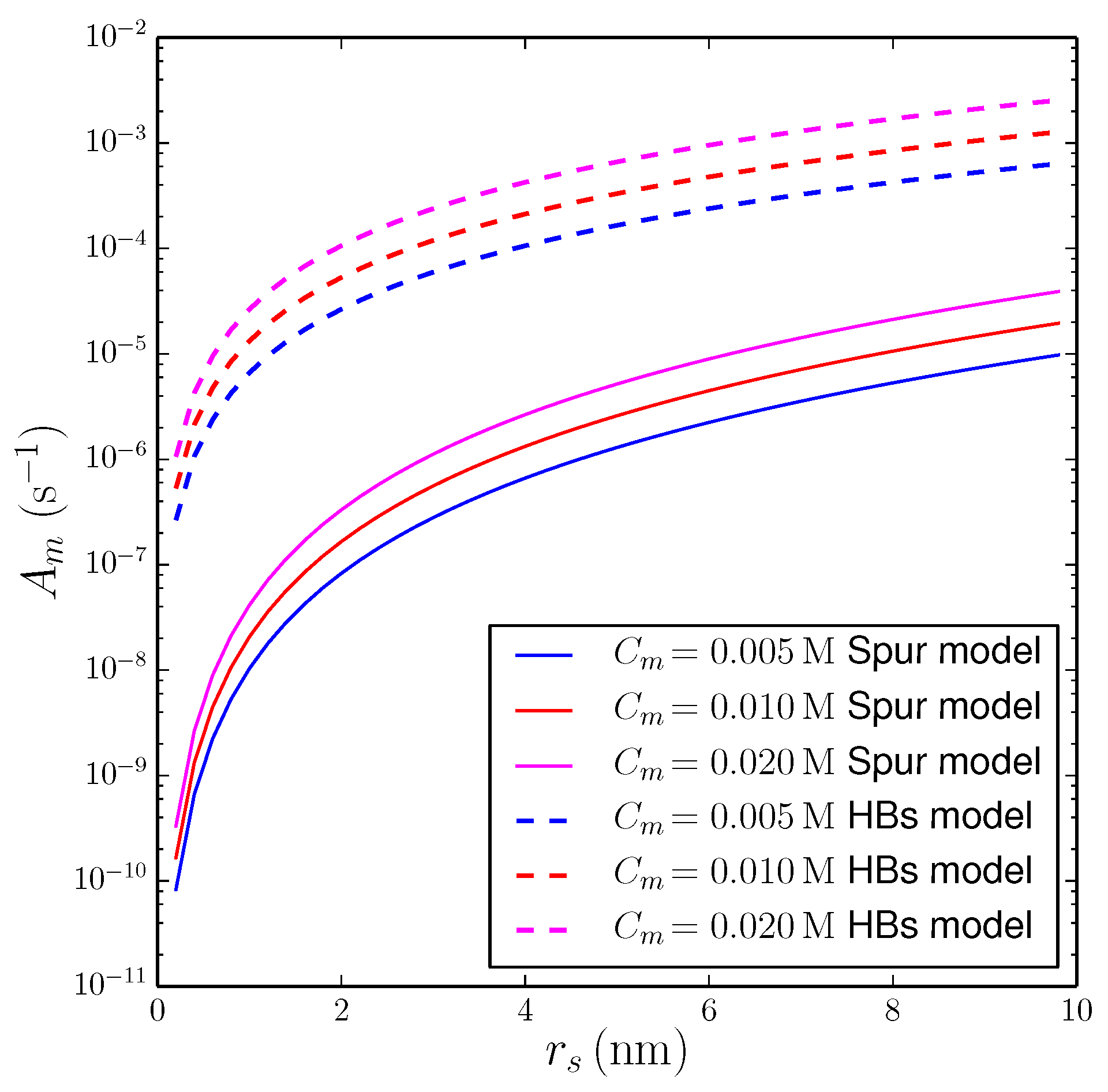
3.2.1. String of Spurs Model
3.2.2. Propagation of Secondary Effects via Hydrogen Bonding
3.3. Non-Diluted Solutions
4. Chiral Effects
4.1. Chiral Noise
4.2. Propagation of Chiral Effects in the Solvent
4.3. Simulating Chiral Effects in Archean Conditions
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Basic Properties of 40 K Decay
Appendix A.1. The β− Decay Route
Appendix A.2. The Electron Capture Route
References
- Oparin, A.I. Proiskhozhdenje Zhisni; Moskowski Rabochii: Moscow, Russia, 1924. [Google Scholar]
- Luisi, P.L. The Emergence of Life From Chemical Origins to Synthetic Biology; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Saladino, R.; Di Mauro, E.; Garcia-Ruiz, J.M. A Universal Geochemical Scenario for Formamide Condensation and Prebiotic Chemistry. Chem. Eur. J. 2019, 25, 3181–3189. [Google Scholar] [CrossRef] [PubMed]
- Bada, J.L.; Lazcano, A. Origin of life. Some like it hot, but not the first biomolecules. Science 2002, 296, 1982–1983. [Google Scholar] [CrossRef] [PubMed]
- Mulkidjanian, A.Y.; Bychkov, A.Y.; Dibrova, D.V.; Galperin, M.Y.; Koonin, E.V. Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl. Acad. Sci. USA 2012, 109, E821–E830. [Google Scholar] [CrossRef] [Green Version]
- Westall, F.; Hickman-Lewis, K.; Hinman, N.; Gautret, P.; Campbell, K.A.; Bréhéret, J.G.; Foucher, F.; Hubert, A.; Sorieul, S.; Dass, A.V.; et al. A Hydrothermal-Sedimentary Context for the Origin of Life. Astrobiology 2018, 18, 259–293. [Google Scholar] [CrossRef]
- Toner, J.D.; Catling, D.C. Alkaline lake settings for concentrated prebiotic cyanide and the origin of life. Geochim. Cosmochim. Acta 2019, 260, 124–132. [Google Scholar] [CrossRef]
- Damer, B.; Deamer, D. The Hot Spring Hypothesis for an Origin of Life. Astrobiol. Apr. 2020, 20, 429–452. [Google Scholar] [CrossRef] [Green Version]
- Draganić, I.G.; Draganić, Z.D.; Adloff, J.-P. (Draganić) Radiation and Radioactivity on Earth and Beyond; CRC Press, Inc.: Boca Raton, FL, USA, 1993. [Google Scholar]
- Anderson, D. New Theory of the Earth, 2nd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Kasting, J.F.; Whitmire, D.P.; Reynolds, R.T. Habitable Zones around Main Sequence Stars. Icarus 1993, 101, 108–128. [Google Scholar] [CrossRef]
- Unterborn, C.T.; Foley, B.J.; Desch, S.J.; Young, P.A.; Vance, G.; Chieffle, L.; Kane, S.R. Mantle Degassing Lifetimes through Galactic Time and the Maximum Age Stagnant-lid Rocky Exoplanets Can Support Temperate Climates. ApJL 2022, 930, L6. [Google Scholar] [CrossRef]
- Choppin, G.; Liljenzin, J.-O.; Rydberg, J. Radiochemistry and Nuclear Chemistry, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 2002; ISBN 0-7506-7463-6. [Google Scholar]
- Magill, J.; Galy, J. Radioactivity Radionuclides Radiation; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Garzón, L.; Garzón, M.L. Radioactivity as a significant energy source in prebiotic synthesis. Orig. Life Evol. Biosph. 2001, 31, 3–13. [Google Scholar] [CrossRef]
- Zagórski, Z.P. Radiation chemistry and origins of life on earth. Radiat. Phys. Chem. 2003, 66, 329–334. [Google Scholar] [CrossRef]
- Parnell, J. Mineral Radioactivity in Sands as a Mechanism for Fixation of Organic Carbon on the Early Earth. Orig. Life Evol. Biosph. 2004, 34, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, Z. Actinides and Life’s Origins. Astrobiology 2007, 7, 852–872. [Google Scholar] [CrossRef] [PubMed]
- Dartnell, L.R. Ionizing Radiation and Life. Astrobiology 2011, 11, 551–582. [Google Scholar] [CrossRef] [Green Version]
- Altair, T.; Sartori, L.M.; Rodrigues, F.; de Avellar, M.G.B.; Galante, D. Natural Radioactive Environments as Sources of Local Disequilibrium for the Emergence of Life. Astrobiology 2020, 20, 1489. [Google Scholar] [CrossRef]
- Noyes, H.P.; Bonner, W.A.; Tomlin, J.A. On the origin of biological chirality via natural beta-decay. Orig. Life 1977, 8, 21–23. [Google Scholar] [CrossRef]
- Draganić, I.G.; Bjergbakke, E.; Draganić, Z.D.; Sehested, K. Decomposition of ocean waters by potassium-40 radiation 3800 Ma ago as a source of oxygen and oxidizing species. Precambrian Res. 1991, 52, 337–345. [Google Scholar] [CrossRef]
- Benner, S.A.; Kim, H.-J.; Carrigan, M.A. Asphalt, Water, and the Prebiotic Synthesis of Ribose, Ribonucleosides, and RNA. Acc. Chem. Res. 2012, 45, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Blackmond, D.G. The Origin of Biological Homochirality. Cold Spring Harb. Perspect Biol. 2019, 11, a032540. [Google Scholar] [CrossRef] [Green Version]
- Joyce, G.F.; Visser, G.M.; Van Boeckel, C.A.; Van Boom, J.H.; Orgel, L.E.; Van Westrenen, J. Chiral selection in poly(C)-directed synthesis of oligo(G). Nature 1984, 310, 602–604. [Google Scholar] [CrossRef]
- Bryliakov, K.P. Chemical Mechanisms of Prebiotic Chirality Amplification. Research 2020, 2020, 5689246. [Google Scholar] [CrossRef]
- Yamagata, Y. A hypothesis for the asymmetric appearance of biomolecules on earth. J. Theoret. Biol. 1966, 11, 495–498. [Google Scholar] [CrossRef]
- Bersuker, G. Chiral symmetry breaking. I. Cooperative effects in solutions. J. Chem. Phys. 1999, 110, 10907. [Google Scholar] [CrossRef]
- Bersuker, G. Chiral symmetry breaking. II. Synthesis in cooperative systems. J. Chem. Phys. 1999, 110, 10923. [Google Scholar] [CrossRef]
- Bada, J.L. Racemization of Amino Acids in Nature. Interdiscip. Sci. Rev. 1982, 7, 30–46. [Google Scholar] [CrossRef]
- Bettini, A. Introduction to Elementary Particle Physics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Martin, B.R.; Shaw, G. Particle Physics, 4th ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2017. [Google Scholar]
- Lee, T.D.; Yang, C.N. Question of Parity Conservation in Weak Interactions. Phys. Rev. 1956, 104, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-S.; Ambler, E.; Hayward, R.; Hoppes, D.; Hudson, R.P. Experimental Test of Parity Conservation in Beta Decay. Phys. Rev. 1957, 105, 1413. [Google Scholar] [CrossRef] [Green Version]
- Keszthelyi, L. Origin of the asymmetry of biomolecules and weak interaction. Orig. Life 1977, 8, 299–340. [Google Scholar] [CrossRef]
- Salam, A. The Role of Chirality in the Origin of Life. J. Mol. Evol. 1991, 33, 105–113. [Google Scholar] [CrossRef]
- Ozturk, S.F.; Sasselov, D.D. On the origins of life’s homochirality: Inducing enentiomeric excess with spin-polarized electrons. Proc. Natl. Acad. Sci. USA 2022, 119, e2204765119. [Google Scholar] [CrossRef]
- Pinti, D.L. The Origin and Evolution of the Oceans. In Lectures in Astrobiology; Gargaud, M., Barbier, B., Martin, H., Reisse, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume I, pp. 83–112. [Google Scholar]
- Westall, F. The Geological Context for the Origin of Life and the mineral Signatures of Fossil Life. In Lectures in Astrobiology; Gargaud, M., Barbier, B., Martin, H., Reisse, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume I, pp. 195–226. [Google Scholar]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. Abundance of the elements in the crust and in the sea. In Handbook of Chemistry and Physics, 97th ed.; CRC: Boca Raton, FL, USA, 2016. [Google Scholar]
- Cameron, J.A.; Singh, B. Nuclear Data Sheets for A=40. Nucl. Data Sheets 2004, 102, 293–513. [Google Scholar] [CrossRef]
- Arevalo, R., Jr.; McDonough, F.; Luong, M. The K/U ratio of the silicate Earth: Insights into mantle composition, structure and thermal evolution. Earth Planet. Sci. Lett. 2009, 278, 361–369. [Google Scholar] [CrossRef]
- Djokic, T.; Van Kranendonk, M.J.; Campbell, K.A.; Havig, J.R.; Walter, M.R.; Guido, D.M. A Reconstructed Subaerial Hot Spring Field in the ∼3.5 Billion-Year-Old Dresser Formation, North Pole Dome, Pilbara Craton, Western Australia. Astrobiology 2021, 21, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Van Kranendonk, M.J.; Baumgartner, R.; Djokic, T.; Ota, T.; Steller, L.; Garbe, U.; Nakamura, E. Elements for the Origin of Life on Land: A Deep-Time Perspective from the Pilbara Craton of Western Australia. Astrobiology 2021, 21, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Hardie, L.A. Secular variations in Precambrian seawater chemistry and the timing of Precambrian aragonite seas and calcite seas. Geology 2003, 31, 785–788. [Google Scholar] [CrossRef]
- Natochin, Y.V. The origin of membranes. Paleontol. J. 2010, 44, 860–869. [Google Scholar] [CrossRef]
- Dubina, M.V.; Vyazmin, S.Y.; Boitsov, V.M.; Nikolaev, E.N.; Popov, I.A.; Kononichin, A.S.; Eliseev, I.E.; Natochin, Y.V. Potassium Ions are More Effective than Sodium Ions in Salt Induced Peptide Formation. Orig. Life Evol. Biosph. 2013, 43, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.D.; Ciulla, R.A.; Roberts, M.F. Osmoadaptation in Archaea. Appl. Environ. Microbiol. 1999, 65, 1815–1825. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.; Zhang, R.; Sun, X. Bustling argon: Biological effect. Med. Gas Res. 2013, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Doyle, D.A.; Morais Cabral, J.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Berg, J.; Tymoczko, J.L.; Stryer, L. Biochemistry, 6th ed.; W. H. Freeman and Company: New York, NY, USA, 2007. [Google Scholar]
- Woese, C.R.; Fox, G.E. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef]
- Macallum, A.B. The paleochemistry of the body fluids and tissues. Physiol. Rev. 1926, 6, 316–357. [Google Scholar] [CrossRef]
- Dibrova, D.V.; Galperin, M.Y.; Koonin, E.V.; Mulkidjanian, A.Y. Ancient Systems of Sodium/Potassium Homeostasis as Predecessors of Membrane Bioenergetics. Biochemistry (Mosc) 2015, 80, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Rugel, G.; Faestermann, T.; Knie, K.; Korschinek, G.; Poutivtsev, M.; Schumann, D.; Kivel, N.; Günther-Leopold, I.; Weinreich, R.; Wohlmuther, M. New Measurement of the 60Fe Half-Life. Phys. Rev. Lett. 2009, 103, 072502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallner, A.; Bichler, M.; Buczak, K.; Dressler, R.; Fifield, L.K.; Schumann, D.; Sterba, J.H.; Tims, S.G.; Wallner, G.; Kutschera, W. Settling the Half-Life of 60Fe: Fundamental for a Versatile Astrophysical Chronometer. Phys. Rev. Lett. 2015, 114, 041101. [Google Scholar] [CrossRef]
- Ostdiek, K.M.; Anderson, T.S.; Bauder, W.K.; Bowers, M.R.; Clark, A.M.; Collon, P.; Lu, W.; Nelson, A.D.; Robertson, D.; Skulski, M.; et al. Activity measurement of 60Fe through the decay of 60mCo and confirmation of its half-life. PhRvC 2017, 95, 055809. [Google Scholar]
- Lichtenberg, T.; Dražkowska, J.; Schönbachler, M.; Golabek, G.J.; Hands, T.O. Bifurcation of planetary building blocks during Solar System formation. Science 2021, 22, 365–370. [Google Scholar] [CrossRef]
- Marcelli, N.; Boezio, M.; Lenni, A.; Menn, W.; Munini, R.; Aslam, O.P.; Bisschoff, D.; Ngobeni, M.D.; Potgieter, M.S.; Adriani, O.; et al. Helium Fluxes Measured by the PAMELA Experiment from the Minimum to the Maximum Solar Activity for Solar Cycle 24. ApJL 2022, 925, L24. [Google Scholar] [CrossRef]
- Jöckel, P.; Brenninkmeijer, C.A.M.; Lawrence, M.G. Atmospheric response time of cosmogenic 14CO to changes in solar activity. J. Geophys. Res. 2000, 105, 6737–6744. [Google Scholar] [CrossRef]
- Cohen, O.; Drake, J.J.; Kóta, J. The cosmic-ray intensity near the Archean Earth. Astrophys. J. 2012, 760, 85. [Google Scholar] [CrossRef] [Green Version]
- Kovaltsov, G.A.; Mishev, A.; Usoskin, I.G. A new model of cosmogenic production of radiocarbon 14C in the atmosphere. Earth Planet. Sci. Let. 2012, 337–338, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Globus, N.; Blandford, D.R. The Chiral Puzzle of Life. Astroph. J. Lett. 2020, 895, 11. [Google Scholar] [CrossRef]
- Vladilo, G.; Hassanali, A. Hydrogen Bonds and Life in the Universe. Life 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation; United Nations: New York, NY, USA, 2010; p. 4. ISBN 978-92-1-142274-0. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford Science Publications Inc.: New York, NY, USA, 1999. [Google Scholar]
- Maréchal, Y. The Hydrogen Bond and the Water Molecule: The Physics and Chemistry of Water, Aqueous and Bio Media; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- MacDermott, A.J.; Tranter, G.E. Electroweak Bioenantioselection. Croatica Chem. Acta 1989, 62, 165–187. [Google Scholar]
- Faglioni, F.; Lazzeretti, P. Understanding parity violation in molecular systems. Phys. Rev. E 2001, 65, 011904. [Google Scholar] [CrossRef]
- Faglioni, F.; Passalacqua, A.; Lazzeretti, P. Parity Violation Energy Of Biomolecules—I: Polypeptides. Orig. Life Evol. Biosph. 2005, 35, 461–475. [Google Scholar] [CrossRef]
- Hegstrom, R. Weak neutral current and β radiolysis effects on the origin of biomolecular chirality. Nature 1985, 315, 749–750. [Google Scholar] [CrossRef]
- Hegstrom, R. Parity violation and chiral symmetry breaking of a racemic mixture. Biosystems 1987, 20, 49–56. [Google Scholar] [CrossRef]
- Garay, A.S. Origin and Role of Optical Isomery of Life. Nature 1968, 219, 338–340. [Google Scholar] [CrossRef]
- Darge, W.; Laczkó, I.; Thiemann, W. Stereoselectivity of β irradiation of D,L-tryptophan in aqueous solution. Nature 1976, 261, 522–524. [Google Scholar] [CrossRef]
- Bonner, W.; Blair, N.; Flores, J. Attempted asymmetric radiolysis of D,L-tryptophan with 32P β radiation. Nature 1979, 281, 150–151. [Google Scholar] [CrossRef]
- Goldhaber, M.; Grodzins, L.; Synyar, A.W. Evidence for Circular Polarization of Bremsstrahlung Produced by Beta Rays. Phys. Rev. 1957, 106, 826–828. [Google Scholar] [CrossRef]
- Garay, A.S.; Keszthelyi, L.; Demeter, I.; Hrasko, P. Differences in the annihilation of positrons in optical isomers. Chem. Phys. Lett. 1973, 23, 549–552. [Google Scholar] [CrossRef]
- Garay, A.S.; Keszthelyi, L.; Demeter, I.; Hrasko, P. Origin of asymmetry in biomolecules. Nature 1974, 250, 332–333. [Google Scholar] [CrossRef]
- Bonner, W.; Dort, M.; Yearian, M. Asymmetric degradation of DL-leucine with longitudinally polarized electrons. Nature 1975, 258, 419–421. [Google Scholar] [CrossRef]
- Gidley, D.W.; Rich, A.; House, J.V.; Zitzewitz, P.W. β Decay and the origins of biological chirality: Experimental results. Nature 1982, 297, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Meiring, W.J. Nuclear β-decay and the origin of biomolecular chirality. Nature 1987, 329, 712–714. [Google Scholar] [CrossRef]
- Atri, D.; Hariharan, B.; Grießmeier, J.-M. Galactic Cosmic Ray-Induced Radiation Dose on Terrestrial Exoplanets. Astrobiology 2013, 13, 910–919. [Google Scholar] [CrossRef] [Green Version]
- Zagórski, Z.P. Facts and artefacts in attempts to separate racemates with the assistance of ionizing radiation. Radiat. Phys. Chem. 1993, 42, 997–1001. [Google Scholar] [CrossRef]
- Rouxel, J.R.; Kowalewski, M.; Mukamel, S. Photoinduced molecular chirality probed by ultrafast resonant X-ray spectroscopy. Struct. Dyn. 2017, 4, 044006. [Google Scholar] [CrossRef]

| Elem. | Abund. in the Crust (mg/kg) | Abund. in the Sea (mg/L) | Role in Biology | Unstable Nuclide | Isotopic Ratio (%) | Half Life (Gyr) | Decay Mode | Decay Product | |
|---|---|---|---|---|---|---|---|---|---|
| K | 20,900 | 399 | yes | K | 0.0117 | 0.893 | Ca | ||
| EC, | 0.107 | Ar | |||||||
| Th | 9.6 | no | Th | 100 | 14 | Ra | |||
| U | no | U | 0.72 | Th | |||||
| U | 99.27 | Th |
| (Ga) | (Bq L) | (Bq L) | (Bq L) |
| 0 | |||
| 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladilo, G. On the Role of 40K in the Origin of Terrestrial Life. Life 2022, 12, 1620. https://doi.org/10.3390/life12101620
Vladilo G. On the Role of 40K in the Origin of Terrestrial Life. Life. 2022; 12(10):1620. https://doi.org/10.3390/life12101620
Chicago/Turabian StyleVladilo, Giovanni. 2022. "On the Role of 40K in the Origin of Terrestrial Life" Life 12, no. 10: 1620. https://doi.org/10.3390/life12101620
APA StyleVladilo, G. (2022). On the Role of 40K in the Origin of Terrestrial Life. Life, 12(10), 1620. https://doi.org/10.3390/life12101620








