Mulberry (Morus alba L.) Leaf Extract and 1-Deoxynojirimycin Improve Skeletal Muscle Insulin Resistance via the Activation of IRS-1/PI3K/Akt Pathway in db/db Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Mulberry Leaf Extract (MLE) Powder Preparation for Metabolite Analysis
2.3. Gas Chromatography-Time of Flight-Mass Spectrometry Analysis (GC-TOF-MS)
2.4. Ultrahigh Performance Liquid Chromatography-Linear Trap Quadrupole-Orbitrap-Mass Spectrometry (UHPLC-LTQ-Orbitrap-MS/MS) Analysis
2.5. Experimental Animals and Diets
2.6. Fasting Blood Glucose Levels (FBGLs), Oral Glucose Tolerance Test (OGTT), and Insulin Tolerance Test (ITT)
2.7. Serum Biochemical Analysis
2.8. Measurement of Glycogen Content in the Skeletal Muscle
2.9. Extraction of Membrane Protein from Skeletal Muscle
2.10. Western Blot Analysis
2.11. Hematoxylin and Eosin (H&E) Staining
2.12. Data Analysis
3. Results
3.1. Metabolites Identified through GC-TOF-MS and UHPLC-LTQ-Orbitrap-MS Analyses
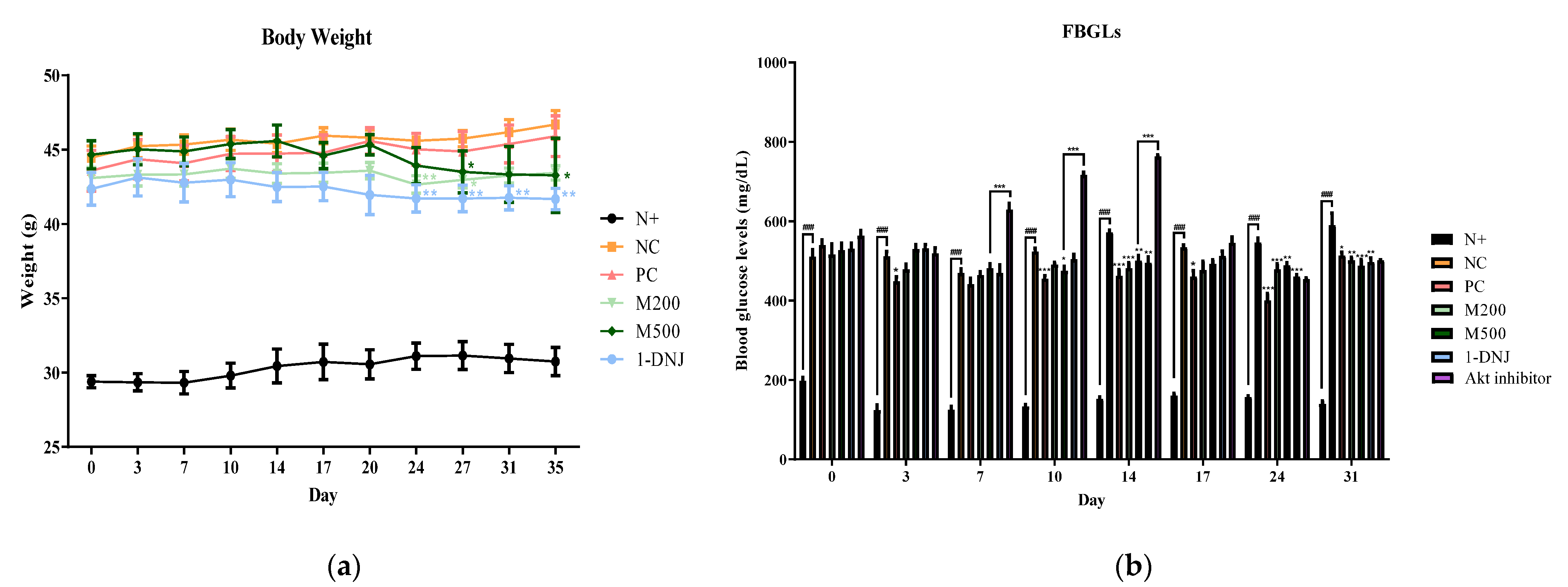
3.2. Effects of MLE or 1-DNJ on Body Weight and FBGLs in Diabetic db/db Mice
3.3. Effects of MLE or 1-DNJ on Oral Glucose Tolerance and Insulin Tolerance in Diabetic db/db Mice
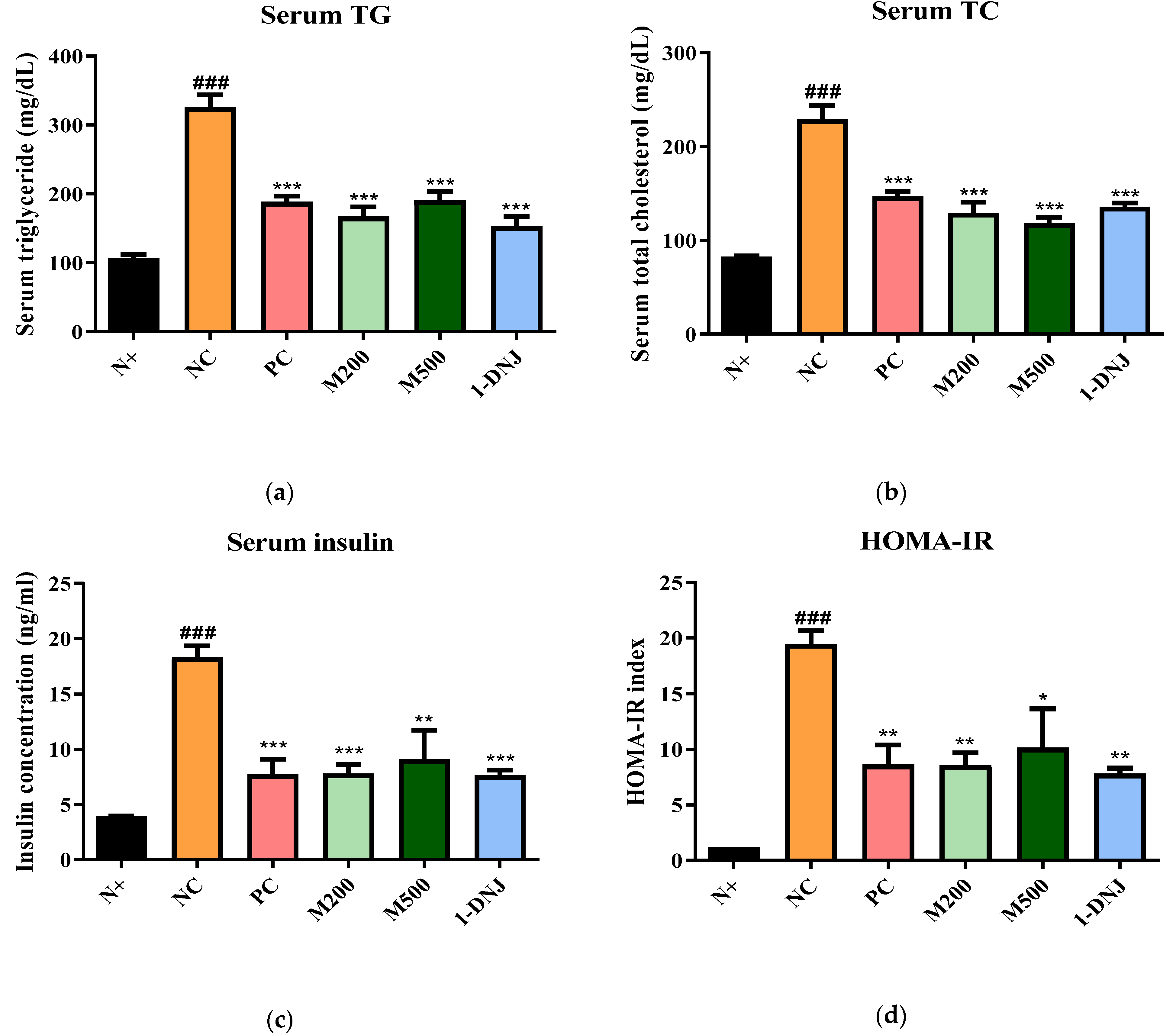
3.4. Effects of MLE or 1-DNJ on Serum Biochemical Parameters in Diabetic db/db Mice
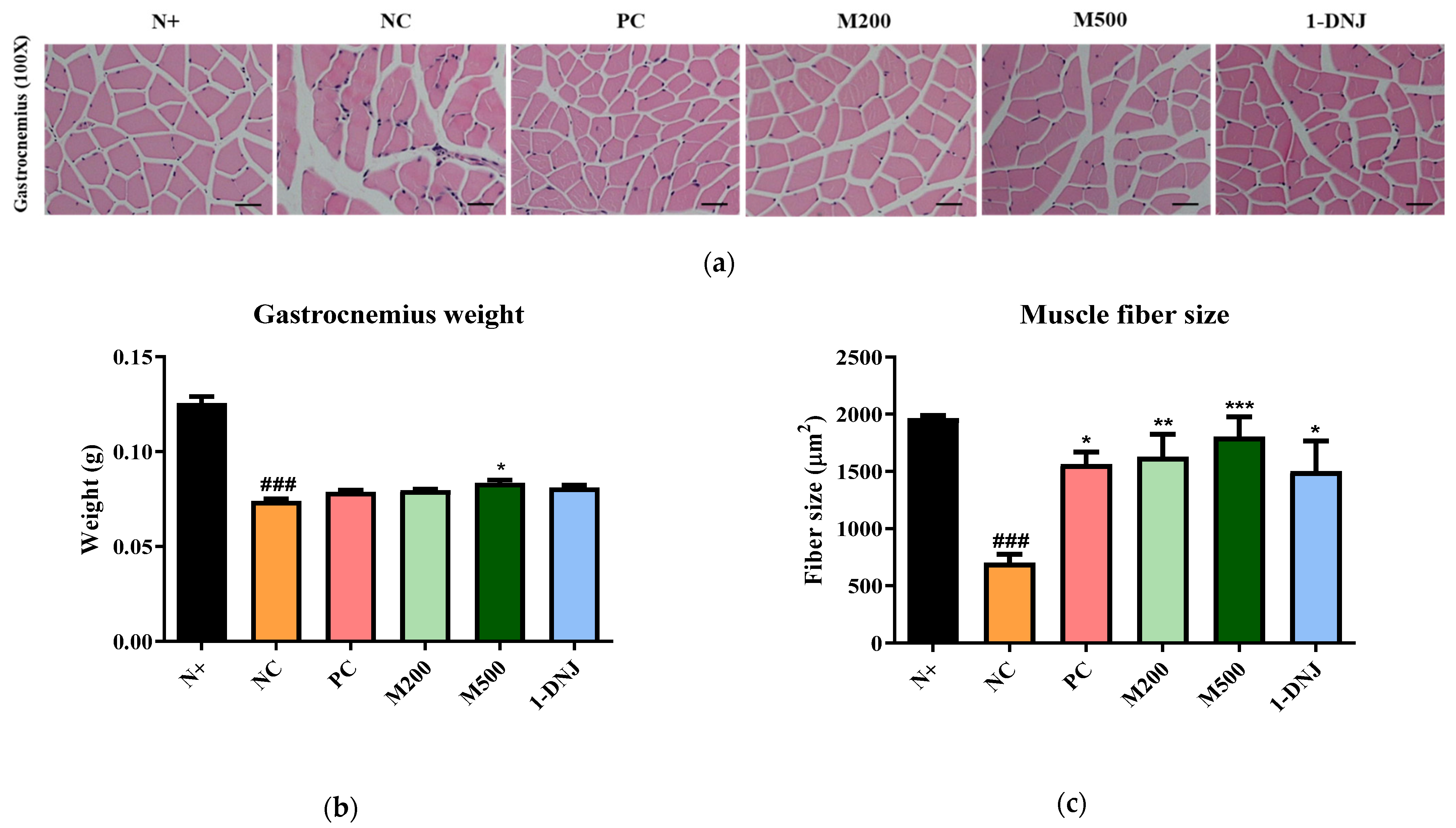
3.5. Effects of MLE or 1-DNJ on the Pathology of Diabetic Skeletal Muscles
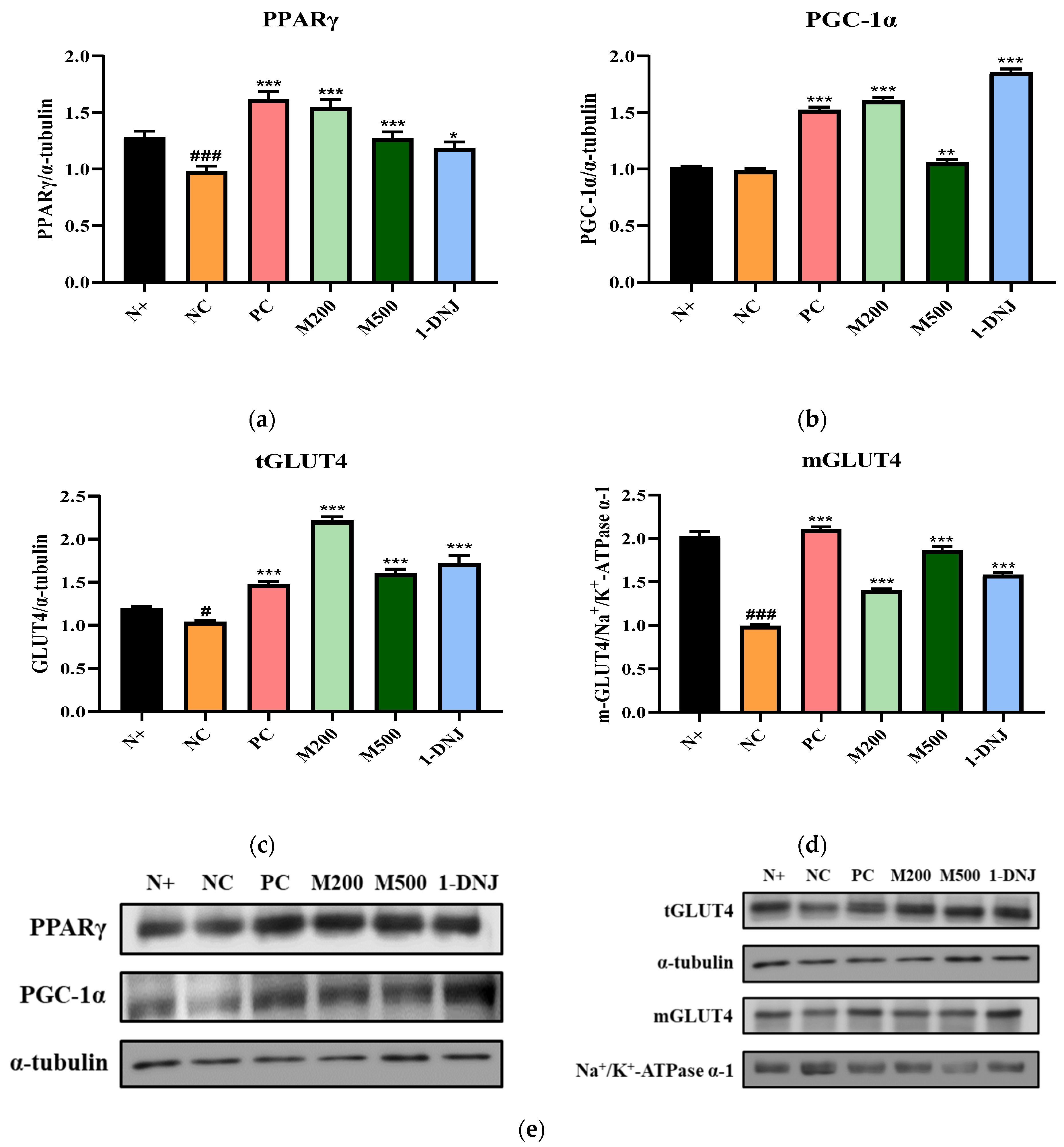
3.6. Effects of MLE or 1-DNJ on Glucose Metabolism in Diabetic Skeletal Muscle
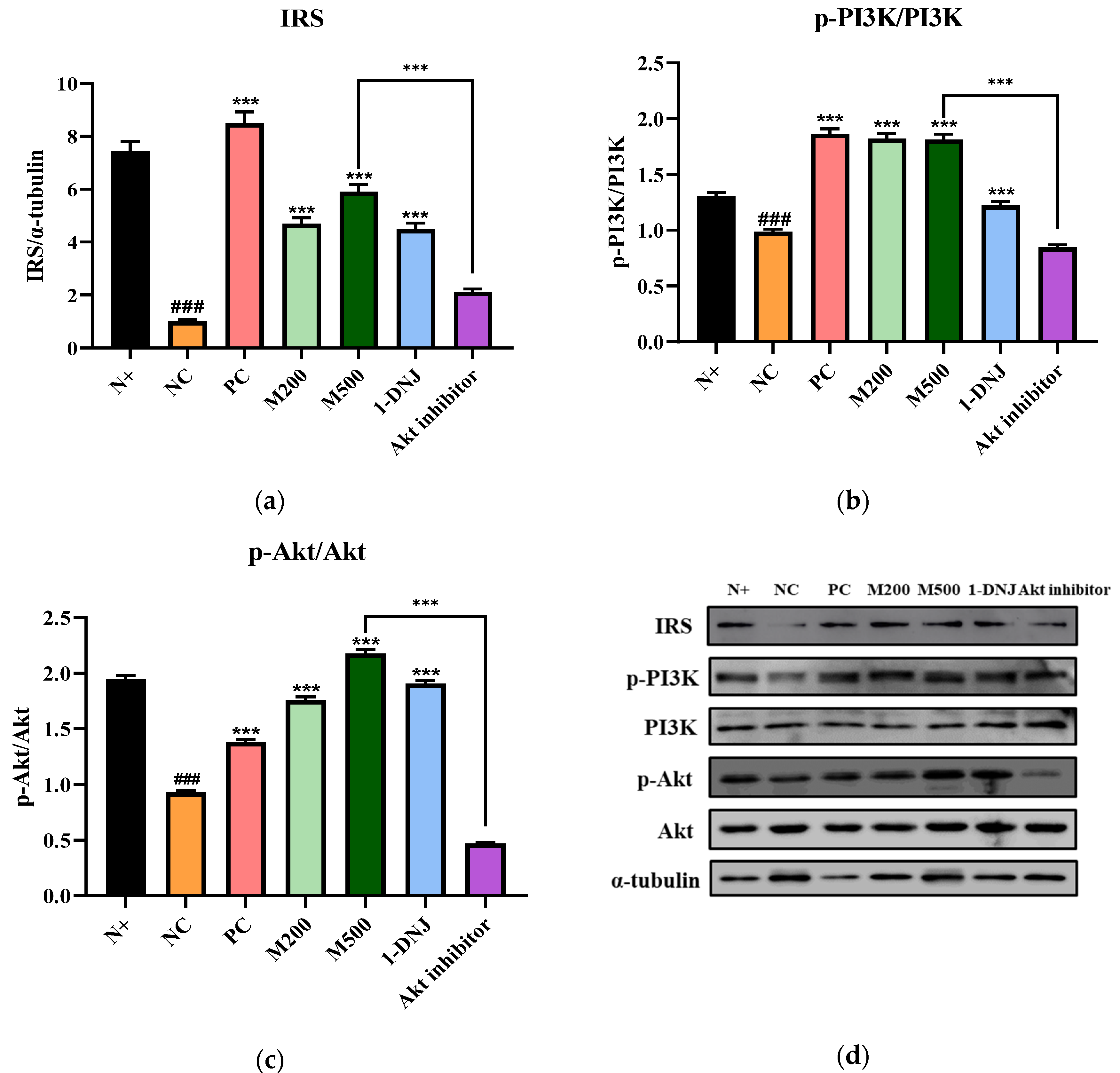
3.7. Effects of MLE or 1-DNJ on the IRS-1/PI3K/Akt Signaling Pathway in Diabetic Skeletal Muscle
3.8. Effects of MLE or 1-DNJ on Glycogen Synthesis in Diabetic Skeletal Muscle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, G.F.; Carpentier, A.; Adeli, K.; Giacca, A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr. Rev. 2002, 23, 201–229. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.M.; Pennings, N. Insulin Resistance; StatPearls [Internet]: Treasure Island, FL, USA, 2022. [Google Scholar]
- Arha, D.; Ramakrishna, E.; Gupta, A.P.; Rai, A.K.; Sharma, A.; Ahmad, I.; Riyazuddin, M.; Gayen, J.R.; Maurya, R.; Tamrakar, A.K. Isoalantolactone derivative promotes glucose utilization in skeletal muscle cells and increases energy expenditure in db/db mice via activating AMPK-dependent signaling. Mol. Cell. Endocrinol. 2018, 460, 134–151. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R. Knockout mice challenge our concepts of glucose homeostasis and the pathogenesis of diabetes. Exp. Diabesity Res. 2003, 4, 169–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, M.E.; Marcucci, M.J.; Cline, G.W.; Bell, K.; Barucci, N.; Lee, D.; Goodyear, L.J.; Kraegen, E.W.; White, M.F.; Shulman, G.I. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 1999, 48, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1057–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, K.F.; Shulman, G.I. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am. J. Cardiol. 2002, 90, 11–18. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Papavassiliou, A.G. Serine phosphorylation of insulin receptor substrate-1: A novel target for the reversal of insulin resistance. Mol. Endocrinol. 2001, 15, 1864–1869. [Google Scholar] [CrossRef]
- White, M.F. The IRS-signalling system: A network of docking proteins that mediate insulin action. Insul. Action 1998, 24, 3–11. [Google Scholar]
- Brazil, D.P.; Hemmings, B.A. Ten years of protein kinase B signalling: A hard Akt to follow. Trends Biochem. Sci. 2001, 26, 657–664. [Google Scholar] [CrossRef]
- Choi, K.; Kim, Y.-B. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J. Intern. Med. 2010, 25, 119. [Google Scholar] [CrossRef]
- Copps, K.; White, M. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.T.; Pessin, J.E. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog. Horm. Res. 2001, 56, 175–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.; Chiang, S.-H.; Saltiel, A.R. Insulin signaling and the regulation of glucose transport. Mol. Med. 2004, 10, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.M.; Staels, B. Peroxisome proliferator-activated receptor γ and adipose tissue—Understanding obesity-related changes in regulation of lipid and glucose metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Nikoulina, S.E.; Ciaraldi, T.P.; Mudaliar, S.; Mohideen, P.; Carter, L.; Henry, R.R. Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes 2000, 49, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacAulay, K.; Woodgett, J.R. Targeting glycogen synthase kinase-3 (GSK-3) in the treatment of Type 2 diabetes. Expert Opin. Ther. Targets 2008, 12, 1265–1274. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Mohamad Razali, U.H.; Saikim, F.H.; Mahyudin, A.; Mohd Noor, N.Q.I. Morus alba L. plant: Bioactive compounds and potential as a functional food ingredient. Foods 2021, 10, 689. [Google Scholar] [CrossRef]
- Andallu, B.; Suryakantham, V.; Srikanthi, B.L.; Reddy, G.K. Effect of mulberry (Morus indica L.) therapy on plasma and erythrocyte membrane lipids in patients with type 2 diabetes. Clin. Chim. Acta 2001, 314, 47–53. [Google Scholar] [CrossRef]
- Wang, H.; Shen, Y.; Zhao, L.; Ye, Y. 1-Deoxynojirimycin and its derivatives: A mini review of the literature. Curr. Med. Chem. 2021, 28, 628–643. [Google Scholar] [CrossRef]
- Huang, S.-S.; Yan, Y.-H.; Ko, C.-H.; Chen, K.-M.; Lee, S.-C.; Liu, C.-T. A comparison of food-grade folium mori extract and 1-deoxynojirimycin for glycemic control and renal function in streptozotocin-induced diabetic rats. J. Tradit. Complement. Med. 2014, 4, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Yang, W.; Liu, L. Study on fingerprint of Mulberry leaves by GC-MS. Zhongguo Zhong Yao Za Zhi= Zhongguo Zhongyao Zazhi= China J. Chin. Mater. Med. 2009, 34, 879–883. [Google Scholar]
- Thaipitakwong, T.; Numhom, S.; Aramwit, P. Mulberry leaves and their potential effects against cardiometabolic risks: A review of chemical compositions, biological properties and clinical efficacy. Pharm. Biol. 2018, 56, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, C.; Lu, G.; Mu, Z.; Cui, W.; Gao, H.; Wang, Y. Anti-diabetic effect of mulberry leaf polysaccharide by inhibiting pancreatic islet cell apoptosis and ameliorating insulin secretory capacity in diabetic rats. Int. Immunopharmacol. 2014, 22, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, Y.; Cui, W.; Lu, G.; Wang, Y.; Gao, H.; Huang, L.; Mu, Z. A polysaccharide extract of mulberry leaf ameliorates hepatic glucose metabolism and insulin signaling in rats with type 2 diabetes induced by high fat-diet and streptozotocin. Int. J. Biol. Macromol. 2015, 72, 951–959. [Google Scholar] [CrossRef]
- He, L.; Xing, Y.; Ren, X.; Zheng, M.; Yu, S.; Wang, Y.; Xiu, Z.; Dong, Y. Mulberry Leaf Extract Improves Metabolic Syndrome by Alleviating Lipid Accumulation In Vitro and In Vivo. Molecules 2022, 27, 5111. [Google Scholar] [CrossRef]
- Li, J.-S.; Ji, T.; Su, S.-L.; Zhu, Y.; Chen, X.-L.; Shang, E.-X.; Guo, S.; Qian, D.-W.; Duan, J.-A. Mulberry leaves ameliorate diabetes via regulating metabolic profiling and AGEs/RAGE and p38 MAPK/NF-κB pathway. J. Ethnopharmacol. 2022, 283, 114713. [Google Scholar] [CrossRef]
- Cai, S.; Sun, W.; Fan, Y.; Guo, X.; Xu, G.; Xu, T.; Hou, Y.; Zhao, B.; Feng, X.; Liu, T. Effect of mulberry leaf (Folium Mori) on insulin resistance via IRS-1/PI3K/Glut-4 signalling pathway in type 2 diabetes mellitus rats. Pharm. Biol. 2016, 54, 2685–2691. [Google Scholar] [CrossRef] [Green Version]
- Marrano, N.; Spagnuolo, R.; Biondi, G.; Cignarelli, A.; Perrini, S.; Vincenti, L.; Laviola, L.; Giorgino, F.; Natalicchio, A. Effects of extra virgin olive oil polyphenols on beta-cell function and survival. Plants 2021, 10, 286. [Google Scholar] [CrossRef]
- Trusheva, B.; Trunkova, D.; Bankova, V. Different extraction methods of biologically active components from propolis: A preliminary study. Chem. Cent. J. 2007, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-Z.; Liu, Q.-H.; Liu, Z.; Tang, J.-W.; Chua, E.-G.; Li, F.; Xiong, X.-S.; Wang, M.-M.; Wen, P.-B.; Shi, X.-Y. Ethanol extract of mulberry leaves partially restores the composition of intestinal microbiota and strengthens liver glycogen fragility in type 2 diabetic rats. BMC Complement. Med. Ther. 2021, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kawale, L.; Nade, V. Effect of Morus alba L.(mulberry) leaves on anxiety in mice. Indian J. Pharmacol. 2008, 40, 32. [Google Scholar] [PubMed]
- Liu, Q.; Li, X.; Li, C.; Zheng, Y.; Peng, G. 1-Deoxynojirimycin alleviates insulin resistance via activation of insulin signaling PI3K/AKT pathway in skeletal muscle of db/db mice. Molecules 2015, 20, 9794. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [Green Version]
- Stenbit, A.E.; Tsao, T.-S.; Li, J.; Burcelin, R.; Geenen, D.L.; Factor, S.M.; Houseknecht, K.; Katz, E.B.; Charron, M.J. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat. Med. 1997, 3, 1096–1101. [Google Scholar] [CrossRef]
- Haber, R.S.; Weinstein, S.P. Role of glucose transporters in glucocorticoid-induced insulin resistance: GLUT4 isoform in rat skeletal muscle is not decreased by dexamethasone. Diabetes 1992, 41, 728–735. [Google Scholar] [CrossRef]
- Houmard, J.A.; Weidner, M.D.; Dolan, P.L.; Leggett-Frazier, N.; Gavigan, K.E.; Hickey, M.S.; Tyndall, G.L.; Zheng, D.; Alshami, A.; Dohm, G.L. Skeletal muscle GLUT4 protein concentration and aging in humans. Diabetes 1995, 44, 555–560. [Google Scholar] [CrossRef]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-talk between PPAR and insulin signaling and modulation of insulin sensitivity. PPAR Res. 2009, 2009, 818945. [Google Scholar] [CrossRef] [Green Version]
- Kintscher, U.; Law, R.E. PPARγ-mediated insulin sensitization: The importance of fat versus muscle. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, E287–E291. [Google Scholar] [CrossRef] [Green Version]
- Gouspillou, G.; Sgarioto, N.; Norris, B.; Barbat-Artigas, S.; Aubertin-Leheudre, M.; Morais, J.A.; Burelle, Y.; Taivassalo, T.; Hepple, R.T. The relationship between muscle fiber type-specific PGC-1α content and mitochondrial content varies between rodent models and humans. PLoS ONE 2014, 9, e103044. [Google Scholar] [CrossRef]
- Wu, H.; Deng, X.; Shi, Y.; Su, Y.; Wei, J.; Duan, H. PGC-1α, glucose metabolism and type 2 diabetes mellitus. J. Endocrinol. 2016, 229, R99–R115. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Louet, J.-F.; Lagouge, M.; Lerin, C.; Antal, M.C.; Meziane, H.; Schoonjans, K.; Puigserver, P.; O’Malley, B.W.; Auwerx, J. The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1α. Proc. Natl. Acad. Sci. USA 2008, 105, 17187–17192. [Google Scholar] [CrossRef]
- Lim, H.H.; Lee, S.O.; Kim, S.Y.; Yang, S.J.; Lim, Y. Anti-inflammatory and antiobesity effects of mulberry leaf and fruit extract on high fat diet-induced obesity. Exp. Biol. Med. 2013, 238, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Tsuduki, T.; Kikuchi, I.; Kimura, T.; Nakagawa, K.; Miyazawa, T. Intake of mulberry 1-deoxynojirimycin prevents diet-induced obesity through increases in adiponectin in mice. Food Chem. 2013, 139, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.; Gadeberg, P.; Brock, B.; Jakobsen, J. Muscular atrophy in diabetic neuropathy: A stereological magnetic resonance imaging study. Diabetologia 1997, 40, 1062–1069. [Google Scholar] [CrossRef]
- Sishi, B.; Loos, B.; Ellis, B.; Smith, W.; du Toit, E.F.; Engelbrecht, A.M. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp. Physiol. 2011, 96, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Leenders, M.; Verdijk, L.B.; van der Hoeven, L.; Adam, J.J.; Van Kranenburg, J.; Nilwik, R.; Van Loon, L.J. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J. Am. Med. Dir. Assoc. 2013, 14, 585–592. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Nyholm, B.; Saltin, B.; Schmitz, O.; Christiansen, J.S. Muscle fiber composition and capillary density in Turner syndrome: Evidence of increased muscle fiber size related to insulin resistance. Diabetes Care 2001, 24, 1668–1673. [Google Scholar] [CrossRef] [Green Version]
- Meex, R.C.; Blaak, E.E.; van Loon, L.J. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes. Rev. 2019, 20, 1205–1217. [Google Scholar] [CrossRef] [Green Version]
- Perry, B.D.; Caldow, M.K.; Brennan-Speranza, T.C.; Sbaraglia, M.; Jerums, G.; Garnham, A.; Wong, C.; Levinger, P.; ul Haq, M.A.; Hare, D.L. Muscle atrophy in patients with Type 2 Diabetes Mellitus: Roles of inflammatory pathways, physical activity and exercise. Exerc. Immunol. Rev. 2016, 22, 94. [Google Scholar]
- Ostler, J.E.; Maurya, S.K.; Dials, J.; Roof, S.R.; Devor, S.T.; Ziolo, M.T.; Periasamy, M. Effects of insulin resistance on skeletal muscle growth and exercise capacity in type 2 diabetic mouse models. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E592–E605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagoshi, T.; Matsui, T.; Aoyama, T.; Leri, A.; Anversa, P.; Li, L.; Ogawa, W.; Del Monte, F.; Gwathmey, J.K.; Grazette, L. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J. Clin. Investig. 2005, 115, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Glass, D.J. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Phosphoinositide 3-Kinase Health Dis. 2010, 346, 267–278. [Google Scholar]
- Li, X.; Zhen, M.; Zhou, C.; Deng, R.; Yu, T.; Wu, Y.; Shu, C.; Wang, C.; Bai, C. Gadofullerene nanoparticles reverse dysfunctions of pancreas and improve hepatic insulin resistance for type 2 diabetes mellitus treatment. ACS Nano 2019, 13, 8597–8608. [Google Scholar] [CrossRef] [PubMed]
- Bouskila, M.; Hirshman, M.F.; Jensen, J.; Goodyear, L.J.; Sakamoto, K. Insulin promotes glycogen synthesis in the absence of GSK3 phosphorylation in skeletal muscle. Am. J. Physiol. -Endocrinol. Metab. 2008, 294, E28–E35. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, C.-W.; Park, M.; Lee, H.-J. Mulberry (Morus alba L.) Leaf Extract and 1-Deoxynojirimycin Improve Skeletal Muscle Insulin Resistance via the Activation of IRS-1/PI3K/Akt Pathway in db/db Mice. Life 2022, 12, 1630. https://doi.org/10.3390/life12101630
Kang C-W, Park M, Lee H-J. Mulberry (Morus alba L.) Leaf Extract and 1-Deoxynojirimycin Improve Skeletal Muscle Insulin Resistance via the Activation of IRS-1/PI3K/Akt Pathway in db/db Mice. Life. 2022; 12(10):1630. https://doi.org/10.3390/life12101630
Chicago/Turabian StyleKang, Chae-Won, Miey Park, and Hae-Jeung Lee. 2022. "Mulberry (Morus alba L.) Leaf Extract and 1-Deoxynojirimycin Improve Skeletal Muscle Insulin Resistance via the Activation of IRS-1/PI3K/Akt Pathway in db/db Mice" Life 12, no. 10: 1630. https://doi.org/10.3390/life12101630
APA StyleKang, C.-W., Park, M., & Lee, H.-J. (2022). Mulberry (Morus alba L.) Leaf Extract and 1-Deoxynojirimycin Improve Skeletal Muscle Insulin Resistance via the Activation of IRS-1/PI3K/Akt Pathway in db/db Mice. Life, 12(10), 1630. https://doi.org/10.3390/life12101630








