Comparison between Enteroscopy-, Laparoscopy- and Endoscopic Ultrasound-Assisted Endoscopic Retrograde Cholangio-Pancreatography in Patients with Surgically Altered Anatomy: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Search Strategy
2.3. Data Abstraction and Quality Assessment
2.4. Outcomes
2.5. Statistical Analysis
2.6. Quality of Evidence
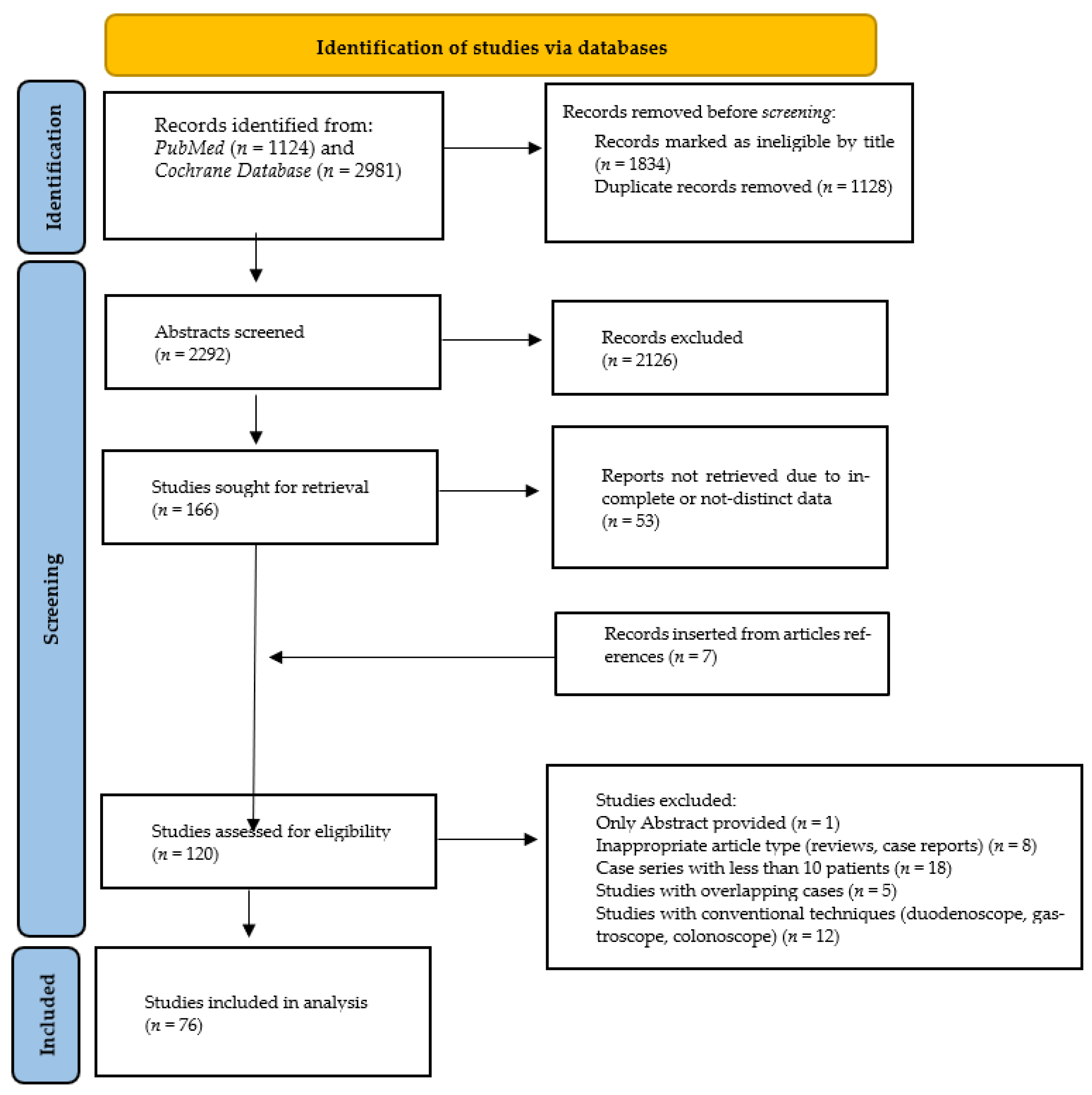
3. Results
3.1. Characteristics of Included Studies
3.2. Quality Assessment
3.3. Primary Outcome—Technical Success in Reaching the Area of Interest (Ampulla or Anastomosis)
3.4. Secondary Outcomes
3.5. Adverse Events
3.6. Quality of Evidence
3.7. Publication Bias
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BA-ERCP | balloon-assisted ERCP |
| CBD | common bile duct |
| DBE | double-balloon enteroscope-assisted ERCP |
| EA-ERCP | enteroscope-assisted ERCP |
| EDEE | entero-enteral anastomosis to perform ERCP |
| EDGE | EUS-directed transgastric ERCP |
| ERCP | endoscopic retrograde cholangiopancreatography |
| EUS | endoscopic ultrasound |
| LA-ERCP | laparoscopy-assisted ERCP |
| LAMS | lumen apposing metal stents |
| SE-ERCP | spiral enteroscope-assisted ERCP |
| RY | Roux-n-Y |
| RYGB | Roux-n-Y gastric bypass |
| SAA | surgically altered anatomy |
| SBE | single-balloon enteroscope-assisted ERCP |
References
- Hasan, M.Y.; Lomanto, D.; Loh, L.L.; So, J.B.Y.; Shabbir, A. Gallstone Disease After Laparoscopic Sleeve Gastrectomy in an Asian Population—What Proportion of Gallstones Actually Becomes Symptomatic? Obes. Surg. 2017, 27, 2419–2423. [Google Scholar] [CrossRef] [PubMed]
- Nagem, R.G.; Lázaro-da-Silva, A.; de Oliveira, R.M.; Morato, V.G. Gallstone-Related Complications after Roux-En-Y Gastric Bypass: A Prospective Study. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 630–635. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y. Related Factors of Postoperative Gallstone Formation after Distal Gastrectomy: A Meta-Analysis. Indian J. Cancer 2017, 54, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Mishra, T.; Lakshmi, K.K.; Peddi, K.K. Prevalence of Cholelithiasis and Choledocholithiasis in Morbidly Obese South Indian Patients and the Further Development of Biliary Calculus Disease After Sleeve Gastrectomy, Gastric Bypass and Mini Gastric Bypass. Obes. Surg. 2016, 26, 2411–2417. [Google Scholar] [CrossRef] [PubMed]
- Groot, V.P.; Rezaee, N.; Wu, W.; Cameron, J.L.; Fishman, E.K.; Hruban, R.H.; Weiss, M.J.; Zheng, L.; Wolfgang, C.L.; He, J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2018, 267, 936–945. [Google Scholar] [CrossRef]
- Wu, W.G.; Mei, J.W.; Zhao, M.N.; Zhang, W.J.; Gu, J.; Tao, Y.J.; Liu, Y.B.; Wang, X.F. Use of the Conventional Side-Viewing Duodenoscope for Successful Endoscopic Retrograde Cholangiopancreatography in Postgastrectomy Patients. J. Clin. Gastroenterol. 2016, 50, 244–251. [Google Scholar] [CrossRef][Green Version]
- Feitoza, A.B.; Baron, T.H. Endoscopy and ERCP in the Setting of Previous Upper GI Tract Surgery. Part I: Reconstruction without Alteration of Pancreaticobiliary Anatomy. Gastrointest. Endosc. 2001, 54, 743–749. [Google Scholar] [CrossRef]
- Bove, V.; Tringali, A.; Familiari, P.; Gigante, G.; Boškoski, I.; Perri, V.; Mutignani, M.; Costamagna, G. ERCP in Patients with Prior Billroth II Gastrectomy: Report of 30 Years’ Experience. Endoscopy 2015, 47, 611–616. [Google Scholar] [CrossRef]
- Park, T.Y.; Kang, J.S.; Song, T.J.; Lee, S.S.; Lee, H.; Choi, J.S.; Kim, H.J.; Jang, J.W. Outcomes of ERCP in Billroth II Gastrectomy Patients. Gastrointest. Endosc. 2016, 83, 1193–1201. [Google Scholar] [CrossRef]
- Li, J.S.; Zou, D.W.; Jin, Z.D.; Chen, J.; Shi, X.G.; Li, Z.S.; Liu, F. Endoscopic Retrograde Cholangiopancreatography in Billroth II Gastrectomy Patients: Outcomes and Potential Factors Affecting Technical Failure. Saudi J. Gastroenterol. 2019, 25, 355–361. [Google Scholar] [CrossRef]
- Liu, K.; Joshi, V.; Saxena, P.; Kaffes, A.J. Predictors of Success for Double Balloon-Assisted Endoscopic Retrograde Cholangiopancreatography in Patients with Roux-En-Y Anastomosis. Dig. Endosc. 2017, 29, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Hintze, R.E.; Adler, A.; Veltzke, W.; Abou-Rebyeh, H. Endoscopic Access to the Papilla of Vater for Endoscopic Retrograde Cholangiopancreatography in Patients with Billroth II or Roux-En-Y Gastrojejunostomy. Endoscopy 1997, 29, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.K.; Lee, M.S.; Chen, K.F.; Lin, C.H.; Sung, K.F.; Wu, C.C. Double-Balloon Enteroscopy-Assisted Endoscopic Retrograde Cholangiography for Roux-En-Y Reconstruction Patients with Papilla of Vater or Bilioenteric Anastomosis. Scand. J. Gastroenterol. 2016, 51, 95–102. [Google Scholar] [CrossRef]
- Nordby, T.; Hugenschmidt, H.; Fagerland, M.W.; Ikdahl, T.; Buanes, T.; Labori, K.J. Follow-up after Curative Surgery for Pancreatic Ductal Adenocarcinoma: Asymptomatic Recurrence Is Associated with Improved Survival. Eur. J. Surg. Oncol. 2013, 39, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Nennstiel, S.; Freivogel, K.; Faber, A.; Schlag, C.; Haller, B.; Blöchinger, M.; Dollhopf, M.; Lewerenz, B.; Schepp, W.; Schirra, J.; et al. Endoscopic and Percutaneous Biliary Interventions in Patients with Altered Upper Gastrointestinal Anatomy—The Munich Multicenter Experience. Surg. Endosc. 2021, 35, 6853–6864. [Google Scholar] [CrossRef]
- Li, K.; Huang, Y.H.; Yao, W.; Chang, H.; Huang, X.B.; Zhang, Y.P.; Song, Z.Q. Adult Colonoscopy or Single-Balloon Enteroscopy-Assisted ERCP in Long-Limb Surgical Bypass Patients. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, B.; Li, Q.; Zhang, X.; Jiang, G.; Ge, X.; Nie, J.; Zhang, X.; Wu, P.; Ji, J.; et al. Endoscopic Retrograde Cholangiopancreatography in Patients with Surgically Altered Anatomy: One Single Center’s Experience. Medicine 2016, 95, e5743. [Google Scholar] [CrossRef]
- Ni, J.B.; Zhu, M.Y.; Li, K.; Dai, W.M.; Lu, L.G.; Wan, X.J.; Wan, R.; Cai, X.B. The Feasibility of Cap-Assisted Routine Adult Colonoscope for Therapeutic Endoscopic Retrograde Cholangiopancreatography in Patients with Roux-En-Y Reconstruction after Total Gastrectomy. J. Dig. Dis. 2021, 22, 721–726. [Google Scholar] [CrossRef]
- Klair, J.S.; Jayaraj, M.; Chandrasekar, V.T.; Priyan, H.; Law, J.; Murali, A.R.; Singh, D.; Larsen, M.; Irani, S.; Kozarek, R.; et al. ERCP with Overtube-Assisted Enteroscopy in Patients with Roux-En-Y Gastric Bypass Anatomy: A Systematic Review and Meta-Analysis. Endoscopy 2020, 52, 824–832. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Kato, H.; Hirao, K.; Mizukawa, S.; Muro, S.; Akimoto, Y.; Uchida, D.; Matsumoto, K.; Tomoda, T.; Horiguchi, S.; et al. Comparison of Two Fluoroscopic Images to Ensure Efficient Scope Insertion for Biliary Intervention in Patients with Roux-En-Y Hepaticojejunostomy. Endoscopy 2017, 49, 1256–1261. [Google Scholar] [CrossRef]
- Inamdar, S.; Slattery, E.; Sejpal, D.V.; Miller, L.S.; Pleskow, D.K.; Berzin, T.M.; Trindade, A.J. Systematic Review and Meta-Analysis of Single-Balloon Enteroscopy-Assisted ERCP in Patients with Surgically Altered GI Anatomy. Gastrointest. Endosc. 2015, 82, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Tanisaka, Y.; Ryozawa, S.; Mizuide, M.; Araki, R.; Fujita, A.; Ogawa, T.; Tashima, T.; Noguchi, T.; Suzuki, M.; Katsuda, H. Status of Single-Balloon Enteroscopy-Assisted Endoscopic Retrograde Cholangiopancreatography in Patients with Surgically Altered Anatomy: Systematic Review and Meta-Analysis on Biliary Interventions. Dig. Endosc. 2021, 33, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Hajibandeh, S.; Hajibandeh, S.; Tarazi, M.; Mansour, M.; Satyadas, T. Procedural Outcomes of Laparoscopic-Assisted Endoscopic Retrograde Cholangiopancreatography in Patients with Previous Roux-En-Y Gastric Bypass Surgery: A Systematic Review and Meta-Analysis. Obes. Surg. 2021, 31, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, B.S.; Dhaliwal, A.; Mohan, B.P.; Mashiana, H.S.; Girotra, M.; Singh, S.; Ohning, G.; Bhat, I.; Adler, D.G. EDGE in Roux-En-Y Gastric Bypass: How Does It Compare to Laparoscopy-Assisted and Balloon Enteroscopy ERCP: A Systematic Review and Meta-Analysis. Endosc. Int. Open 2020, 8, E163–E171. [Google Scholar] [CrossRef] [PubMed]
- da Ponte-Neto, A.M.; Bernardo, W.M.; Lara, L.M.; Josino, I.R.; Brunaldi, V.O.; Moura, D.T.H.; Sakai, P.; Kuga, R.; de Moura, E.G.H. Comparison between Enteroscopy-Based and Laparoscopy-Assisted ERCP for Accessing the Biliary Tree in Patients with Roux-En-Y Gastric Bypass: Systematic Review and Meta-Analysis. Obes. Surg. 2018, 28, 4064–4076. [Google Scholar] [CrossRef]
- Ayoub, F.; Brar, T.S.; Banerjee, D.; Abbas, A.M.; Wang, Y.; Yang, D.; Draganov, P.V. Laparoscopy-Assisted versus Enteroscopy-Assisted Endoscopic Retrograde Cholangiopancreatography (ERCP) in Roux-En-Y Gastric Bypass: A Meta-Analysis. Endosc. Int. Open 2020, 8, E423–E436. [Google Scholar] [CrossRef] [PubMed]
- Aiolfi, A.; Asti, E.; Rausa, E.; Bernardi, D.; Bonitta, G.; Bonavina, L. Trans-Gastric ERCP After Roux-En-Y Gastric Bypass: Systematic Review and Meta-Analysis. Obes. Surg. 2018, 28, 2836–2843. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a Knowledge Representation for Clinical Questions. In AMIA Annual Symposium Proceedings; American Medical Informatics Association: Bethesda, MD, USA, 2006; pp. 359–363. [Google Scholar]
- Cantrell, A.; Croot, E.; Johnson, M.; Wong, R.; Chambers, D.; Baxter, S.K.; Booth, A. Access to Primary and Community Health-Care Services for People 16 Years and over with Intellectual Disabilities: A Mapping and Targeted Systematic Review. Health Serv. Deliv. Res. 2020, 8, 1–142. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.S.; Scholten, R.J.P.M.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate Analysis of Sensitivity and Specificity Produces Informative Summary Measures in Diagnostic Reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Falck-Ytter, Y.; Jaeschke, R.; Vist, G.; et al. GRADE Guidelines: 8. Rating the Quality of Evidence—Indirectness. J. Clin. Epidemiol. 2011, 64, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Trindade, A.J.; Mella, J.M.; Slattery, E.; Cohen, J.; Dickstein, J.; Garud, S.S.; Chuttani, R.; Pleskow, D.K.; Sawhney, M.S.; Berzin, T.M. Use of a Cap in Single-Balloon Enteroscopy-Assisted Endoscopic Retrograde Cholangiography. Endoscopy 2014, 77, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, Y.; Sullivan, C.T.; Gelrud, A. Single Balloon Enteroscopy (SBE) Assisted Therapeutic Endoscopic Retrograde Cholangiopancreatography (ERCP) in Patients with Roux-En-Y Anastomosis. Dig. Dis. Sci. 2014, 59, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, T.; Kato, H.; Miyamoto, K.; Matsumi, A.; Ueta, E.; Fujii, Y.; Saragai, Y.; Yamazaki, T.; Uchida, D.; Matsumoto, K.; et al. Outcomes of Endoscopic Treatment for Malignant Biliary Obstruction in Patients with Surgically Altered Anatomy: Analysis of Risk Factors for Clinical Failure. Surg. Endosc. 2021, 35, 232–238. [Google Scholar] [CrossRef]
- Uchida, D.; Tsutsumi, K.; Kato, H.; Matsumi, A.; Saragai, Y.; Tomoda, T.; Matsumoto, K.; Horiguchi, S.; Okada, H. Potential Factors Affecting Results of Short-Type Double-Balloon Endoscope-Assisted Endoscopic Retrograde Cholangiopancreatography. Dig. Dis. Sci. 2020, 65, 1460–1470. [Google Scholar] [CrossRef]
- Wang, A.Y.; Sauer, B.G.; Behm, B.W.; Ramanath, M.; Cox, D.G.; Ellen, K.L.; Shami, V.M.; Kahaleh, M. Single-Balloon Enteroscopy Effectively Enables Diagnostic and Therapeutic Retrograde Cholangiography in Patients with Surgically Altered Anatomy. Gastrointest. Endosc. 2010, 71, 641–649. [Google Scholar] [CrossRef]
- Wu, W.G.; Qin, L.C.; Song, X.L.; Zhao, M.N.; Zhang, W.J.; Gu, J.; Weng, H.; Liu, Y.B.; Zhang, Y.; Qu, C.Y.; et al. Application of Single Balloon Enteroscopy-Assisted Therapeutic Endoscopic Retrograde Cholangiopancreatography in Patients after Bilioenteric Roux-En-Y Anastomosis: Experience of Multi-Disciplinary Collaboration. World J. Gastroenterol. 2019, 25, 5505–5514. [Google Scholar] [CrossRef]
- Yamada, A.; Kogure, H.; Nakai, Y.; Takahara, N.; Mizuno, S.; Tada, M.; Koike, K. Performance of a New Short-Type Double-Balloon Endoscope with Advanced Force Transmission and Adaptive Bending for Pancreaticobiliary Intervention in Patients with Surgically Altered Anatomy: A Propensity-Matched Analysis. Dig. Endosc. 2019, 31, 86–93. [Google Scholar] [CrossRef]
- Yamauchi, H.; Kida, M.; Okuwaki, K.; Miyazawa, S.; Iwai, T.; Takezawa, M.; Kikuchi, H.; Watanabe, M.; Imaizumi, H.; Koizumi, W. Short-Type Single Balloon Enteroscope for Endoscopic Retrograde Cholangiopancreatography with Altered Gastrointestinal Anatomy. World J Gastroenterol. 2013, 19, 1728–1735. [Google Scholar] [CrossRef]
- Zamora-Nava, L.E.; Teran-Ellis, S.M.Y.; Zepeda-Gómez, S.; Pérez-Cuadrado-Robles, E.; Miranda-Lora, A.L.; Valdovinos-Andraca, F.; López-Méndez, D.P. Endoscopic Retrograde Cholangiopancreatography by Double-Balloon Enteroscopy in Patients with Surgically Altered Gastrointestinal Anatomy. Rev. Esp. Enferm. Dig. 2020, 112, 278–283. [Google Scholar] [CrossRef]
- Zouhairi, M. El Rotational Assisted Endoscopic Retrograde Cholangiopancreatography in Patients with Reconstructive Gastrointestinal Surgical Anatomy. World J. Gastrointest. Endosc. 2015, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.; Modayil, R.; Gurram, K.C.; Brathwaite, C.E.M.; Friedel, D.; Stavropoulos, S.N. Spiral Enteroscopy–Assisted ERCP in Bariatric-Length Roux-En-Y Anatomy: A Large Single-Center Series and Review of the Literature (with Video). Gastrointest. Endosc. 2018, 87, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, Y.; Yu, D.; Gao, G.; Li, L. Endoscopic Retrograde Cholangiopancreatography with Balloon-Assisted Enteroscopy in Patients with Roux-En-Y Anastomosis and Whipple Operation. Medicine 2020, 99, e22653. [Google Scholar] [CrossRef] [PubMed]
- Tanisaka, Y.; Ryozawa, S.; Mizuide, M.; Fujita, A.; Ogawa, T.; Harada, M.; Noguchi, T.; Suzuki, M.; Araki, R. Biliary Cannulation in Patients with Roux-En-y Gastrectomy: An Analysis of the Factors Associated with Successful Cannulation. Intern. Med. 2020, 59, 1687–1693. [Google Scholar] [CrossRef]
- Osoegawa, T.; Motomura, Y.; Akahoshi, K.; Higuchi, N.; Tanaka, Y.; Hisano, T.; Itaba, S.; Gibo, J.; Yamada, M.; Kubokawa, M.; et al. Improved Techniques for Double-Balloon-Enteroscopy-Assisted Endoscopic Retrograde Cholangiopancreatography. World J. Gastroenterol. 2012, 18, 6843–6849. [Google Scholar] [CrossRef]
- Parlak, E.; Çiçek, B.; Dişibeyaz, S.; Cengiz, C.; Yurdakul, M.; Akdoǧan, M.; Kiliç, M.Z.Y.; Şaşmaz, N.; Cumhur, T.; Şahin, B. Endoscopic Retrograde Cholangiography by Double Balloon Enteroscopy in Patients with Roux-En-Y Hepaticojejunostomy. Surg. Endosc. 2010, 24, 466–470. [Google Scholar] [CrossRef]
- Saleem, A.; Levy, M.J.; Petersen, B.T.; Que, F.G.; Baron, T.H. Laparoscopic Assisted ERCP in Roux-En-Y Gastric Bypass (RYGB) Surgery Patients. J. Gastrointest. Surg. 2012, 16, 203–208. [Google Scholar] [CrossRef]
- Shimatani, M.; Matsushita, M.; Takaoka, M.; Koyabu, M.; Ikeura, T.; Kato, K.; Fukui, T.; Uchida, K.; Okazaki, K. Effective Short Double-Balloon Enteroscope for Diagnostic and Therapeutic ERCP in Patients with Altered Gastrointestinal Anatomy: A Large Case Series. Endoscopy 2009, 41, 849–854. [Google Scholar] [CrossRef]
- Shimatani, M.; Takaoka, M.; Ikeura, T.; Mitsuyama, T.; Okazaki, K. Evaluation of Endoscopic Retrograde Cholangiopancreatography Using a Newly Developed Short-Type Single-Balloon Endoscope in Patients with Altered Gastrointestinal Anatomy. Dig. Endosc. 2014, 26, 147–155. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Chaaya, A.; Shelton, C.; Marmion, J.; Kowalski, T.E.; Loren, D.E.; Heller, S.J.; Haluszka, O.; Adler, D.G.; Tokar, J.L. Utility of the Short Double-Balloon Enteroscope to Perform Pancreaticobiliary Interventions in Patients with Surgically Altered Anatomy in a US Multicenter Study. Dig. Dis. Sci. 2013, 58, 858–864. [Google Scholar] [CrossRef]
- Takasaki, Y.; Ishii, S.; Shibuya, T.; Fujisawa, T.; Ushio, M.; Takahashi, S.; Ito, K.; Yamagata, W.; Suzuki, A.; Okahara, K.; et al. Endoscopic Ultrasound-Guided Antegrade Procedures for Managing Bile Duct Stones in Patients with Surgically Altered Anatomy: Comparison with Double-Balloon Enteroscopy-Assisted Endoscopic Retrograde Cholangiography (with Video). Dig. Endosc. 2021, 33, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Tanisaka, Y.; Ryozawa, S.; Itoi, T.; Yamauchi, H.; Katanuma, A.; Okabe, Y.; Irisawa, A.; Nakahara, K.; Iwasaki, E.; Ishii, K.; et al. Efficacy and Factors Affecting Procedure Results of Short-Type Single-Balloon Enteroscopy–Assisted ERCP for Altered Anatomy: A Multicenter Cohort in Japan. Gastrointest. Endosc. 2022, 95, 310–318.e1. [Google Scholar] [CrossRef] [PubMed]
- Tanisaka, Y.; Ryozawa, S.; Mizuide, M.; Kobayashi, M.; Fujita, A.; Minami, K.; Kobatake, T.; Omiya, K.; Iwano, H.; Araki, R. Usefulness of the “Newly Designed” Short-Type Single-Balloon Enteroscope for ERCP in Patients with Roux-En-Y Gastrectomy: A Pilot Study. Endosc. Int. Open 2018, 6, E1417–E1422. [Google Scholar] [CrossRef]
- Izawa, N.; Tsuchida, K.; Tominaga, K.; Fukushi, K.; Sakuma, F.; Kashima, K.; Kunogi, Y.; Kanazawa, M.; Tanaka, T.; Nagashima, K.; et al. Factors Affecting Technical Difficulty in Balloon Enteroscopy-Assisted Endoscopic Retrograde Cholangiopancreatography in Patients with Surgically Altered Anatomy. J. Clin. Med. 2021, 10, 1100. [Google Scholar] [CrossRef] [PubMed]
- Aabakken, L.; Bretthauer, M.; Line, P.D. Double-Balloon Enteroscopy for Endoscopic Retrograde Cholangiography in Patients with a Roux-En-Y Anastomosis. Endoscopy 2007, 39, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.S.; Li, M.K.K.; Yip, W.M.; Choi, W.L.; Fong, M.C. Endoscopic Retrograde Cholangiopancreatography Using Short-Type Double-Balloon Enteroscope: Experience in Hong Kong. J. Dig. Dis. 2021, 22, 545–550. [Google Scholar] [CrossRef]
- Cho, S.; Kamalaporn, P.; Kandel, G.; Kortan, P.; Marcon, N.; May, G. “Short” Double-Balloon Enteroscope for Endoscopic Retrograde Cholangiopancreatography in Patients with a Surgically Altered Upper Gastrointestinal Tract. Can. J. Gastroenterol. 2011, 25, 615–619. [Google Scholar] [CrossRef]
- Díez, J.E.; Ramos, M.E.P. Single-Balloon Enteroscopy-Assisted ERCP in Patients with Roux-En-Y Anatomy and Choledocholithiasis: Do Technical Improvements Mean Better Outcomes? Rev. Esp. Enferm. Dig. 2021, 112, 929–934. [Google Scholar] [CrossRef]
- Emmett, D.S.; Mallat, D.B. Double-Balloon ERCP in Patients Who Have Undergone Roux-En-Y Surgery: A Case Series. Gastrointest. Endosc. 2007, 66, 1038–1041. [Google Scholar] [CrossRef]
- Hakuta, R.; Kogure, H.; Nakai, Y.; Hamada, T.; Sato, T.; Suzuki, Y.; Inokuma, A.; Kanai, S.; Nakamura, T.; Noguchi, K.; et al. Feasibility of Balloon Endoscope-Assisted Endoscopic Retrograde Cholangiopancreatography for the Elderly. Endosc. Int. Open 2020, 8, E1202–E1211. [Google Scholar] [CrossRef]
- Itoi, T.; Ishii, K.; Sofuni, A.; Itokawa, F.; Tsuchiya, T.; Kurihara, T.; Tsuji, S.; Ikeuchi, N.; Umeda, J.; Moriyasu, F. Single-Balloon Enteroscopy-Assisted Ercp in Patients with Billroth II Gastrectomy or Roux-En-y Anastomosis (with Video). Am. J. Gastroenterol. 2010, 105, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, F.; Itoi, T.; Ishii, K.; Sofuni, A.; Moriyasu, F. Single- and Double-Balloon Enteroscopy-Assisted Endoscopic Retrograde Cholangiopancreatography in Patients with Roux-En-Y plus Hepaticojejunostomy Anastomosis and Whipple Resection. Dig. Endosc. 2014, 26, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T.; Kida, M.; Yamauchi, H.; Imaizumi, H.; Koizumi, W. Short-Type and Conventional Single-Balloon Enteroscopes for Endoscopic Retrograde Cholangiopancreatography in Patients with Surgically Altered Anatomy: Single-Center Experience. Dig. Endosc. 2014, 26, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.; Abboud, G.; Lo, S.; Jamil, L. Double Balloon Enteroscopy-Assisted Endoscopic Retrograde Cholangiopancreatography in Roux-En-Y Gastric Bypass Anatomy: Expert vs. Novice Experience. Endosc. Int. Open 2018, 6, E885–E891. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Uno, K.; Suzuki, A.; Mandai, K.; Nakase, K.; Tanaka, K.; Yasuda, K. Clinical Usefulness of a Short-Type, Prototype Single-Balloon Enteroscope for Endoscopic Retrograde Cholangiopancreatography in Patients with Altered Gastrointestinal Anatomy: Preliminary Experiences. Dig. Endosc. 2015, 27, 82–86. [Google Scholar] [CrossRef]
- Kogure, H.; Sato, T.; Nakai, Y.; Ishigaki, K.; Hakuta, R.; Saito, K.; Saito, T.; Takahara, N.; Hamada, T.; Mizuno, S.; et al. Endoscopic Management of Pancreatic Diseases in Patients with Surgically Altered Anatomy: Clinical Outcomes of Combination of Double-Balloon Endoscopy- and Endoscopic Ultrasound-Guided Interventions. Dig. Endosc. 2021, 33, 441–450. [Google Scholar] [CrossRef]
- Lenze, F.; Meister, T.; Matern, P.; Heinzow, H.S.; Domschke, W.; Ullerich, H. Single-Balloon Enteroscopy-Assisted Endoscopic Retrograde Cholangiopancreaticography in Patients with Surgically Altered Anatomy: Higher Failure Rate in Malignant Biliary Obstruction-a Prospective Single Center Cohort Analysis. Scand. J. Gastroenterol. 2014, 49, 766–771. [Google Scholar] [CrossRef]
- Kianička, B.; Lata, J.; Novotný, I.; Dítě, P.; Vaníček, J. Single Balloon Enteroscopy for Endoscopic Retrograde Cholangiography in Patients with Roux-En-Y Hepaticojejuno Anastomosis. World J. Gastroenterol. 2013, 19, 8047–8055. [Google Scholar] [CrossRef]
- Mönkemüller, K.; Fry, L.C.; Bellutti, M.; Neumann, H.; Malfertheiner, P. ERCP with the Double Balloon Enteroscope in Patients with Roux-En-Y Anastomosis. Surg. Endosc. 2009, 23, 1961–1967. [Google Scholar] [CrossRef]
- Neumann, H.; Fry, L.C.; Meyer, F.; Malfertheiner, P.; Mönkemüller, K. Endoscopic Retrograde Cholangiopancreatography Using the Single Balloon Enteroscope Technique in Patients with Roux-En-y Anastomosis. Digestion 2009, 80, 52–57. [Google Scholar] [CrossRef]
- Obana, T.; Fujita, N.; Ito, K.; Noda, Y.; Kobayashi, G.; Horaguchi, J.; Koshita, S.; Kanno, Y.; Ogawa, T.; Hashimoto, S.; et al. Therapeutic Endoscopic Retrograde Cholangiography Using a Single-Balloon Enteroscope in Patients with Roux-En-Y Anastomosis. Dig. Endosc. 2013, 25, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Tsutsumi, K.; Kato, H.; Ueki, T.; Miyamoto, K.; Yamazaki, T.; Matsumi, A.; Fujii, Y.; Matsumoto, K.; Horiguchi, S.; et al. Balloon Enteroscopy-Assisted Endoscopic Retrograde Cholangiopancreatography for the Treatment of Common Bile Duct Stones in Patients with Roux-En-y Gastrectomy: Outcomes and Factors Affecting Complete Stone Extraction. Clin. Med. 2021, 10, 3314. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.M.; Strong, A.T.; Diehl, D.L.; Brauer, B.C.; Lee, I.H.; Burbridge, R.; Zivny, J.; Higa, J.T.; Falcão, M.; El Hajj, I.I.; et al. Multicenter Evaluation of the Clinical Utility of Laparoscopy-Assisted ERCP in Patients with Roux-En-Y Gastric Bypass. Gastrointest. Endosc. 2018, 87, 1031–1039. [Google Scholar] [CrossRef]
- AlMasri, S.; Zenati, M.S.; Papachristou, G.I.; Slivka, A.; Sanders, M.; Chennat, J.; Rabinowitz, M.; Khalid, A.; Gelrud, A.; Nasr, J.; et al. Laparoscopic-Assisted ERCP Following RYGB: A 12-Year Assessment of Outcomes and Learning Curve at a High-Volume Pancreatobiliary Center. Surg. Endosc. 2022, 36, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Bowman, E.; Greenberg, J.; Garren, M.; Guda, N.; Rajca, B.; Benson, M.; Pfau, P.; Soni, A.; Walker, A.; Gopal, D. Laparoscopic-Assisted ERCP and EUS in Patients with Prior Roux-En-Y Gastric Bypass Surgery: A Dual-Center Case Series Experience. Surg. Endosc. 2016, 30, 4647–4652. [Google Scholar] [CrossRef] [PubMed]
- De Benito Sanz, M.; Carbajo, A.Y.; Hernández, R.S.O.; Chavarria, C.; De Rozas, S.B.P.; García-Alonso, F.J.; De La Serna Higuera, C.; Perez-Miranda, M. Endoscopic Ultrasound-Directed Transgastric ERCP in Patients with Rouxen- y Gastric Bypass Using Lumen-Apposing Metal Stents or Duodenal Selfexpandable Metal Stents. A European Single-Center Experience. Rev. Esp. Enferm. Dig. 2020, 112, 211–215. [Google Scholar] [CrossRef]
- Falcão, M.; Campos, J.M.; Neto, M.G.; Ramos, A.; Secchi, T.; Alves, E.; Franca, E.; Maluf-Filho, F.; Ferraz, Á. Transgastric Endoscopic Retrograde Cholangiopancreatography for the Management of Biliary Tract Disease after Roux-En-Y Gastric Bypass Treatment for Obesity. Obes. Surg. 2012, 22, 872–876. [Google Scholar] [CrossRef]
- Frederiksen, N.A.; Tveskov, L.; Helgstrand, F.; Naver, L.; Floyd, A. Treatment of Common Bile Duct Stones in Gastric Bypass Patients with Laparoscopic Transgastric Endoscopic Retrograde Cholangiopancreatography. Obes. Surg. 2017, 27, 1409–1413. [Google Scholar] [CrossRef]
- Habenicht Yancey, K.; McCormack, L.K.; McNatt, S.S.; Powell, M.S.; Fernandez, A.Z.; Westcott, C.J. Laparoscopic-Assisted Transgastric ERCP: A Single-Institution Experience. J. Obes. 2018, 2018, 8275965. [Google Scholar] [CrossRef]
- Ichkhanian, Y.; Yang, J.; James, T.W.; Baron, T.H.; Irani, S.; Nasr, J.; Sharaiha, R.Z.; Law, R.; Wannhoff, A.; Khashab, M.A. EUS-Directed Transenteric ERCP in Non–Roux-En-Y Gastric Bypass Surgical Anatomy Patients (with Video). Gastrointest. Endosc. 2020, 91, 1188–1194.e2. [Google Scholar] [CrossRef]
- James, T.W.; Baron, T.H. Endoscopic Ultrasound-Directed Transgastric ERCP (EDGE): A Single-Center US Experience with Follow-up Data on Fistula Closure. Obes. Surg. 2019, 29, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Koggel, L.M.; Wahab, P.J.; Robijn, R.J.; Aufenacker, T.J.; Witteman, B.P.L.; Groenen, M.J.M.; Vrolijk, J.M. Efficacy and Safety of 100 Laparoscopy-Assisted Transgastric Endoscopic Retrograde Cholangiopancreatography Procedures in Patients with Roux-En-Y Gastric Bypass. Obes. Surg. 2021, 31, 987–993. [Google Scholar] [CrossRef] [PubMed]
- May, D.; Vogels, E.; Parker, D.; Petrick, A.; Diehl, D.; Gabrielsen, J. Overall Outcomes of Laparoscopic-Assisted ERCP after Roux-En-Y Gastric Bypass and Sphincter of Oddi Dysfunction Subgroup Analysis. Endosc. Int. Open 2019, 7, E1276–E1280. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, B.; Richard, M.N.; Pandit, A.; Zuccala, K.; Brandwein, S. Outcomes of Laparoscopic-Assisted ERCP in Gastric Bypass Patients at a Community Hospital Center. Surg. Endosc. 2020, 34, 5259–5264. [Google Scholar] [CrossRef] [PubMed]
- Ngamruengphong, S.; Nieto, J.; Kunda, R.; Kumbhari, V.; Chen, Y.I.; Bukhari, M.; El Zein, M.H.; Bueno, R.P.; Hajiyeva, G.; Ismail, A.; et al. Endoscopic Ultrasound-Guided Creation of a Transgastric Fistula for the Management of Hepatobiliary Disease in Patients with Roux-En-Y Gastric Bypass. Endoscopy 2017, 49, 549–552. [Google Scholar] [CrossRef]
- Runge, T.M.; Chiang, A.L.; Kowalski, T.E.; James, T.W.; Baron, T.H.; Nieto, J.; Diehl, D.L.; Krafft, M.R.; Nasr, J.Y.; Kumar, V.; et al. Endoscopic Ultrasound-Directed Transgastric ERCP (EDGE): A Retrospective Multicenter Study. Endoscopy 2021, 53, 611–618. [Google Scholar] [CrossRef]
- Richardson, J.F.; Lee, J.G.; Smith, B.R.; Nguyen, B.; Pham, K.P.; Nguyen, N.T. Laparoscopic Transgastric Endoscopy after Roux-En-Y Gastric Bypass: Case Series and Review of the Literature. Am. Surg. 2012, 78, 1182–1186. [Google Scholar] [CrossRef]
- Schreiner, M.A.; Chang, L.; Gluck, M.; Irani, S.; Gan, S.I.; Brandabur, J.J.; Thirlby, R.; Moonka, R.; Kozarek, R.A.; Ross, A.S. Laparoscopy-Assisted versus Balloon Enteroscopy-Assisted ERCP in Bariatric Post-Roux-En-Y Gastric Bypass Patients. Gastrointest. Endosc. 2012, 75, 748–756. [Google Scholar] [CrossRef]
- Snauwaert, C.; Laukens, P.; Dillemans, B.; Himpens, J.; De Looze, D.; Deprez, P.; Badaoui, A. Laparoscopy-Assisted Transgastric Endoscopic Retrograde Cholangiopancreatography in Bariatric Roux-En-Y Gastric Bypass Patients. Endosc. Int. Open 2015, 3, E458–E463. [Google Scholar] [CrossRef][Green Version]
- Telfah, M.M.; Noble, H.; Mahon, D.; Mason, M.; Hollyman, M.; Matull, R.; Welbourn, R. Laparoscopic-Assisted Endoscopic Retrograde Cholangiopancreatography (ERCP) for Bile Duct Stones After Roux-En-Y-Gastric Bypass: Single-Centre Experience. Obes. Surg. 2020, 30, 4953–4957. [Google Scholar] [CrossRef]
- Azeem, N.; Tabibian, J.H.; Baron, T.H.; Orhurhu, V.; Rosen, C.B.; Petersen, B.T.; Gostout, C.J.; Topazian, M.D.; Levy, M.J. Use of a Single-Balloon Enteroscope Compared with Variable-Stiffness Colonoscopes for Endoscopic Retrograde Cholangiography in Liver Transplant Patients with Roux-En-Y Biliary Anastomosis. Gastrointest. Endosc. 2013, 77, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Mbatshi, G.; Macken, E.J.; De Schepper, H.U.; Piessevaux, H.; Deprez, P.H.; Moreels, T.G. Comparison of Side-Viewing Duodenoscope and Single-Balloon Enteroscope to Perform ERCP in Patients with Billroth II Gastrectomy. Acta Gastroenterol. Belg. 2017, 80, 493–497. [Google Scholar] [PubMed]
- Wang, T.J.; Cortes, P.; Jirapinyo, P.; Thompson, C.C.; Ryou, M. A Comparison of Clinical Outcomes and Cost Utility among Laparoscopy, Enteroscopy, and Temporary Gastric Access-Assisted ERCP in Patients with Roux-En-Y Gastric Bypass Anatomy. Surg. Endosc. 2021, 35, 4469–4477. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, C.J.; Young, J.; Glomsaker, T.; Mala, T.; Loberg, M.; Bretthauer, M.; Refsum, E.; Aabakken, L. Laparoscopy-Assisted versus Balloon Enteroscopy-Assisted ERCP after Roux-En-Y Gastric Bypass. Endoscopy 2020, 52, 654–661. [Google Scholar] [CrossRef]
- Sirin, G. Double Balloon Enteroscopy Improves ERCP Successfulness in Patients with Modified Small Bowel Anatomy. North Clin. Istanb. 2020, 7, 131–139. [Google Scholar] [CrossRef]
- Bukhari, M.; Kowalski, T.; Nieto, J.; Kunda, R.; Ahuja, N.K.; Irani, S.; Shah, A.; Loren, D.; Brewer, O.; Sanaei, O.; et al. An International, Multicenter, Comparative Trial of EUS-Guided Gastrogastrostomy-Assisted ERCP versus Enteroscopy-Assisted ERCP in Patients with Roux-En-Y Gastric Bypass Anatomy. Gastrointest. Endosc. 2018, 88, 486–494. [Google Scholar] [CrossRef]
- Choi, E.K.; Chiorean, M.V.; Coté, G.A.; Hajj, I.E.; Ballard, D.; Fogel, E.L.; Watkins, J.L.; McHenry, L.; Sherman, S.; Lehman, G.A. ERCP via Gastrostomy vs. Double Balloon Enteroscopy in Patients with Prior Bariatric Roux-En-Y Gastric Bypass Surgery. Surg. Endosc. 2013, 27, 2894–2899. [Google Scholar] [CrossRef]
- Kedia, P.; Tarnasky, P.R.; Nieto, J.; Steele, S.L.; Siddiqui, A.; Xu, M.M.; Tyberg, A.; Gaidhane, M.; Kahaleh, M. EUS-Directed Transgastric ERCP (EDGE) Versus Laparoscopy-Assisted ERCP (LA-ERCP) for Roux-En-Y Gastric Bypass (RYGB) Anatomy. J. Clin. Gastroenterol. 2019, 53, 304–308. [Google Scholar] [CrossRef]
- Kochhar, G.S.; Mohy-ud-din, N.; Grover, A.; Carleton, N.; Kulkarni, A.; Farah, K.; Dhawan, M.; Thakkar, S. EUS-Directed Transgastric Endoscopic Retrograde Cholangiopancreatography versus Laparoscopic-Assisted ERCP versus Deep Enteroscopy-Assisted ERCP for Patients with RYGB. Endosc. Int. Open 2020, 8, E877–E882. [Google Scholar] [CrossRef]
- Lennon, A.M.; Corless, E.; Kapoor, S.; Amateau, S.; Chandrasekhara, V.; Khashab, M.; Dunbar, K.; Singh, V.; Okolo, P.I. Spiral Assisted ERCP Is Equivalent to Single Balloon Assisted ERCP in Patients with Roux-En-Y Anatomy. Dig. Dis. Sci. 2012, 57, 1391–1398. [Google Scholar] [CrossRef]
- Shah, R.J.; Smolkin, M.; Yen, R.; Ross, A.; Kozarek, R.A.; Howell, D.A.; Bakis, G.; Jonnalagadda, S.S.; Al-Lehibi, A.A.; Hardy, A.; et al. A Multicenter, U.S. Experience of Single-Balloon, Double-Balloon, and Rotational Overtube-Assisted Enteroscopy ERCP in Patients with Surgically Altered Pancreaticobiliary Anatomy (with Video). Gastrointest. Endosc. 2013, 77, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Sawas, T.; Storm, A.C.; Bazerbachi, F.; Fleming, C.J.; Vargas, E.J.; Chandrasekhara, V.; Andrews, J.C.; Levy, M.J.; Martin, J.A.; Petersen, B.T.; et al. An Innovative Technique Using a Percutaneously Placed Guidewire Allows for Higher Success Rate for ERCP Compared to Balloon Enteroscopy Assistance in Roux-En-Y Gastric Bypass Anatomy. Surg. Endosc. 2020, 34, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Baron, T.; Gostout, C.; Topazian, M.; Levy, M.; Petersen, B.; Wong Kee Song, L. Endoscopic Retrograde Cholangiopancreatography Using a Single-Balloon Enteroscope in Patients with Altered Roux-En-Y Anatomy. Endoscopy 2010, 42, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Anvari, S.; Lee, Y.; Patro, N.; Soon, M.S.; Doumouras, A.G.; Hong, D. Double-Balloon Enteroscopy for Diagnostic and Therapeutic ERCP in Patients with Surgically Altered Gastrointestinal Anatomy: A Systematic Review and Meta-Analysis. Surg. Endosc. 2021, 35, 18–36. [Google Scholar] [CrossRef]
- Beyna, T.; Moreels, T.; Arvanitakis, M.; Pioche, M.; Saurin, J.-C.; May, A.; Knabe, M.; Agnholt, J.S.; Bjerregaard, N.C.; Puustinen, L.; et al. Motorized Spiral Enteroscopy: Results of an International, Multicenter, Prospective Observational Clinical Study on Patients with Normal and Altered Gastrointestinal Anatomy. Endoscopy 2022. online ahead of print. [Google Scholar] [CrossRef]
- Schneider, M.; Neuhaus, H.; Beyna, T. Motorized Spiral Enteroscopy-Assisted ERCP in Altered Gastrointestinal Anatomy: First Clinical Series. Endoscopy 2021, 53 (Suppl. 1), S65–S66. [Google Scholar]
- Beyna, T.; Schneider, M.; Höllerich, J.; Neuhaus, H. Motorized Spiral Enteroscopy–Assisted ERCP after Roux-En-Y Reconstructive Surgery and Bilioenteric Anastomosis: First Clinical Case. VideoGIE 2020, 5, 311–313. [Google Scholar] [CrossRef]
- Kedia, P.; Kumta, N.A.; Widmer, J.; Sundararajan, S.; Cerefice, M.; Gaidhane, M.; Sharaiha, R.; Kahaleh, M. Endoscopic Ultrasound-Directed Transgastric ERCP (EDGE) for Roux-En-Y Anatomy: A Novel Technique. Endoscopy 2015, 307, 491–497. [Google Scholar] [CrossRef]
- Shinn, B.; Boortalary, T.; Raijman, I.; Nieto, J.; Khara, H.S.; Kumar, S.V.; Confer, B.; Diehl, D.L.; El Halabi, M.; Ichkhanian, Y.; et al. Maximizing Success in Single-Session EUS-Directed Transgastric ERCP: A Retrospective Cohort Study to Identify Predictive Factors of Stent Migration. Gastrointest. Endosc. 2021, 94, 727–732. [Google Scholar] [CrossRef]
- Schiemer, M.; Bettinger, D.; Mueller, J.; Schultheiss, M.; Schwacha, H.; Hasselblatt, P.; Thimme, R.; Schmidt, A.; Kuellmer, A. Reduction of Esophageal Stent Migration Rate with a Novel Over-the-Scope Fixation Device (with Video). Gastrointest. Endosc. 2022, 96, 1–8. [Google Scholar] [CrossRef]
- James, H.J.; James, T.W.; Wheeler, S.B.; Spencer, J.C.; Baron, T.H. Cost-Effectiveness of Endoscopic Ultrasound-Directed Transgastric ERCP Compared with Device-Assisted and Laparoscopic-Assisted ERCP in Patients with Roux-En-Y Anatomy. Endoscopy 2019, 51, 1051–1058. [Google Scholar] [CrossRef] [PubMed]

| Comparison (Sig.) | |||
|---|---|---|---|
| EDGE | LA-ERCP | ||
| Technical success rate (95%CI) | |||
| EA-ERCP | 87.3 (85.3–89.4) | 0.001 * | <0.001 * |
| EDGE | 97.9 (96.4–99.4) | 0.43 | |
| LA-ERCP | 99.1 (98.6–99.7) | ||
| Cannulation success rate (95%CI) | |||
| EA-ERCP | 74.7 (71.3–78.0) | <0.001 * | <0.001 * |
| EDGE | 98 (96.5–99.6) | 0.92 | |
| LA-ERCP | 98.6 (97.9–99.2) | ||
| Therapeutic success rate (95%CI) | |||
| EA-ERCP | 69.1 (65.3–72.9) | <0.001 * | <0.001 * |
| EDGE | 97.9 (96.3–99.4) | 0.80 | |
| LA-ERCP | 98.5 (97.8–99.2) | ||
| Adverse Events rate (95%CI) | |||
| EA-ERCP | 5.7 (4.50–6.80) | 0.04 * | 0.003 * |
| EDGE | 13.1 (7.50–18.8) | 0.75 | |
| LA-ERCP | 15.1 (9.40–20.8) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkolfakis, P.; Papaefthymiou, A.; Facciorusso, A.; Tziatzios, G.; Ramai, D.; Dritsas, S.; Florou, T.; Papanikolaou, I.S.; Hassan, C.; Repici, A.; et al. Comparison between Enteroscopy-, Laparoscopy- and Endoscopic Ultrasound-Assisted Endoscopic Retrograde Cholangio-Pancreatography in Patients with Surgically Altered Anatomy: A Systematic Review and Meta-Analysis. Life 2022, 12, 1646. https://doi.org/10.3390/life12101646
Gkolfakis P, Papaefthymiou A, Facciorusso A, Tziatzios G, Ramai D, Dritsas S, Florou T, Papanikolaou IS, Hassan C, Repici A, et al. Comparison between Enteroscopy-, Laparoscopy- and Endoscopic Ultrasound-Assisted Endoscopic Retrograde Cholangio-Pancreatography in Patients with Surgically Altered Anatomy: A Systematic Review and Meta-Analysis. Life. 2022; 12(10):1646. https://doi.org/10.3390/life12101646
Chicago/Turabian StyleGkolfakis, Paraskevas, Apostolis Papaefthymiou, Antonio Facciorusso, Georgios Tziatzios, Daryl Ramai, Spyridon Dritsas, Theodosia Florou, Ioannis S. Papanikolaou, Cesare Hassan, Alessandro Repici, and et al. 2022. "Comparison between Enteroscopy-, Laparoscopy- and Endoscopic Ultrasound-Assisted Endoscopic Retrograde Cholangio-Pancreatography in Patients with Surgically Altered Anatomy: A Systematic Review and Meta-Analysis" Life 12, no. 10: 1646. https://doi.org/10.3390/life12101646
APA StyleGkolfakis, P., Papaefthymiou, A., Facciorusso, A., Tziatzios, G., Ramai, D., Dritsas, S., Florou, T., Papanikolaou, I. S., Hassan, C., Repici, A., Triantafyllou, K., Aabakken, L., Devière, J., Beyna, T., & Arvanitakis, M. (2022). Comparison between Enteroscopy-, Laparoscopy- and Endoscopic Ultrasound-Assisted Endoscopic Retrograde Cholangio-Pancreatography in Patients with Surgically Altered Anatomy: A Systematic Review and Meta-Analysis. Life, 12(10), 1646. https://doi.org/10.3390/life12101646











