Organic Amendments for Mitigation of Salinity Stress in Plants: A Review
Abstract
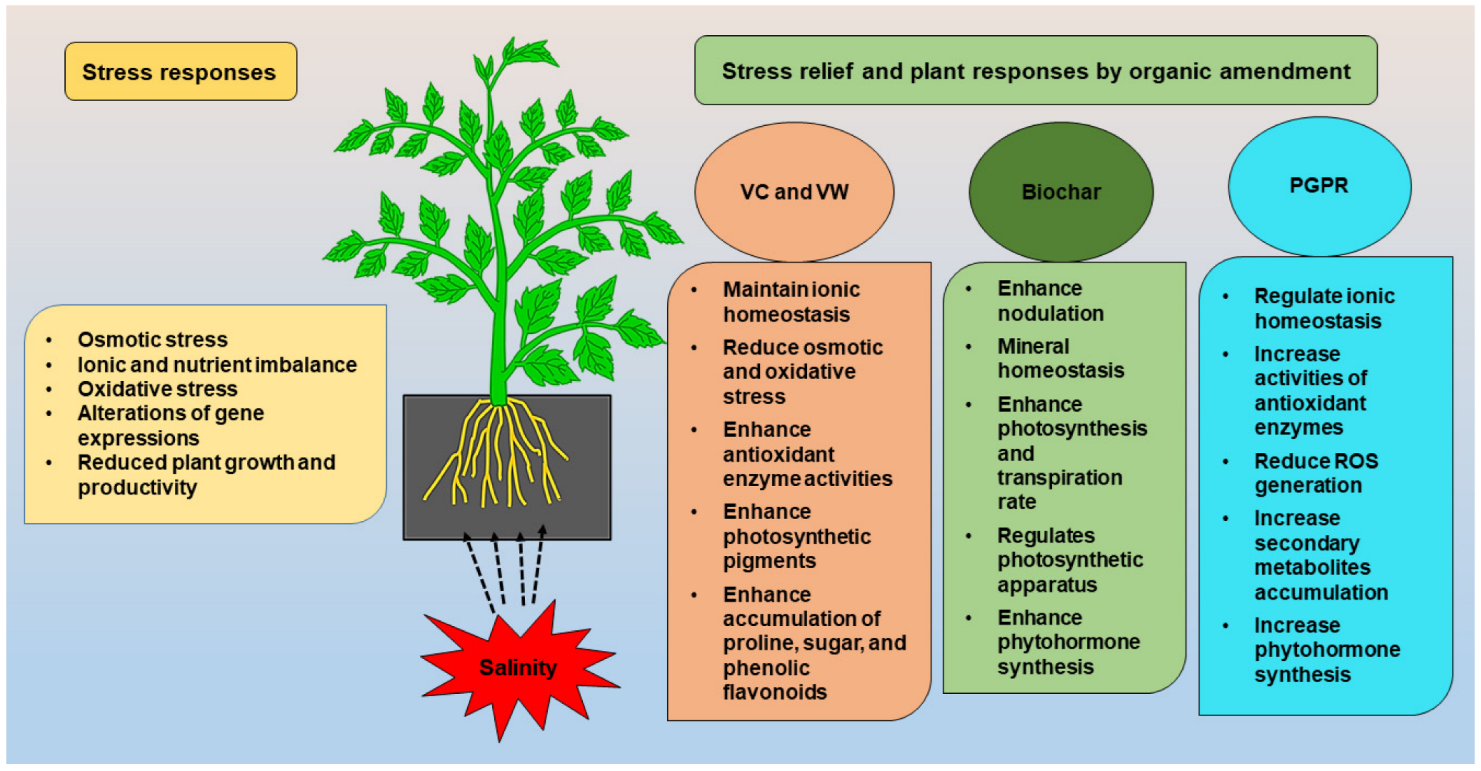
:1. Introduction
2. Organic Amendments for Salinity Stress Mitigation
2.1. Vermicompost and Vermiwash
| Plant Species | Stress Level | Treatment and Application Methods | Effects of Amendments | References |
|---|---|---|---|---|
| Tomato (Solanum lycopersicum L.) | NaCl @ 150 mM | VC @ 6 mL/L | Improved foliar growth, increased water content of the leaves, reduced osmotic potential at the root level and Na content of the leaves; promoted the accumulation of proline and total sugars. | [69] |
| NaCl @ 125 mM | VCL @ 18 mLL−1 | Improved plant growth and lowered Na+ deposition in salt-stressed plants; delayed young leaf senescence. | [70] | |
| NaCl @ 0, 50 and 150 mM | VC @ 10, and 20% | Increased shoot length, stem diameter, leaves number, root length, shoot and root fresh, dry weight, Chl a, Chl b and carotenoid; increased Cat; decreased MDA; improved salinity tolerance. | [61] | |
| Potato (Solanum tuberosum L.) | NaCl @ 15, 20, and 25 mM | VC @ 300, 580, and 860 g plant−1; VW @ 5-, 10-, and 15-mL plant−1 | The addition of VC and VW increased the height of the plant and the diameter of the stem. VC reduced salinity effects on the plant. | [35] |
| 2.85 dSm−1 | Exogenous VC, proline and glycine betaine | Increased growth, yield, bio-constituents and antioxidant enzymatic activity. Improved salt tolerance of potatoes | [71] | |
| Maize (Zea mays L.) | NaCl @ 0, 50, 100, 150 and 200 mM | VC with bacteria having ACC deaminase activity | Improved seed germination and the growth of seedlings; increased proline, chlorophyll content and alleviated the salt stress. | [72] |
| Coastal saline soil | VC with humic acid fertilizer | Increased soil macro-aggregates, improved soil nutrient availability and maize nutrient uptake. | [62] | |
| NaCl @ 6, and 12 dS m−1 | VC @ 5, and 10% | Increased root, shoot fresh and dry weight; increased Chl a, Chl b, total Chl, carotenoids; increased CAT, SOD, POD activities; decreased H2O2, MDA content; increased salinity tolerance. | [27] | |
| Moldavian dragonhead (Dracocephalum moldavica L.) | NaCl @ 0,50, 100 and 150 mM | VC @ 0, 5, 10 and 15% (v/v) | Increased plant biomass, chlorophyll content and proline accumulation. Reduced the effects of high sodium chloride concentrations. | [59] |
| Lemon verbena (Lippia citriodora) | NaCl @70 mM | VC @ 0%, 10% and 30% of pot volume | Alleviated salt stress by improving the growth and phenolic compounds of the plants. | [73] |
| Basil (Ocimum basilicum L.) | NaCl @ 0, 50 and 100 mM | Humates VC @ 0 and 1/60 v/v | Enhanced shoots and roots length, fresh and dry biomass of root, stem, leaf and leaf area. Reduced salinity. | [74] |
| Smoke tree (Cotinus coggygria Scop.) | NaCl @ 1, 4 and 7 dS.m−1 | VC @ 80% v/v soil + 20% v/v | Increased fresh and dry weight of shoots, increased leaf area; Reduced sodium and chloride of leaf and increased potassium. Increased salt tolerance of plant. | [58] |
| Fenugreek (Trigonellafoenum-graecum L.) | NaCl @ 0, 100 and 200 mM | VC @ 0, 5 and 10 weight% | Increased number of seed per pod, number of pods, number of sub branch and plant height. Reduced salinity effects. | [57] |
| Wheat (Triticum durum Desf. cv. Yelken) | High salt stress | VC and fish flour (1:1) | Enhanced growth, seed vigor and total phenolic-flavonoids, chlorophyl-carotenoids contents, and increased phenylalanine ammonialyase (PAL), peroxidase (POD) activities. VC decreased salinity effects. | [75] |
| Coastal salinity | Soil amendment of VC | Increased soil macro-aggregates; Improved shoot biomass, grain yield, soil physical, chemical and biological properties. Ameliorated salt-induced stress. | [76] | |
| Saline soil | VC @ 10.0-ton ha−1; Biochar @ 10-ton ha−1 | Improved relative water content, total chlorophyll, stomatal conductance, leaf K+ concentration; Reduced oxidative stress, leaf Na+ concentration, and proline content; improved yield related traits, productivity, soil water level and chemical properties. Eliminated the detrimental effects of soil salinity. | [68] | |
| Rice (Oryza sativa) | Soil salinity | VC and rice husk ash @ 1000 kg per Rai for both | Increased exchangeable K+, Ca2+ and Mg2+ in soil; reduced electrical conductivity and risen tillers per clump; improved the physiological and biochemical responses. Increased the rice growth in salt affected area. | [44] |
| Lettuce (Lactuca sativa) | NaCl @ 0, 4 and 8 dS m−1 | VC @ 0, 2.5 and 5% (w/w) | Enhanced soil organic matter, available P, total N, available K and the cation exchange capacity of the soils; Increased field capacity, available water capacity, saturated hydraulic conductivity, total porosity, and aggregate stability; Decreased EC values and the bulk density of the soils. | [56] |
| NaCl @ 8.32 dS/m | VC 50% and pulverized eggshell 12.5% | Decreased soil salinity for about 77%; fasten the seed germination and seedling growth. | [77] | |
| NaCl @ 4, 8 dSm−1 | VC 5% (w/w) | Increased P, K, Mg, Fe, Mn and Zn concentrations; decreased Na contents. Reduced toxic effects of salinity on the plant. | [65] | |
| NaCl @ 4 and 8 dSm–1 | VC @ 0, 2.5 and 5% (w/w) | Increased relative water content, stomatal conductance, chlorophyll a content; decreased electrolyte leakage, malondialdehyde (MDA) contents; increased superoxide dismutase (SOD) and catalase (CAT) activities. Alleviated the salt stress. | [67] | |
| Pot marigold (Calendula officinalis L.) | NaCl @ 0, 50, 100, 150 and 200 mM | VC @ 0%, 5%, 10%, 15% and 20% | Increased the activity of the antioxidant system; increased proline and chlorophyll content. Reduced salinity impacts and boost-up yield. | [78] |
| Noni (Morinda citrifolia L.) | Salinity stress @ 0.5, 1.5, 3.0 and 4.5 dS m−1 | Substrates with humus; 33.33 and 66.66% of humus | Decreased the intensification of electrical conductivity of irrigation water; mitigated the negative effects of salts on plants. | [79] |
| Bean (Phaseolus vulgaris L.) | NaCl @ 20, 40, 60 and 80 mmol l−1 | VC: Sand = 0:100; 10:90; 25:75; 50:50 and 75:25 | Increased photosynthetic rate and potassium (K+) and calcium (Ca2+) concentration in leaf and root; improved the growth of bean plants. Alleviated negative effects of salinity. | [49] |
| Pomegranate (Punica granatum L.) | NaCl @ 0, 30, and 60 mM | Vermicompost leachate (VCL) foliar spray | Leaf area, photosynthetic efficiency, and shoot and root fresh and dry weight significantly increased; improved the activity of antioxidant enzymes; reduced oxidative stress and electrolyte leakage. VCL alleviated the damage caused by salt stress | [42] |
| Tall fescue turfgrass (Festuca arundinacea cv Queen) | NaCl @ 0, 3, 6 and 12 dS/m | VC @ 0, 100, 200 and 300 g | Activities of CAT and APX were increased; leaf area, shoot length and dry shoot weight were highest. Reduced the effects of high concentrations of sodium chloride in saline soils. | [66] |
| Onion (Allium cepa L. cv. Metan) | NaCl @ 50 and 100 mM | Seed Priming with VC | Higher germination, seedling growth, CAT, SOD and APX activities were found in VC treated seeds. VC mitigated salinity effects | [55] |
| Bell pepper (Capsicum annuum L.) | NaCl @ 160 mM | Addition of 7 mL/L VCL | Increased sugar concentration in roots and proline content in leaves; increased leaf fresh weight. VCL enhanced the property of salt-stress resistance in bell peppers. | [52] |
| Medicago (Medicago rigidula L.) | NaCl @ 0, 50 and 100 mM | VC @ 0, 10, 20 and 30%. | Increased plant survival capacity, chlorophyll contents, shoot dry weight; maximize leaf area values. | [80] |
| Sunflower (Helianthus annuus L.) | EC: 0.5, 4.8 and 8.6 dS/m | VC @ 1 kg/pot | Increased plant growth, yield, nitrate and protein content; decreased sodium (Na+), chloride (Cl−), ammonium; Increased N-assimilation. | [64] |
| Borage (Borago officinalis) | NaCl @ 0, 4, 8 and 12 dSm−1 | VC @ 0, 6, 12 and 18% (w/w) of soil | Increased chlorophyll b, carotenoids and MDA contents and reduced the negative effects of salinity. | [81] |
| Milk thistle (Silybum marianum L.) | NaCl @ 0, −2, −4, −6, and −8 bar | Superabsorbent polymers with VC coats | Increased seedlings emergence rate, plant vigor index, shoot dry weight, leaf area, specific leaf area, relative water content, and total chlorophyll. | [54] |
| Sugarcane commercial variety of ‘Bululawang (BL)’ | NaCl @ 4.12 dS/m | VC @ 0, 10, 20 t/ha) and nitrogen fertilizer @ 50, 75 and 100 kg N/ha | Increased N, K uptakes and the growth of sugarcane and alleviated salinity effects. | [82] |
| Rapeseed (Brassica napus L.) | NaCl @ 100 mM | VCL (1:10, v/v) | VCL was shown to improve seed germination and management of oxidative stress. | [51] |
| Fountain Grass (Pennisetum alopecuroides) | NaCl @ 5.0 g per kg soil | VC | Enhanced Na+ exclusion and K+ accumulation, relieved stomatal limitation, increased leaf pigment contents, enhanced electron transport efficiency and net photosynthesis, improved root activity, and minimized the oxidative damage. | [60] |
2.2. Biochar
| Plant Species | Stress Level | Treatment and Application Methods | Effects of Amendments | References |
|---|---|---|---|---|
| Wheat (Triticum aestivum L.) | Saline water irrigation @ 10 dSm−1 | BC @ 10, 20, 30 t/ha | Increased and relative water content, photosynthesis. Decreased Na+/K+, and leaf senescence. | [84] |
| NaCl @ 3000 ppm | Soybean straw BC | Increased plant growth, grain yield and biomass production; increased leaf chlorophyll content, water use efficiency, PSII efficiency, and net photosynthesis rate; decreased electrolyte leakage, H2O2, MDA; increased CAT, APX, SOD, GR activities; improved salinity tolerance. | [95] | |
| Quinoa (Chenopodium quinoa L.) | Saline water irrigation @ 400 mM | BC@ 5% (w/w) | Increased photosynthesis, stomatal conductance, WUE and K+ content. Decreased ABA and Na+ content. | [83] |
| Eggplant (Solanum melongena L.) | Saline water irrigation @ 2 and 4 dSm−1 | Hardwood BC @ 5%, Softwood BC @ 5% | Increased biomass, photosynthesis and stomatal conductance. Decreased leaf temperature and electrolyte leakage in leaf tissue. | [32] |
| Maize (Zea mays L.) | Saline soil | Wheat straw BC @ 12 t/ha | Increased LAI, Chlorophyll content, K, P and N content. Reduced MDA, soluble sugar, ascorbic acid and proline content. | [88] |
| Saline soil | BC @ 5% (w/w) | Increased photosynthesis and stomatal conductance, K+ content and K+/Na+. Decreased ABA and Na+ content. | [96] | |
| Soybean (Glycine max L.) | NaCl @ 5 and 10 dSm−1 | BC @ 50 and 100 g kg−1 soil | Improved nodulation, chlorophyll content, N content, rubisco activity, GDH, GS, GOGAT, and NR activities. | [85] |
| Bean (Phaseolus vulgaris L.) | NaCl @ 6 and 12 dSm−1 | BC @ 10% and 20% w/w | Decreased Na+ concentration, PAO activity, polyamines, ABA, ACC and JA; enhanced IAA content. | [93] |
| Mungbean (Vigna radiata L.) | NaCl @ 5 and 10 dS m−1 | BC @ 50 and 100 g kg−1 | Increased and relative water content, IAA content, vascular cylinder, cortical parenchyma areas, IAA/ABA and IAA/ACC ratios; decreased ABA and ACC. | [94] |
| Sorghum (Sorghum bicolor L.) | NaCl @ 0.26, 5.8 and 12.6 dSm−1 | Soil mixer @ 2.5%, 5% and 10% (w/w) of total mass | Increased photosynthesis, stomatal conductance, transpiration rate CAT, POD, and SOD activity. | [87] |
| NaCl @ 0.8, 4.1, and 7.7 dS m−1 | BC @ 0, 2.5, 5, and 10% (w/w) | Increased saddling emergence percentage, dry matter accumulation, and relative water content. Mitigated salinity stress. | [86] | |
| Potato (Solanum tuberosum L.) | NaCl @ 25 and 50 mM | BC @ 5% w/w of total mass | Increased photosynthesis, stomatal conductance, leaf water potential, K+ content; decreased Na+, Na+/K+ ratio and ABA concentration. | [97] |
| Rice (Oryza sativa) | Saline soil | BC @ 0%, 1.5%, 3.0% and 4.5% w/w | Increased biomass, grain yield; decreased in leaf Na+ concentration and Na+/K+ ratio; increased in leaf K+ concentration; decreased ABA, MDA content; increased leaf photosynthesis rates (Pn), transpiration rates (Tr), stomatal conductance (Gs); improved salinity tolerance. | [91] |
| NaCl @ 3 g per kg soil | BC application | Decreased the value of EC, soluble Na+ and Cl− contents; increased CEC, SOM, HA, total nitrogen, and total phosphorus contents in the soil; increased soil microbial community. | [92] | |
| Cabbage (Brassica oleracea) | NaCl @ 0 and 150 mM | BC @ 0%, 2.5%, and 5% | Increased stem diameter, leaf area, shoot fresh weight, root fresh weight, shoot dry weight, and root dry weight; decreased malondialdehyde (MDA), hydrogen peroxide (H2O2), proline, and sucrose content; reduced Cl and Na concentration, and reactive oxygen species (ROS) production; increased CAT and SOD activities. | [29] |
| Borage (Borago officinalis) | NaCl @ 1250, 2500, 5000, and 7500 mg per kg soil | BC @ 5% | Decreased leaf water potential (Yw), osmotic potential (Ys), water saturation deficit (WSD); increased relative water content (RWC), water content (WC), and water retention capacity (WTC); increased K+, and K+/Na+ ratio; decreased MDA, H2O2; increased POD, SOD activities; improved salinity tolerance. | [98] |
2.3. Bio-Fertilizer
| Plant Species | Stress Level | Treatment and Application Methods | Effects of Amendments | References |
|---|---|---|---|---|
| Wheat (Triticum aestivum L.) | NaCl @ 0, 3000, 6000, 9000 ppm | Cerealien, Phosphorien and Cerealien + Phosphorien in addition mix-up with wheat grains. | Increased growth, dry matter accumulation, and yields. Decreased proline content. Improved salinity tolerance. | [37] |
| NaCl @ 0, 2.76, 5.53, and 8.3 dSm−1 | Four (04) biofertilizer treatments were applied: not at all biofertilizer; seed injection by Azotobacter chroococcum Beijerinck strain 5; Pseudomonas putida (Trevisan) Migula strain 186; joint inoculation of Azotobacter + Pseudomonas | Increased chlorophyll index, relative water content, and grain yield. Concentrated dry matter, stem reserve mobilizations to grain yield and decreased proline content. | [28] | |
| Lettuce (Lactuca sativa L.) | Irrigated with saline water @ 1.2 dSm−1 | Biofertilizer @ 5 kg/ha | Increased POD, CAT, MDA, SOD activities. Decreased disruption of endohormones, osmotic stress and mitigates salinity stress. | [108] |
| Geranium plant (Pelargonium graveolens L.) | Irrigated with saline water NaCl1: NaC12 (1:1) | (Half dose of compost + Bio) & (full dose of peanut compost + Bio) added to the pot. | Increased oil percentage but N, P, K contents remained unchanged. Improved yield and mitigated salinity stress. | [112] |
| Okra (Abelmoschus esculentus L.) | Irrigated with saline water with 3 levels 0.47, 2, & 4 dSm−1 | Biofertilizers + Ascorbic acid @ 100 & 200 mgL−1 was applied. | Increased chlorophyll content, growth and yield but deceased ascorbic acid and proline content in okra plants. | [38] |
| Barley (Hordeum vulgare) & Broad beans (Vicia faba) | Irrigated with saline water @ 0, −1, −3, −5 Mpa | Seeds were presoaked with biofertilizer (2 mL of nanomaterial + 10 mL cyanobacterial (algal culture) + 10 mL rhizobacterial strain + 10 mL MeSA) for one day and 12 h and then added to the saline soil. | Increased bioavailability of nutrients, production of growth hormones and bio-stimulants. Decreased Na+, Cl−, and proline concentrations ultimately reduced salinity. | [113] |
| Yellow passion fruit (Passiflora edulis) | Irrigated with saline water (EC 0.35 & 4 dSm−1) | Soil applied biofertilizer @ 0 and 50% | Increased absolute growth rate, period for pruning the side branches, and yield, and decreased the adverse effect of salinity. | [104] |
| Soybean (Glycine max L.) | Saline water @ 3.13, 6.25, 9.38 dSm−1 | Seeds were inoculated with bio-fertilizers and applied on the field. | Increased ascorbic acid, total indoles, a- amylase activity and polyphenol oxidase, decreased total soluble phenols, total soluble sugars and free proline. Decreased the salinity effects. | [114] |
| Safflower (Carthamus tinctorius L.) | NaCl @ 250 mM | Coated seeds with biofertilizers & sugars were applied to the pot. | Increased antioxidant enzymes (SOD, CAT, POD, and APX), decreased proline and malondialdehyde (MDA). Improved salinity tolerance | [109] |
| Peanut (Arachis hypogaea L.) | Irrigated with saline water @ 0.5, 1.5, 2.5, 3.5, 4.5 and 5.5 dSm−1 | Biofertilizer @ 15, 30 and 45 | In the peanut, it promoted higher vegetative growth and improved photosynthesis rate. Decreased soil salinity and improved yield. | [115] |
| Cowpea (Vigna unguiculata L.) | NaCl @ 25, 50, 100, 200, and 300 mM | Biofertilizers mixed with sand @ 0.8 g/Kg | Increased growth parameters, total pigments, protein, proline contents and activities of SOD and CAT. Reduced H2O2 production and alleviated salinity stress. | [105] |
| Pitombeira seedlings (Talisia esculenta) | NaCl @ 0.8, 2, 4, 6, 8 dSm−1 | Biofertilizer @ 10% of the total volume | Increased plant height, stem diameter, number of leaves, leaf area, total leaf area, Dickson quality index, dry mass of root and stem. Mitigated the harmful effects of salinity. | [116] |
| Cotton (Gossypium hirsutum L.) | NaCl @ 15 dSm−1 | Seeds were coated with biofertilizers. | Increased shoot growth, root growth and yield. Decreased leaf gas exchange characteristics. | [117] |
| Corn (Zea mays L.) | Irrigated with saline water @ 0.47, 2.50, and 3.90 dSm−1 | Biofertilizer “Halix” was applied as an inoculum to corn seeds before cultivation. | Increased the concentrations of macro and micronutrients, total chlorophyll, and ascorbic acid in maize plants, as well as mitigated the negative effects of salinity on corn. | [104] |
| Olive (Olea europaea L.) | Irrigated with saline water @ 2000, 3000 and 4000 ppm | Biofertilization treatments control, Azotobacter chroococcum, Mycorrhizae (Glomus macrocarbium) and mix of Azotobacter chroococcum + Mycorrhizae | Enhanced growth and plant biomass, improved microbial activity in the rhizosphere zone. Decreased intensity of salt toxic effects. | [106] |
| Papaya (Carica papaya L.) | Irrigated with saline water @ 0.5, 1, 2, 3 and 4 dSm−1 | Biofertilizer applied @ 10% of the substrate volume. | Enhanced growth and plant biomass, provided greater osmotic adjustments between root and soil solution, increased absorption efficiency of water and essential nutrients stimulating plants to grow. Decreased intensity of salt toxic effects on growth. | [107] |
| Amaranth (Amaranthus tricolor L.) | NaCl @ 0, 2500, 5000, 7500, and 10,000 ppm | Bacillus sp., Lactobacillus sp., Saccharomyces sp., Streptomyces sp., Azospirillum sp., Pseudomonas sp., Azotobacter sp., Rhizobium sp. | Increased plant height, number of leaves, and stem metaxylem diameter. | [101] |
| Lavender (Lavandula angustifolia) | NaCl @ 0, 50, and 100 mM | Azotobacter, Azospirillum, and a combination of Azotobacter and Azospirillum | Increased plant height, stem length, root length, fresh weight, dry weight, relative water content, chlorophyll a, chlorophyll b, total chlorophyll, and essential oil yield; improved salinity tolerance. | [102] |
2.4. PGPR
| Plant Species | PGPR Inoculation | Salinity Stress | Effects of Inoculation | References |
|---|---|---|---|---|
| Wheat (Triticum aestivum) | Pseudomonas fluorescence, Bacillus pumilus, and Exiguobacterium aurantiacum | 10% NaCl solution | Maximum root growth and dry biomass was observed; higher in proline and total soluble proteins contents; antioxidant activity improved; improved water and osmotic potential. | [122] |
| Enterobacter cloacae | 10% and 15% NaCl solution | Decreased the accumulation of Na+ and increased K+ uptake in shoots and roots; higher K+/Na+ ratios; improved antioxidant activity. | [123] | |
| Bacillus subtilis and Arthrobacter sp. | 2–6 dSm−1 | Improved antioxidant activity; increased in dry biomass, total soluble sugars and proline content. | [135] | |
| Dietzia natronolimnaea | 100 and 150 mM NaCl | Modulated the expression of stress responsive genes; improved ion transporters TaNHX1, TaHAK, and TaHKT1; improved the activities of antioxidant enzymes. | [143] | |
| Serratia marcescens | 150–200 mM NaCl | Higher osmo-protectants and growth parameters; higher K+/Na+ ratios; increased SOD, APX, and CAT activity. | [127] | |
| Maize (Zea mays) | Kocuria rhizophila | 100 and 200 mM NaCl | Improved IAA and the ABA activity; upregulation of salt tolerant genes ZmNHX1, ZmNHX2, ZmNHX3, ZmWRKY58 and ZmDREB2A; higher K+/Na+ ratios; improved the growth parameters; higher chlorophyll, proline, and total soluble sugar content. | [146] |
| Azotobacter chroococcum | 0, 2.93 and 5.85 g NaCl/kg soil | Increased in biomass and stomatal conductance; higher K+/Na+ ratios; improved antioxidant enzyme activity. | [140] | |
| Enterobacter cloacae | 0, 300, 600, and 900 mM NaCl | Enhanced plant growth, biomass, and photosynthetic pigments under salinity stress; enhanced radical scavenging capacity, RWC, soluble sugars, proteins, secondary metabolite content. | [124] | |
| Piriformospora indica | 500 μM KCl and 100 μM CaCl2 | Higher biomass and stomatal conductance; lower K+ efflux from roots and higher potassium content in shoots. | [139] | |
| Soybean (Glycine max) | Rhizobium sp. Bradyrhizobium japonicum and Hydrogenophaga sp. | 100, 250, and 500 mM NaCl solution | Higher shoot biomass at the vegetative stage, reproductive stages; improved seed weight and shoot K+/Na+ ratio. | [130] |
| Methylobacterium aminovorans and Methylobacterium rhodinum; Bradyrhizobium japonicum and Bacillus megaterium | 0.170 dSm−1 | Increased nodule numbers and dry weight of nodules; significantly increased in N, P and K; higher number of pods, seed index and seed yield. | [132] | |
| Rice (Oryza sativa) | Pseudomonas pseudoalcaligenes and Bacillus pumilus | 5, 10, 15, 20, and 25 g NaCl L−1 | Reduced lipid peroxidation and superoxide dismutase activity; reduced plant cell membrane index cell caspase-like protease activity, and programmed cell death. | [137] |
| Bacillus amyloliquefaciens | 120 and 250 mM NaCl | Higher synthesis of amino acids; improved endogenous SA and ABA; improved plant physiology. | [142] | |
| Mung bean (Vigna radiate) | Rhizobium sp. and Enterococcus mundtii | 10% NaCl solution | Higher seed germination and seedling growth and biomass; enhanced chlorophyll content and macro-micronutrient uptake; improved soil physical, chemical and biological parameters. | [120] |
| Barley (Hordeum vulgare) | Bacillus megatherium, Pseudomonas fluorescens, Bacillus circulans, Paenibacillus polymyxa, Azotobacter chroococcum, Azospirillum sp. Paenibacillus polymyxa2, Azospirillum brasilense, Hyderella sp. | 250, 500 or 1000 mM NaCl | Alleviated the deleterious effect of salinity; higher dry masses and relative water content. | [133] |
| Pea (Pisum sativum) | Acinetobacter bereziniae, Enterobacter ludwigii, and Alcaligenes faecalis | 75 mM, 100 mM and 150 mM NaCl | Improved the growth parameters; higher chlorophyll, proline, and total soluble sugar content; improved electrolyte leakage; improved the activities of antioxidant enzymes. | [136] |
| Pepper (Capsicum annuum) | Azospirillum brasilense and Pantoea dispersa | 40, 80 and 120 mM NaCl | Higher K+ /Na+ ratio; improved leaf photosynthesis and stomatal conductance. | [141] |
| Burclover (Medicago sp.) | Bacillus megaterium, E. medicae, Ensifer Medicae and B. megaterium | 0–2000 mM NaCl | Improved IAA and the ACC deaminase activity; higher chlorophyll, proline, and total soluble sugar content. | [125] |
| Okra (Abelmoschus esculentus) | Bacillus megaterium and Enterobacter sp. | 75 mM NaCl | Enhanced ROS-scavenging enzyme activity; increased antioxidant enzyme SOD, APX, and CAT; upregulation of ROS pathway genes CAT, APX, GR, and DHAR. | [147] |
| Suaeda fruticosa | Glutamicibacter sp. and Pseudomonas sp. | 600 mM NaCl | Increased shoot K+ and Ca2+ content; lowered shoot MDA concentration and less accumulation of Na+ and Cl− in shoots. | [138] |
| Rapeseed (Brassica napus) | Enterobacter cloacae | 50 and 100 mM NaCl | Promoted seed germination and seedling growth; improved chlorophyll, water potential and other physiological activity. | [128] |
| Avena sativa, Medicago sativa, and Cucumis sativus | Advenella incenata, Providencia re- Ttgeri, Acinetobacter calcoaceticus, and Serratia plymuthica | Salinity stress | Enhanced ROS-scavenging enzyme activity; increased SOD, APX, and CAT activity; enhanced plant growth, and photosynthetic pigments. enhanced RWC and proteins, content | [145] |
| Tomato (Solanum lycopersicum) | Bacillus megaterium | 200 mM NaCl | Improved the growth parameters and biomass; higher chlorophyll, proline, and total soluble sugar content. | [126] |
3. Limitation of Organic Amendments and Future Perspectives
- Its preparations, which are organically altered as natural weathering processes, need more labor, time, space, and raw resources.
- More experienced and skilled people, as well as scientific understanding, are required to maintain environmental conditions such as temperature, moisture, and respiration.
- Some organic methods, such as vermicomposting, biochar, and bio-fertilizer, emit a foul stench and attract flies. On the worm-feeding materials, harmful molds and bacteria are frequently produced in some cases.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Imran, S.; Katsuhara, M.; Tada, Y. Na+ transporter SvHKT1; 1 from a halophytic turf grass is specifically upregulated by high Na+ concentration and regulates shoot Na+ concentration. Int. J. Mol. Sci. 2020, 21, 6100. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Kaur, M.; Kaushik, P.; Alyemeni, M.N.; Alsahli, A.A.; Ahmad, P. Arbuscular mycorrhiza in combating abiotic stresses in vegetables: An eco-friendly approach. Saudi J. Biol. Sci. 2021, 28, 1465–1476. [Google Scholar] [CrossRef]
- Imran, S.; Sarker, P.; Hoque, M.N.; Paul, N.C.; Mahamud, M.A.; Chakrobortty, J.; Tahjib-Ul-Arif, M.; Latef, A.A.; Hasanuzzaman, M.; Rhaman, M.S. Biochar actions for the mitigation of plant abiotic stress. Crop Pasture Sci. 2022. [Google Scholar] [CrossRef]
- Hoque, M.N.; Hannan, A.; Imran, S.; Paul, N.C.; Mondal, M.; Sadhin, M.; Rahman, M.; Bristi, J.M.; Dola, F.S.; Hanif, M.; et al. Plant Growth-Promoting Rhizobacteria-Mediated Adaptive Responses of Plants Under Salinity Stress. J. Plant Growth Regul. 2022, 28, 1–20. [Google Scholar] [CrossRef]
- Wallender, W.W.; Tanji, K.K. Nature and extent of agricultural salinity and sodicity. In Agricultural Salinity and Management, 2nd ed.; American Society of Civil Engineers: Reston, VA, USA, 2012; pp. 1–25. [Google Scholar] [CrossRef]
- Alqahtani, M.; Roy, S.J.; Tester, M. Increasing Salinity Tolerance of Crops. In Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2019. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, G.; Akhtar, M.S.; Abdullah, R. Global concern for salinity on various agro-ecosystems. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution; Springer: Singapore, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Introduction to soil salinity, sodicity and diagnostics techniques. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 1–42. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant salt stress: Adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Imran, S.; Tsuchiya, Y.; Tran, S.T.; Katsuhara, M. Identification and Characterization of Rice OsHKT1; 3 Variants. Plants 2021, 10, 2006. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Hewedy, O.A.; Battaglia, M.L.; Jalal, R.S.; Alhammad, B.A.; Schillaci, C.; Ali, N.; Al-Doss, A. Field crop responses and management strategies to mitigate soil salinity in modern agriculture: A review. Agronomy 2021, 11, 2299. [Google Scholar] [CrossRef]
- Islam, W.; Waheed, A.; Naveed, H.; Zeng, F. MicroRNAs mediated plant responses to salt stress. Cells 2022, 11, 2806. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Long, R.; Zhang, T.; Kang, J.; Wang, Z.; Wang, P.; Sun, H.; Yu, J.; Yang, Q. Genome-wide identification of microRNAs in response to salt/alkali stress in medicagotruncatula through high-throughput sequencing. Int. J. Mol. Sci. 2018, 19, 4076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, L.; Shen, Q.; Wu, L.; Yu, J.; Fu, L.; Wu, D.; Zhang, G. Identification of microRNAs responding to salt stress in barley by high-throughput sequencing and degradome analysis. Environ. Exp. Bot. 2019, 160, 59–70. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, H.; Zhang, F.; Jiang, Q.; Yang, S.; Yue, P.; Wang, F.; Zhang, Y.; Li, L.; He, P.; et al. The miR156/SPL module regulates apple salt stress tolerance by activating MdWRKY100 expression. Plant Biotechnol. J. 2021, 19, 311–323. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Salinity and sodicity adaptation and mitigation options. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 55–89. [Google Scholar] [CrossRef]
- Shilev, S. Plant-growth-promoting bacteria mitigating soil salinity stress in plants. Appl. Sci. 2020, 10, 7326. [Google Scholar] [CrossRef]
- Meena, K.K.; Bitla, U.M.; Sorty, A.M.; Singh, D.P.; Gupta, V.K.; Wakchaure, G.C.; Kumar, S. Mitigation of salinity stress in wheat seedlings due to the application of phytohormone-rich culture filtrate extract of methylotrophic actinobacterium Nocardioides sp. NIMMe6. Front. Microbiol. 2020, 11, 2091. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, U.; Kibria, M.G.; Rhaman, M.S.; Murata, Y.; Hoque, M.A. Screening of rice genotypes for salt tolerance by physiological and biochemical characters. Plant Sci. Today 2021, 8, 467–472. [Google Scholar] [CrossRef]
- Kanwal, S.; Ilyas, N.; Shabir, S.; Saeed, M.; Gul, R.; Zahoor, M.; Batool, N.; Mazhar, R. Application of biochar in mitigation of negative effects of salinity stress in wheat (Triticum aestivum L.). J. Plant Nutr. 2018, 41, 526–538. [Google Scholar] [CrossRef]
- Rasheed, F.; Anjum, N.A.; Masood, A.; Sofo, A.; Khan, N.A. The key roles of salicylic acid and sulfur in plant salinity stress tolerance. J. Plant Growth Regul. 2020, 30, 1–4. [Google Scholar] [CrossRef]
- Hannan, A.; Hoque, M.N.; Hassan, L.; Robin, A.H. Adaptive Mechanisms of Root System of Rice for Withstanding Osmotic Stress. In Recent Advances Rice Research; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Ali, M.; Kamran, M.; Abbasi, G.H.; Saleem, M.H.; Ahmad, S.; Parveen, A.; Malik, Z.; Afzal, S.; Ahmar, S.; Dawar, K.M.; et al. Melatonin-induced salinity tolerance by ameliorating osmotic and oxidative stress in the seedlings of two tomato (Solanum lycopersicum L.) cultivars. J. Plant Growth Regul. 2021, 40, 2236–2248. [Google Scholar] [CrossRef]
- Alamer, K.H.; Perveen, S.; Khaliq, A.; Zia Ul Haq, M.; Ibrahim, M.U.; Ijaz, B. Mitigation of salinity stress in maize seedlings by the application of vermicompost and sorghum water extracts. Plants 2022, 11, 2548. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, R.; Seyed, S.R.; Jalilian, J. Growth, physiological status, and yield of salt stressed wheat (Triticum aestivum L.) plants affected by biofertilizer and cycocel applications. Arid. Land Res. Manag. 2017, 32, 71–90. [Google Scholar] [CrossRef]
- Ekinci, M.; Turan, M.; Yildirim, E. Biochar mitigates salt stress by regulating nutrient uptake and antioxidant activity, alleviating the oxidative stress and abscisic acid content in cabbage seedlings. Turk. J. Agric. For. 2022, 46, 28–37. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Lata, C.; Tiwari, S.; Chauhan, A.S.; Mishra, S.K.; Agrawal, L.; Chakrabarty, D.; Nautiyal, C.S. Transcriptional alterations reveal Bacillus amyloliquefaciens-rice cooperation under salt stress. Sci. Rep. 2019, 9, 11912. [Google Scholar] [CrossRef] [Green Version]
- Parkash, V.; Singh, S. Potential of biochar application to mitigate salinity stress in eggplant. Hortc. Sci. 2020, 55, 1946–1955. [Google Scholar] [CrossRef]
- Kerbab, S.; Silini, A.; Chenari, B.A.; Cherif-Silini, H.; Eshelli, M.; El Houda, R.N.; Belbahri, L. Mitigation of NaCl stress in wheat by rhizosphere engineering using salt habitat adapted PGPR halotolerant bacteria. Appl. Sci. 2021, 11, 1034. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.; Hung, S.H.; Huang, E.; Huang, C.C. Roles of plant growth-promoting rhizobacteria (PGPR) in stimulating salinity stress defense in plants: A review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gómez, J.D.; Abud-Archila, M.; Villalobos-Maldonado, J.J.; Enciso-Saenz, S.; Hernández de, L.H.; Ruiz-Valdiviezo, V.M.; Gutiérrez-Miceli, F.A. Vermicompost and vermiwash minimized the influence of salinity stress on growth parameters in potato plants. Compost Sci. Util. 2017, 25, 282–287. [Google Scholar] [CrossRef]
- Ruiz-Lau, N.; Oliva-Llaven, M.A.; Montes-Molina, J.A.; Gutiérrez-Miceli, F.A. Mitigation of Salinity Stress by Using the Vermicompost and Vermiwash. In Ecological and Practical Applications for Sustainable Agriculture; Springer: Singapore, 2020; pp. 345–356. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Mohamed, H.F. Impact of biofertilizers application on improving wheat (Triticum aestivum L.) resistance to salinity. Res. J. Agric. Biol. Sci. 2008, 4, 520–528. [Google Scholar]
- Mahdy, A.M.; Nieven, O.F. Interactive effects between biofertilizer and antioxidant on salinity mitigation and nutrition and yield of okra plants (Abelmoschus esculentus L.). J. Soil Sci. Agric. Eng. 2012, 3, 189–205. [Google Scholar] [CrossRef]
- Sinha, R.K.; Valani, D.; Chauhan, K.; Agarwal, S. Embarking on a second green revolution for sustainable agriculture by vermiculture biotechnology using earthworms: Reviving the dreams of Sir Charles Darwin. Int. J. Agric. Health Saf. 2014, 1, 50–64. [Google Scholar]
- Kiyasudeen, K.; Ibrahim, M.H.; Quaik, S.; Ismail, S.A. Vermicompost, its applications and derivatives. In Prospects of Organic Waste Management and the Significance of Earthworms; Springer: Cham, Switzerland, 2016; pp. 201–230. [Google Scholar]
- Arancon, N.Q.; Lee, S.; Edwards, C.A.; Atiyeh, R. Effects of humic acids derived from cattle, food and paper-waste vermicomposts on growth of greenhouse plants: The 7th international symposium on earthworm ecology · Cardiff · Wales · 2002. Pedobiologia 2003, 47, 741–744. [Google Scholar] [CrossRef] [Green Version]
- Bidabadi, S.S.; Dehghanipoodeh, S.; Wright, G.C. Vermicompost leachate reduces some negative effects of salt stress in pomegranate. Int. J. Recycl. Org. Waste Agric. 2017, 6, 255–263. [Google Scholar] [CrossRef]
- Koozehgar, K.M.; Ardakani, M.R. Effects of vermicomposting and compost tea on nitrogen, phosphorus, and potassium yield and uptake of Mentha aquatic L. inoculated with mycorrhizal fungi Glomus moseae. Iran. J. Plant Physiol. 2017, 11, 10–19. [Google Scholar]
- Pengkam, C.; Iwai, C.B.; Kume, T. Effects of Vermicompost and Rice Husk Ash on the Change of Soil Chemical Properties and the Growth of Rice in Salt Affected Area. Int. J. Environ. Rural. Dev. 2019, 10, 129–132. [Google Scholar] [CrossRef]
- Demir, Z.; Tursun, N.; Işık, D. Role of different cover crops on DTPA-extractable micronutrients in an apricot orchard. Turk. J. Agric. Food Sci. Technol. 2019, 7, 698–706. [Google Scholar] [CrossRef] [Green Version]
- Ansari, A.A.; Ismail, S.A. Role of earthworms in vermitechnology. J. Agric. Technol. 2012, 8, 403–415. [Google Scholar]
- Khan, M.H.; Meghvansi, M.K.; Gupta, R.; Veer, V.; Singh, L.; Kalita, M.C. Foliar spray with vermiwash modifies the arbuscular mycorrhizal dependency and nutrient stoichiometry of Bhut jolokia (Capsicum assamicum). PLoS ONE 2014, 9, e92318. [Google Scholar] [CrossRef]
- Nath, G.; Singh, K. Effect of vermiwash of different vermicomposts on the kharif crops. J. Cent. Eur. Agric. 2012, 13, 379–402. Available online: https://hrcak.srce.hr/83274 (accessed on 10 September 2022). [CrossRef] [Green Version]
- Beykkhormizi, A.; Abrishamchi, P.; Ganjeali, A.; Parsa, M. Effect of vermicompost on some morphological, physiological and biochemical traits of bean (Phaseolus vulgaris L.) under salinity stress. J. Plant Nutr. 2016, 39, 883–893. [Google Scholar] [CrossRef]
- Chinsamy, M.; Kulkarni, M.G.; Van Staden, J. Garden-waste-vermicompost leachate alleviates salinity stress in tomato seedlings by mobilizing salt tolerance mechanisms. Plant Growth Regul. 2013, 71, 41–47. [Google Scholar] [CrossRef]
- Benazzouk, S.; Djazouli, Z.E.; Lutts, S. Vermicompost leachate as a promising agent for priming and rejuvenation of salt-treated germinating seeds in Brassica napus. Commun. Soil Sci. Plant Anal. 2019, 50, 1344–1357. [Google Scholar] [CrossRef]
- Ahmadi, N.; Akbari, E. The preventive impact of vermicompost on bell pepper (Capsicum annuum L.) salinity resistance: An evaluation. Afr. J. Agric. Res. 2021, 17, 46–56. [Google Scholar] [CrossRef]
- Bassaco, A.C.; Antoniolli, Z.I.; Júnior, B.D.; Eckhardt, D.P.; Montagner, D.F.; Bassaco, G.P. Chemistry characterization from animal origin residues and Eisenia andrei behaviour. Ciênc. Natura 2015, 37, 45–51. [Google Scholar] [CrossRef]
- Ebrahimi, M.H.; Taghvaei, M.; Sadeghi, H.; Zarei, M. Effect of organic coats with superabsorbent polymers on improving the germination and early vigor Milk thistle (Silybum marianum L.) seeds under salinity stress. Desert 2019, 24, 207–215. [Google Scholar] [CrossRef]
- Muhie, S.H.; Yildirim, E.; Memis, N.; Demir, I. Vermicompost priming stimulated germination and seedling emergence of onion seeds against abiotic stresses. Seed Sci. Technol. 2020, 48, 153–157. [Google Scholar] [CrossRef]
- Demir, Z. Alleviation of adverse effects of sodium on soil physicochemical properties by application of vermicompost. Compost Sci. Util. 2020, 28, 100–116. [Google Scholar] [CrossRef]
- Barahouee, M.; Sabbagh, E. Influence of vermicompost and salt stress on some characteristics of fenugreek (Trigonellafoenum-graecum L.). Int. J. Agric. Biosci. 2017, 6, 60–63. [Google Scholar]
- Banadkooki, A.M.; Ardakani, M.D.; Shirmardi, M.; Momenpour, A. Effects of cow manure and vermicompost on growth characteristics of smoke tree (Cotinus coggygria Scop.) under salt stress under greenhouse. Iranian J. Poplar Res. 2019, 26, 483–495. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Duathi, K.H. Effect of vermicompost on some growth and biochemical characteristic of Dracocephalum moldavica L. under water salinity stress. J. Agric. Sci Sustain. Prod. 2019, 29, 151–168. [Google Scholar]
- Song, X.; Li, H.; Song, J.; Chen, W.; Shi, L. Biochar/vermicompost promotes Hybrid Pennisetum plant growth and soil enzyme activity in saline soils. Plant Physiol. Biochem. 2022, 183, 96–110. [Google Scholar] [CrossRef]
- Bziouech, S.A.; Dhen, N.; Helaoui, S.; Ammar, I.B.; Dridi, B.A.M. Effect of vermicompost soil additive on growth performance, physiological and biochemical responses of tomato plants (Solanum lycopersicum L. var. Firenze) to salt stress. Emir. J. Food Agric. 2022, 34, 316–328. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Wang, F.; Xie, Y. Maize (Zea mays) growth and nutrient uptake following integrated improvement of vermicompost and humic acid fertilizer on coastal saline soil. Appl. Soil Ecol. 2019, 142, 147–154. [Google Scholar] [CrossRef]
- Oo, A.N.; Iwai, C.B.; Saenjan, P. Soil properties and maize growth in saline and nonsaline soils using cassava-industrial waste compost and vermicompost with or without earthworms. Land Degrad. Dev. 2015, 26, 300–310. [Google Scholar] [CrossRef]
- Jabeen, N.; Ahmad, R. Growth response and nitrogen metabolism of sunflower (Helianthus annuus L.) to vermicompost and biogas slurry under salinity stress. J. Plant Nutr. 2017, 40, 104–114. [Google Scholar] [CrossRef]
- Demir, Z.; Kiran, S. Effect of vermicompost on macro and micro nutrients of lettuce (Lactuca sativa var. Crispa) under salt stress conditions. Kahramanmaraş. Sütçü. İmam. Üniversitesi. Tarım. Doğa. Dergisi. 2020, 23, 33–43. [Google Scholar] [CrossRef]
- Adamipour, N.; Heiderianpour, M.B.; Zarei, M. Application of vermicompost for reducing the destructive effects of salinity stress on tall fescue turfgrass (Festuca arundinacea Schreb. ‘Queen’). J. Soil Plant Interact. Isfahan Uni. Technol. 2016, 7, 35–47. [Google Scholar] [CrossRef]
- Kiran, S. Alleviation of adverse effects of salt stress on lettuce (Lactuca sativa var. crispa) by application of vermicompost. Acta Sci. Pol. Hortorum Cultus 2019, 5, 153–160. [Google Scholar] [CrossRef]
- Hafez, E.M.; Omara, A.E.; Alhumaydhi, F.A.; El-Esawi, M.A. Minimizing hazard impacts of soil salinity and water stress on wheat plants by soil application of vermicompost and biochar. Physiol. Plant 2020, 172, 587–602. [Google Scholar] [CrossRef]
- Benazzouk, S.; Lutts, S.; Djazouli, Z.E. Alleviation of salinity stress by Vermicompost extract in Solanum lycopersicum L. by mobilizing salt tolerance mechanisms. AgroBiologia 2018, 8, 1136–1144. [Google Scholar]
- Benazzouk, S.; Dobrev, P.I.; Djazouli, Z.E.; Motyka, V.; Lutts, S. Positive impact of vermicompost leachate on salt stress resistance in tomato (Solanum lycopersicum L.) at the seedling stage: A phytohormonal approach. Plant Soil. 2020, 446, 145–162. [Google Scholar] [CrossRef]
- Ezzat, A.S.; Badway, A.S.; Abdelkader, A.E. Sequenced vermicompost, glycine betaine, proline treatments elevate salinity tolerance in potatoes. Middle East J. Agric. Res. 2019, 8, 126–138. [Google Scholar]
- Zhou, J.; Ahmed, N.; Cheng, Y.; Qin, C.; Chen, P.; Zhang, C.; Zhang, L. Effect of inoculation of strains with acc deaminase isolated from vermicompost on seed germination and some physiological attributes in maize (Zea mays L.) exposed to salt stress. Pak. J. Bot. 2019, 51, 1169–1177. [Google Scholar] [CrossRef]
- Mohsenzadeh, S.; Zamanpour, S.H. Evaluation of Municipal Solid Waste Compost and Agricultural Waste Vermicompost by Growth of Lippia citriodora Under Salinity Stress. J. Environ. Sci. Stud. 2019, 4, 2135–2143. [Google Scholar]
- Reyes-Pérez, J.J.; Murillo-Amador, B.; Nieto-Garibay, A.; Troyo-Diéguez, E.; Rueda-Puente, E.O.; Hernández-Montiel, L.G.; Preciado, R.P.; Beltrán, M.A.; Rodríguez, F.F.; López Bustamante, R.J. Use of humates of vermicompost to reduce the effect of salinity on growth and development of basil (Ocimum basilicum L.). Rev. Mex. Cienc. Agrí. 2016, 7, 1375–1387. [Google Scholar]
- Yücel, N.C.; Chİtİlova, M. Improving wheat performance by fish flour and vermicompost priming against salt stress. Int. J. Agric. Biol. 2017, 19, 1483–1488. [Google Scholar]
- Liu, M.; Wang, C.; Wang, F.; Xie, Y. Vermicompost and humic fertilizer improve coastal saline soil by regulating soil aggregates and the bacterial community. Arch. Agron. Soil Sci. 2019, 65, 281–293. [Google Scholar] [CrossRef]
- Zurbano, L.Y. Response of lettuce (Lactuca sativa) on saline soil amended with vermicompost and pulverized eggshell. Indian J. Sci. Technol. 2018, 11, 1–8. [Google Scholar] [CrossRef]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H.; Rho, H. Effect of vermicompost on morphological and physiological performances of pot marigold (Calendula officinalis L.) under salinity conditions. Adv. Hortic. Sci. 2019, 33, 345–358. [Google Scholar] [CrossRef]
- Santos, D.G.; Diniz, B.L.; Diniz, M.A.; Silva, J.H.; Oliveira, W.N.; Ferreira, R.M. Growth and chlorophyll in noni seedlings irrigated with saline water in substrate with vermicompost. Rev. Bras. Eng. Agricola Ambient. 2019, 23, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Akhzari, D.; Pessarakli, M.; Khedmati, M. Effects of vermicompost and salinity stress on growth and physiological traits of Medicago rigidula L . J. Plant Nutr. 2016, 39, 2106–2114. [Google Scholar] [CrossRef]
- Sorkhi, F. Effect of vermicompost fertilizer on antioxidant enzymes and chlorophyll contents in Borago officinalis under salinity stress. Iran. J. Plant Physiol. 2021, 11, 3589–3598. [Google Scholar] [CrossRef]
- Djajadi, D.; Syaputra, R.; Hidayati, S.N.; Khairiyah, Y. Effect of vermicompost and nitrogen on N, K, Na uptakes and growth of sugarcane in saline soil. Agrivita. J. Agric. Sci. 2020, 42, 110–119. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Li, L.; Fu, Q.; Li, Q.; Naeem, M.A.; He, X.; Zhang, Z.; Jacobsen, S.E. Biochar mitigates combined effects of drought and salinity stress in quinoa. J. Agron. 2020, 10, 912. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Z.; Zhai, Y.; Lu, P.; Zhu, C. Effect of straw biochar on soil properties and wheat production under saline water irrigation. Agronomy 2019, 9, 457. [Google Scholar] [CrossRef] [Green Version]
- Farhangi-Abriz, S.; Torabian, S. Biochar improved nodulation and nitrogen metabolism of soybean under salt stress. Symbiosis 2018, 74, 215–223. [Google Scholar] [CrossRef]
- Ibrahim, M.E.H.; Ali, A.Y.A.; Elsiddig, A.M.I.; Zhou, G.; Nimir, N.E.A.; Agbna, G.H.; Zhu, G. Mitigation effect of biochar on sorghum seedling growth under salinity stress. Pak. J. Bot. 2021, 53, 387–392. [Google Scholar] [CrossRef]
- Ibrahim, M.E.H.; Ali, A.Y.A.; Zhou, G.; Elsiddig, A.M.I.; Zhu, G.; Nimir, N.E.A.; Ahmad, I. Biochar application affects forage sorghum under salinity stress. Chil. J. Agric. Res. 2020, 80, 317–325. [Google Scholar] [CrossRef]
- Lashari, M.S.; Ye, Y.; Ji, H.; Li, L.; Kibue, G.W.; Lu, H.; Zheng, J.; Pan, G. Biochar–manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: A 2-year field experiment. J. Sci. Food Agric. 2015, 95, 1321–1327. [Google Scholar] [CrossRef]
- Usman, A.R.; Al-Wabel, M.I.; Abdulaziz, A.H.; Mahmoud, W.A.; EL-Naggar, A.H.; Ahmad, M.; Abdulelah, A.F.; Abdulrasoul, A.O. Conocarpus biochar induces changes in soil nutrient availability and tomato growth under saline irrigation. Pedosphere 2016, 26, 27–38. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Piao, J.; Che, W.; Li, X.; Zhang, C.; Wang, Q.; Hua, S. Peanut shell biochar increases rice yield in highly saline-alkali paddy fields by regulating of leaf ionic concentration and improving leaf photosynthesis rate. Plant Soil 2022, preprint. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, C.; Kong, Y.; Cao, X.; Zhu, L.; Zhang, Y.; Ning, Y.; Tian, W.; Zhang, H.; Yu, Y.; et al. Biochar Application Alleviated Rice Salt Stress via Modifying Soil Properties and Regulating Soil Bacterial Abundance and Community Structure. Agronomy 2022, 12, 409. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Biochar increased plant growth-promoting hormones and helped to alleviates salt stress in common bean seedlings. J. Plant Growth Regul. 2018, 37, 591–601. [Google Scholar] [CrossRef]
- Nikpour-Rashidabad, N.; Tavasolee, A.; Torabian, S.; Farhangi-Abriz, S. The effect of biochar on the physiological, morphological and anatomical characteristics of mung bean roots after exposure to salt stress. Arch. Biol. Sci. 2019, 71, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Soliman, M.H.; Alnusairi, G.S.; Khan, A.A.; Alnusaire, T.S.; Fakhr, M.A.; Abdulmajeed, A.M.; Aldesuquy, H.S.; Yahya, M.; Najeeb, U. Biochar and selenium nanoparticles induce water transporter genes for sustaining carbon assimilation and grain production in salt-stressed wheat. J. Plant Growth Regul. 2022, 1–22. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Naveed, M.; Zahir, Z.A.; Liu, F. Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant. Biol. 2015, 42, 770–781. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar mitigates salinity stress in potato. J. Agron. Crop Sci. 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Farouk, S.; AL-Huqail, A.A. Sustainable biochar and/or melatonin improve salinity tolerance in borage plants by modulating osmotic adjustment, antioxidants, and ion homeostasis. Plants 2022, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Hegde, D.M.; Dwivedi, B.S.; Sudhakara Babu, S.N. Biofertilizers for cereal production in India: A review. Indian J. Agric. Sci. 1999, 69, 73–83. [Google Scholar]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Riesty, O.S.; Siswanti, D.U. Effect of biofertilizer on growth and metaxylem diameter of Amaranthus tricolor L. in salinity stress condition. Biogenes. J. Ilm. Biol. 2021, 9, 178–188. [Google Scholar] [CrossRef]
- Khatami, S.A.; Kasraie, P.; Oveysi, M.; Moghadam, H.R.T.; Ghooshchi, F. Mitigating the adverse effects of salinity stress on lavender using biodynamic preparations and bio-fertilizers. Ind. Crops Prod. 2022, 183, 114985. [Google Scholar] [CrossRef]
- Mahdy, A.M.; Fathi, N.O.; Kandil, M.M.; Elnamas, A.E. Synergistic effects of biofertilizers and antioxidants on growth and nutrients content of corn under salinity and water-deficit stresses. Alex. Sci. Exch. J. 2012, 33, 292–304. [Google Scholar] [CrossRef]
- Souza, J.T.; Cavalcante, L.F.; Nunes, J.C.; Bezerra, F.T.; da Silva, N.J.A.; Silva, A.R.; Oresca, D.; Cavalcante, A.G. Effect of saline water, bovine biofertilizer and potassium on yellow passion fruit growth after planting and on soil salinity. Afr. J. Agric. Res. 2016, 11, 2994–3003. [Google Scholar] [CrossRef] [Green Version]
- AlAbdallah, N.M.; Basalah, M.O.; Roushdy, S.S. The promotive effect of algal biofertilizers on growth and some metabolic activities of (Vigna unguiculata L.) under salt stress conditions. Egypt. J. Exp. Biol. 2017, 13, 187–195. [Google Scholar] [CrossRef]
- El-Shazly, M.; Ghieth, W.M. Effect of some biofertilizers and humic acid application on olive seedlings growth under irrigation with saline water. Alex. Sci. Exch. J. 2019, 40, 263–279. [Google Scholar] [CrossRef] [Green Version]
- de Lima-Neto, A.J.; Cavalcante, L.F.; Mesquita, F.D.; Souto, A.G.; dos Santos, G.P.; dos Santos, J.Z.; de Mesquita, E.F. Papaya seedlings irrigation with saline water in soil with bovine fertilizer. Chil. J. Agric. Res. 2015, 76, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Al-Taey, D.K.; Majid, Z.Z. Study effect of kinetin, bio-fertilizers and organic matter application in lettuce under salt stress. J. Glob. Pharma. Technol. 2018, 10, 148–164. [Google Scholar]
- Yasmin, H.; Mazher, J.; Azmat, A.; Nosheen, A.; Naz, R.; Hassan, M.N.; Noureldeen, A.; Parvaiz, A.P. Combined application of zinc oxide nanoparticles and biofertilizer to induce salt resistance in safflower by regulating ion homeostasis and antioxidant defence responses. Ecotoxicol. Environ. Saf. 2021, 218, 112262. [Google Scholar] [CrossRef] [PubMed]
- Albdaiwi, R.N.; Khyami-Horani, H.; Ayad, J.Y. Plant growth-promoting Rhizobacteria: An emerging method for the enhancement of wheat tolerance against salinity stress. Jordan J. Biol. Sci. 2019, 12, 525–534. [Google Scholar]
- Goswami, M.; Deka, S. Isolation of a novel rhizobacteria having multiple plant growth promoting traits and antifungal activity against certain phytopathogens. Microbiol. Res. 2020, 240, 126516. [Google Scholar] [CrossRef] [PubMed]
- Leithy, S.; Gaballah, M.S.; Gomaa, A.M. Associative impact of bio- and organic fertilizers on geranium plants grown under saline conditions. Int. J. Acad. Res. 2009, 1, 17–23. [Google Scholar]
- El Semary, N.A.; Alouane, M.H.; Nasr, O.; Aldayel, M.F.; Alhaweti, F.H.; Ahmed, F. Salinity Stress Mitigation using encapsulated biofertilizers for sustainable agriculture. Sustainability 2020, 12, 9218. [Google Scholar] [CrossRef]
- Abdelhamid, M.; Gaballah, M.S.; Rady, M.; Gomaa, A. Biofertilizer and ascorbic acid alleviated the detrimental effects of soil salinity on growth and yield of soybean. In Proceedings of the Second Science with Africa Conference, Addis Ababa, Ethiopia, 22–25 June 2010; pp. 73–81. [Google Scholar]
- Oliveira, D.S.; Dias, T.J.; Edvania Pereira de Oliveira, E.P.; Santos, H.C.; Magalhaes, W.B.; Matos, B.F.; Sousa, L.M.C.; Filho, J.S.M. Growth and physiology of peanut (Arachis hypogaea L.) irrigated with saline water and biofertilizer application times. Afr. J. Agric. Res. 2016, 11, 4517–4524. [Google Scholar] [CrossRef] [Green Version]
- Véras, M.L.M.; da Silva, A.R.; de Sousa, A.L.; de Melo, F.J.S.; da Silva, I.T.H.; Dias, T.J. Growth and dry matter of pitombeira seedlings under salinity levels and application of biofertilizer. Com. Sci. 2017, 8, 486–492. [Google Scholar] [CrossRef] [Green Version]
- Amjad, M.; Akhtar, J.; Rashid, M.S. Evaluating the effectiveness of biofertilizer on salt tolerance of cotton (Gossypium hirsutum L.). Arch. Agron. Soil Sci. 2014, 61, 1165–1177. [Google Scholar] [CrossRef]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture: Perspectives and challenges. In PGPR Amelioration in Sustainable Agriculture; Woodhead Publishing: Sawston, UK, 2019; pp. 129–157. [Google Scholar] [CrossRef]
- Gao, Y.; Zou, H.; Wang, B.; Yuan, F. Progress and applications of plant growth-promoting bacteria in salt tolerance of crops. Int. J. Mol. Sci. 2022, 23, 7036. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, P.; Nagpal, S.; Gupta, R.K.; Sirari, A.; Nair, R.M.; Bindumadhava, H.; Singh, S. Dual microbial inoculation, a game changer?—Bacterial biostimulants with multifunctional growth promoting traits to mitigate salinity stress in Spring Mungbean. Front. Microbiol. 2021, 11, 3491. [Google Scholar] [CrossRef]
- Simonin, K.A.; Burns, E.; Choat, B.; Barbour, M.M.; Dawson, T.E.; Franks, P.J. Increasing leaf hydraulic conductance with transpiration rate minimizes the water potential drawdown from stem to leaf. J. Exp. Bot. 2015, 66, 1303–1315. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, A.; Shahbaz, M.; Imran, A.; Marghoob, M.U.; Imtiaz, M.; Mubeen, F. Potential of salt tolerant PGPR in growth and yield augmentation of wheat (Triticum aestivum L.) under saline conditions. Front. Microbiol. 2020, 11, 2019. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.; Jha, P.N. Bio-inoculation of plant growth-promoting rhizobacterium Enterobacter cloacae ZNP-3 increased resistance against salt and temperature stresses in wheat plant (Triticum aestivum L.). J. Plant Growth Regul. 2017, 36, 783–798. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Sumaira; Hafeez, A.; Afridi, M.S.; Khan, S.; Zaib-Un-Nisa; Ullah, I.; AmaralJúnior, A.T.D.; et al. PGPR-Mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Chinnaswamy, A.; Coba de la Peña, T.; Stoll, A.; de la Peña Rojo, D.; Bravo, J.; Rincón, A.; Lucas, M.M.; Pueyo, J.J. A nodule endophytic Bacillus megaterium strain isolated from Medicago polymorpha enhances growth, promotes nodulation by Ensifer medicae and alleviates salt stress in alfalfa plants. Ann. Appl. Biol. 2018, 172, 295–308. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Hernández, A.G.; Glick, B.R.; Rossi, M.J. Plant growth-promoting activities and genomic analysis of the stress-resistant Bacillus megaterium STB1, a bacterium of agricultural and biotechnological interest. Biotechnol. Rep. 2020, 25, e00406. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lei, P.; Pang, X.; Li, S.; Xu, H.; Xu, Z.; Feng, X. Enhanced tolerance to salt stress in canola (Brassica napus L.) seedlings inoculated with the halotolerant Enterobacter cloacae HSNJ4. Appl. Soil Ecol. 2017, 119, 26–34. [Google Scholar] [CrossRef]
- Bal, H.B.; Nayak, L.; Das, S.; Adhya, T.K. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil 2013, 366, 93–105. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Schwinghamer, T.D.; Smith, D.L. Rhizobacteria from root nodules of an indigenous legume enhance salinity stress tolerance in soybean. Front. Sustain. Food. Syst. 2021, 4, 617978. [Google Scholar] [CrossRef]
- Matse, D.T.; Huang, C.H.; Huang, Y.M.; Yen, M.Y. Effects of coinoculation of Rhizobium with plant growth promoting rhizobacteria on the nitrogen fixation and nutrient uptake of Trifolium repens in low phosphorus soil. J. Plant Nutr. 2020, 43, 739–752. [Google Scholar] [CrossRef]
- Omara, A.E.; Hauka, F.; Afify, A.; Nour, E.M.; Kassem, M. The role of some PGPR strains to biocontrol Rhizoctonia solani in soybean and enhancement the growth dynamics and seed yield. Env. Biodivers. Soil Secur. 2017, 1, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Kasim, W.A.; Gaafar, R.M.; Abou-Ali, R.M.; Omar, M.N.; Hewait, H.M. Effect of biofilm forming plant growth promoting rhizobacteria on salinity tolerance in barley. Ann. Agric. Sci. 2016, 6, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Bharti, N.; Barnawal, D. Amelioration of salinity stress by PGPR: ACC deaminase and ROS scavenging enzymes activity. In PGPR Amelioration in Sustainable Agriculture; Woodhead Publishing: Sawston, UK, 2019; pp. 85–106. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 2012, 14, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Plant growth-promoting rhizobacteria ameliorates salinity stress in pea (Pisum sativum). J. Plant Growth Regul. 2022, 41, 647–656. [Google Scholar] [CrossRef]
- Jha, Y.; Subramanian, R.B. PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol. Mol. Biol. Plants 2014, 20, 201–207. [Google Scholar] [CrossRef]
- Hidri, R.; Mahmoud, O.M.-B.; Zorrig, W.; Mahmoudi, H.; Smaoui, A.; Abdelly, C.; Azcon, R.; Debez, A. Plant growth-promoting rhizobacteria alleviate high salinity impact on the halophyte Suaeda fruticosa by modulating antioxidant defense and soil biological activity. Front. Plant Sci. 2022, 13, 821475. [Google Scholar] [CrossRef] [PubMed]
- Yun, P.; Xu, L.; Wang, S.S.; Shabala, L.; Shabala, S.; Zhang, W.Y. Piriformospora indica improves salinity stress tolerance in Zea mays L. plants by regulating Na+ and K+ loading in root and allocating K+ in shoot. Plant Growth Regul. 2018, 86, 323–331. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- del Amor, F.M.; Cuadra-Crespo, P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct. Plant Biol. 2011, 39, 82–90. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.M.; Lee, I.J. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ. Exp. Bot. 2017, 136, 68–77. [Google Scholar] [CrossRef]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef] [Green Version]
- Kabiraj, A.; Majhi, K.; Halder, U.; Let, M.; Bandopadhyay, R. Role of Plant Growth-Promoting Rhizobacteria (PGPR) for crop stress management. In Sustainable Agriculture in the Era of Climate Change; Springer: Cham, Switzerland, 2020; pp. 367–389. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020, 199, 104577. [Google Scholar] [CrossRef]
- Li, X.; Sun, P.; Zhang, Y.; Jin, C.; Guan, C. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 2020, 174, 104023. [Google Scholar] [CrossRef]
- Habib, S.H.; Kausar, H.; Saud, H.M. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed Res. Int. 2016, 2016, 6284547. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jang, Y.J.; Lee, S.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol. Cells 2014, 37, 109. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoque, M.N.; Imran, S.; Hannan, A.; Paul, N.C.; Mahamud, M.A.; Chakrobortty, J.; Sarker, P.; Irin, I.J.; Brestic, M.; Rhaman, M.S. Organic Amendments for Mitigation of Salinity Stress in Plants: A Review. Life 2022, 12, 1632. https://doi.org/10.3390/life12101632
Hoque MN, Imran S, Hannan A, Paul NC, Mahamud MA, Chakrobortty J, Sarker P, Irin IJ, Brestic M, Rhaman MS. Organic Amendments for Mitigation of Salinity Stress in Plants: A Review. Life. 2022; 12(10):1632. https://doi.org/10.3390/life12101632
Chicago/Turabian StyleHoque, Md. Najmol, Shahin Imran, Afsana Hannan, Newton Chandra Paul, Md. Asif Mahamud, Jotirmoy Chakrobortty, Prosenjit Sarker, Israt Jahan Irin, Marian Brestic, and Mohammad Saidur Rhaman. 2022. "Organic Amendments for Mitigation of Salinity Stress in Plants: A Review" Life 12, no. 10: 1632. https://doi.org/10.3390/life12101632
APA StyleHoque, M. N., Imran, S., Hannan, A., Paul, N. C., Mahamud, M. A., Chakrobortty, J., Sarker, P., Irin, I. J., Brestic, M., & Rhaman, M. S. (2022). Organic Amendments for Mitigation of Salinity Stress in Plants: A Review. Life, 12(10), 1632. https://doi.org/10.3390/life12101632











