Oxidative Stress Management in Cardiorenal Diseases: Focus on Novel Antidiabetic Agents, Finerenone, and Melatonin
Abstract
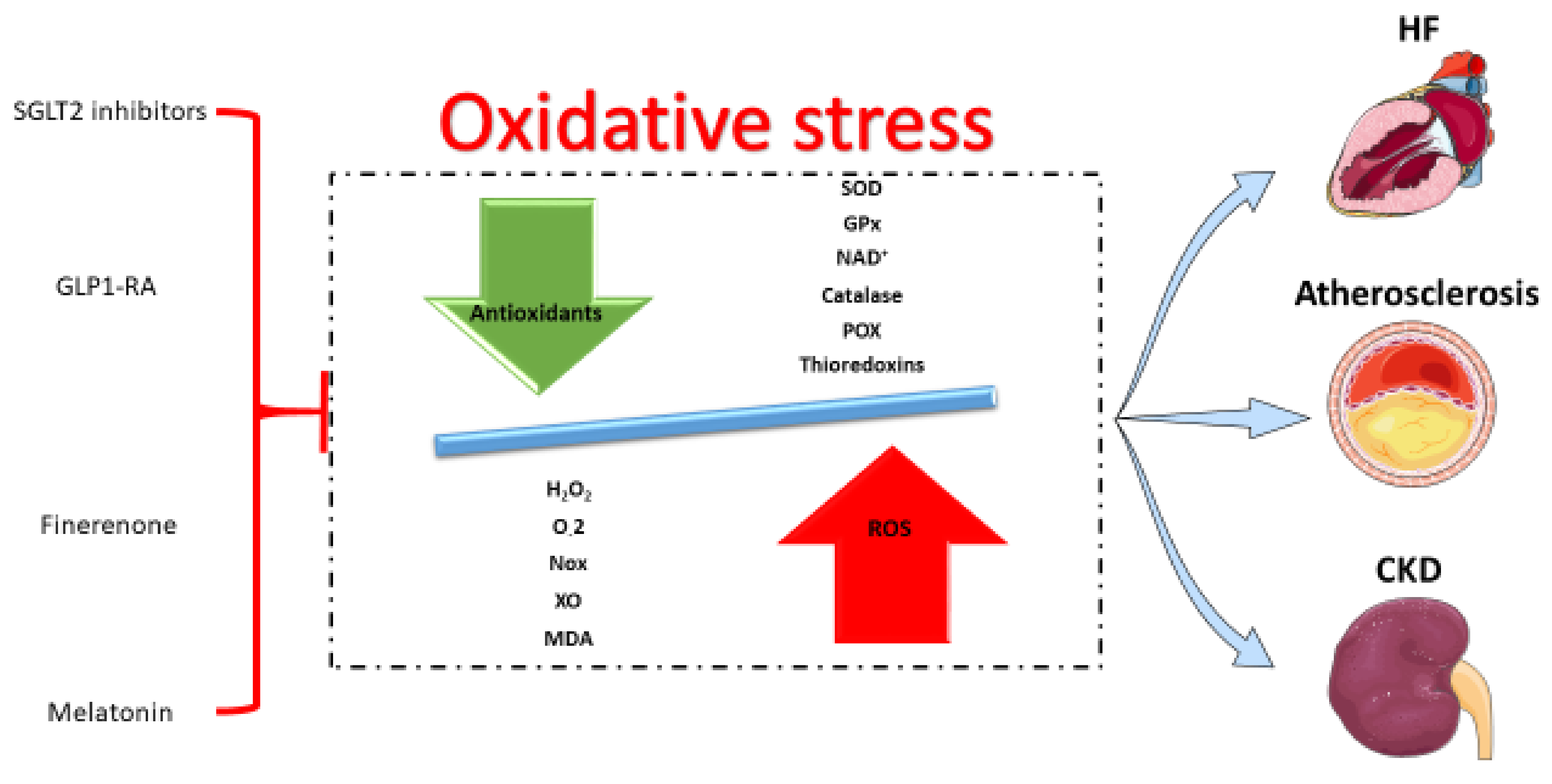
:1. Introduction
2. Oxidative Stress in Cardiorenal Diseases
3. Antioxidant Pharmacotherapies in Cardiorenal Diseases
3.1. SGLT2 Inhibitors
3.2. GLP1 Receptor Agonists
3.3. Finerenone
3.4. Melatonin
4. Clinical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D. Epidemiological Features of Cardiovascular Disease in Asia. JACC 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [Green Version]
- Ago, T.; Kitazono, T.; Ooboshi, H.; Iyama, T.; Han, Y.H.; Takada, J.; Wakisaka, M.; Ibayashi, S.; Utsumi, H.; Iida, M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 2004, 109, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorlach, A.; Brandes, R.P.; Nguyen, K.; Amidi, M.; Dehghani, F.; Busse, R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ. Res. 2000, 87, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Ellmark, S.H.; Dusting, G.J.; Fui, M.N.; Guzzo-Pernell, N.; Drummond, G.R. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc. Res. 2005, 65, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Lassegue, B.; Sorescu, D.; Szocs, K.; Yin, Q.; Akers, M.; Zhang, Y.; Grant, S.L.; Lambeth, J.D.; Griendling, K.K. Novel gp91(phox) homologues in vascular smooth muscle cells: Nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ. Res. 2001, 88, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Sobey, C.G.; Judkins, C.P.; Rivera, J.; Lewis, C.V.; Diep, H.; Lee, H.W.; Kemp-Harper, B.K.; Broughton, B.R.; Selemidis, S.; Gaspari, T.A.; et al. NOX1 deficiency in apolipoprotein E-knockout mice is associated with elevated plasma lipids and enhanced atherosclerosis. Free Radic. Res. 2015, 49, 186–198. [Google Scholar] [CrossRef]
- Douglas, G.; Bendall, J.K.; Crabtree, M.J.; Tatham, A.L.; Carter, E.E.; Hale, A.B.; Channon, K.M. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE(-)/(-) mice. Cardiovasc. Res. 2012, 94, 20–29. [Google Scholar] [CrossRef]
- Violi, F.; Carnevale, R.; Loffredo, L.; Pignatelli, P.; Gallin, J.I. NADPH Oxidase-2 and Atherothrombosis: Insight from Chronic Granulomatous Disease. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Gray, S.P.; Di Marco, E.; Kennedy, K.; Chew, P.; Okabe, J.; El-Osta, A.; Calkin, A.C.; Biessen, E.A.; Touyz, R.M.; Cooper, M.E.; et al. Reactive Oxygen Species Can Provide Atheroprotection via NOX4-Dependent Inhibition of Inflammation and Vascular Remodeling. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 295–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schurmann, C.; Rezende, F.; Kruse, C.; Yasar, Y.; Lowe, O.; Fork, C.; van de Sluis, B.; Bremer, R.; Weissmann, N.; Shah, A.M.; et al. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur. Heart J. 2015, 36, 3447–3456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langbein, H.; Brunssen, C.; Hofmann, A.; Cimalla, P.; Brux, M.; Bornstein, S.R.; Deussen, A.; Koch, E.; Morawietz, H. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Eur. Heart J. 2016, 37, 1753–1761. [Google Scholar] [CrossRef] [Green Version]
- Landmesser, U.; Spiekermann, S.; Preuss, C.; Sorrentino, S.; Fischer, D.; Manes, C.; Mueller, M.; Drexler, H. Angiotensin II induces endothelial xanthine oxidase activation: Role for endothelial dysfunction in patients with coronary disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 943–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNally, J.S.; Davis, M.E.; Giddens, D.P.; Saha, A.; Hwang, J.; Dikalov, S.; Jo, H.; Harrison, D.G. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2290–H2297. [Google Scholar] [CrossRef] [Green Version]
- Battelli, M.G.; Polito, L.; Bolognesi, A. Xanthine oxidoreductase in atherosclerosis pathogenesis: Not only oxidative stress. Atherosclerosis 2014, 237, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Tribble, D.L.; Gong, E.L.; Leeuwenburgh, C.; Heinecke, J.W.; Carlson, E.L.; Verstuyft, J.G.; Epstein, C.J. Fatty streak formation in fat-fed mice expressing human copper-zinc superoxide dismutase. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1734–1740. [Google Scholar] [CrossRef]
- Neves, A.L.; Mohammedi, K.; Emery, N.; Roussel, R.; Fumeron, F.; Marre, M.; Velho, G. Allelic variations in superoxide dismutase-1 (SOD1) gene and renal and cardiovascular morbidity and mortality in type 2 diabetic subjects. Mol. Genet. Metab. 2012, 106, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Mollsten, A.; Jorsal, A.; Lajer, M.; Vionnet, N.; Tarnow, L. The V16A polymorphism in SOD2 is associated with increased risk of diabetic nephropathy and cardiovascular disease in type 1 diabetes. Diabetologia 2009, 52, 2590–2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, H.; Taguchi, J.; Imai, Y.; Ayabe, S.; Hashimoto, H.; Kobayashi, H.; Ogasawara, K.; Aizawa, T.; Yamakado, M.; Nagai, R.; et al. Manganese superoxide dismutase polymorphism affects the oxidized low-density lipoprotein-induced apoptosis of macrophages and coronary artery disease. Eur. Heart J. 2008, 29, 1267–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakko, S.; Paivansalo, M.; Koistinen, P.; Kesaniemi, Y.A.; Kinnula, V.L.; Savolainen, M.J. The signal sequence polymorphism of the MnSOD gene is associated with the degree of carotid atherosclerosis. Atherosclerosis 2003, 168, 147–152. [Google Scholar] [CrossRef]
- Marklund, S.L.; Nilsson, P.; Israelsson, K.; Schampi, I.; Peltonen, M.; Asplund, K. Two variants of extracellular-superoxide dismutase: Relationship to cardiovascular risk factors in an unselected middle-aged population. J. Intern. Med. 1997, 242, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Nemoto, M.; Nishimura, R.; Sasaki, T.; Hiki, Y.; Miyashita, Y.; Nishioka, M.; Fujimoto, K.; Sakuma, T.; Ohashi, T.; Fukuda, K.; et al. Genetic association of glutathione peroxidase-1 with coronary artery calcification in type 2 diabetes: A case control study with multi-slice computed tomography. Cardiovasc. Diabetol. 2007, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Yang, S.; Xu, H.; Liu, D.; Zhang, Y.; Wang, G. Superoxide Dismutase Gene Polymorphism is Associated with Ischemic Stroke Risk in the China Dali Region Han Population. Neurologist 2021, 26, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Heslop, C.L.; Tebbutt, S.J.; Podder, M.; Ruan, J.; Hill, J.S. Combined polymorphisms in oxidative stress genes predict coronary artery disease and oxidative stress in coronary angiography patients. Ann. Hum. Genet. 2012, 76, 435–447. [Google Scholar] [CrossRef]
- Hamanishi, T.; Furuta, H.; Kato, H.; Doi, A.; Tamai, M.; Shimomura, H.; Sakagashira, S.; Nishi, M.; Sasaki, H.; Sanke, T.; et al. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in japanese type 2 diabetic patients. Diabetes 2004, 53, 2455–2460. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.X.; Wang, Z.M.; Zhang, J.J.; Zhu, L.L.; Gao, X.F.; Chen, S.L. Association of glutathione peroxidase-1 (GPx-1) rs1050450 Pro198Leu and Pro197Leu polymorphisms with cardiovascular risk: A meta-analysis of observational studies. J. Geriatr. Cardiol. 2014, 11, 141–150. [Google Scholar] [CrossRef]
- Al-Kateb, H.; Boright, A.P.; Mirea, L.; Xie, X.; Sutradhar, R.; Mowjoodi, A.; Bharaj, B.; Liu, M.; Bucksa, J.M.; Arends, V.L.; et al. Multiple superoxide dismutase 1/splicing factor serine alanine 15 variants are associated with the development and progression of diabetic nephropathy: The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Genetics study. Diabetes 2008, 57, 218–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammedi, K.; Maimaitiming, S.; Emery, N.; Bellili-Munoz, N.; Roussel, R.; Fumeron, F.; Hadjadj, S.; Marre, M.; Velho, G. Allelic variations in superoxide dismutase-1 (SOD1) gene are associated with increased risk of diabetic nephropathy in type 1 diabetic subjects. Mol. Genet. Metab. 2011, 104, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Jerotic, D.; Matic, M.; Suvakov, S.; Vucicevic, K.; Damjanovic, T.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Coric, V.; Stefanovic, A.; Ivanisevic, J.; et al. Association of Nrf2, SOD2 and GPX1 Polymorphisms with Biomarkers of Oxidative Distress and Survival in End-Stage Renal Disease Patients. Toxins 2019, 11, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, C.T.; Huang, J.W.; Chiang, C.K.; Chen, Y.C.; Fang, C.C.; Hu, F.C.; Chang, C.C.; Yen, C.J. Diabetes mellitus, superoxide dismutase and peroxisome proliferator activated receptor gamma polymorphisms modify the outcome of end-stage renal disease patients of Han Chinese origin. Nephrology 2018, 23, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Mohammedi, K.; Bellili-Munoz, N.; Driss, F.; Roussel, R.; Seta, N.; Fumeron, F.; Hadjadj, S.; Marre, M.; Velho, G. Manganese superoxide dismutase (SOD2) polymorphisms, plasma advanced oxidation protein products (AOPP) concentration and risk of kidney complications in subjects with type 1 diabetes. PLoS ONE 2014, 9, e96916. [Google Scholar] [CrossRef] [Green Version]
- Cardona-Sanclemente, L.E.; Born, G.V. Effect of inhibition of nitric oxide synthesis on the uptake of LDL and fibrinogen by arterial walls and other organs of the rat. Br. J. Pharmacol. 1995, 114, 1490–1494. [Google Scholar] [CrossRef] [Green Version]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Binder, C.J.; Papac-Milicevic, N.; Witztum, J.L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 2016, 16, 485–497. [Google Scholar] [CrossRef]
- Lehoux, S. Redox signalling in vascular responses to shear and stretch. Cardiovasc. Res. 2006, 71, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [Green Version]
- Jha, J.C.; Banal, C.; Chow, B.S.; Cooper, M.E.; Jandeleit-Dahm, K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxid. Redox Signal. 2016, 25, 657–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, R.M.; Brown, T.M.; Muntner, P. Epidemiology of obesity, the metabolic syndrome, and chronic kidney disease. Curr. Hypertens. Rep. 2012, 14, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Kang, K.S.; Kwak, M.K. Effect of redox modulating NRF2 activators on chronic kidney disease. Molecules 2014, 19, 12727–12759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gondouin, B.; Jourde-Chiche, N.; Sallee, M.; Dou, L.; Cerini, C.; Loundou, A.; Morange, S.; Berland, Y.; Burtey, S.; Brunet, P.; et al. Plasma Xanthine Oxidase Activity Is Predictive of Cardiovascular Disease in Patients with Chronic Kidney Disease, Independently of Uric Acid Levels. Nephron 2015, 131, 167–174. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Baylis, C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int. 2000, 58, 1261–1266. [Google Scholar] [CrossRef] [Green Version]
- Norris, K.C.; Olabisi, O.; Barnett, M.E.; Meng, Y.X.; Martins, D.; Obialo, C.; Lee, J.E.; Nicholas, S.B. The Role of Vitamin D and Oxidative Stress in Chronic Kidney Disease. Int. J. Environ. Res. Public Health 2018, 15, 2701. [Google Scholar] [CrossRef] [Green Version]
- Noronha, I.L.; Fujihara, C.K.; Zatz, R. The inflammatory component in progressive renal disease—Are interventions possible? Nephrol. Dial. Transplant. 2002, 17, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Karin, M. Is NF-kappaB the sensor of oxidative stress? FASEB J. 1999, 13, 1137–1143. [Google Scholar] [CrossRef]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Simmons, E.M.; Langone, A.; Sezer, M.T.; Vella, J.P.; Recupero, P.; Morrow, J.D.; Ikizler, T.A.; Himmelfarb, J. Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end-stage renal disease patients. Transplantation 2005, 79, 914–919. [Google Scholar] [CrossRef]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubina, P.; Lahera, V.; Luno, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. Suppl. 2008, 74, S4–S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Jhund, P.S.; Solomon, S.D.; Docherty, K.F.; Heerspink, H.J.L.; Anand, I.S.; Bohm, M.; Chopra, V.; de Boer, R.A.; Desai, A.S.; Ge, J.; et al. Efficacy of Dapagliflozin on Renal Function and Outcomes in Patients with Heart Failure with Reduced Ejection Fraction: Results of DAPA-HF. Circulation 2021, 143, 298–309. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Theofilis, P.; Antonopoulos, A.S.; Katsimichas, T.; Oikonomou, E.; Siasos, G.; Aggeli, C.; Tsioufis, K.; Tousoulis, D. The impact of SGLT2 inhibition on imaging markers of cardiac function: A systematic review and meta-analysis. Pharmacol. Res. 2022, 180, 106243. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Ala, M. SGLT2 Inhibition for Cardiovascular Diseases, Chronic Kidney Disease, and NAFLD. Endocrinology 2021, 162, bqab157. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, K.; Tousoulis, D. The impact of SGLT2 inhibitors on inflammation: A systematic review and meta-analysis of studies in rodents. Int. Immunopharmacol. 2022, 111, 109080. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, K.; Tousoulis, D. Pleiotropic effects of SGLT2 inhibitors and heart failure outcomes. Diabetes Res. Clin. Pract. 2022, 188, 109927. [Google Scholar] [CrossRef]
- Uthman, L.; Li, X.; Baartscheer, A.; Schumacher, C.A.; Baumgart, P.; Hermanides, J.; Preckel, B.; Hollmann, M.W.; Coronel, R.; Zuurbier, C.J.; et al. Empagliflozin reduces oxidative stress through inhibition of the novel inflammation/NHE/[Na(+)]c/ROS-pathway in human endothelial cells. Biomed. Pharmacother. 2022, 146, 112515. [Google Scholar] [CrossRef]
- Ashrafi Jigheh, Z.; Ghorbani Haghjo, A.; Argani, H.; Roshangar, L.; Rashtchizadeh, N.; Sanajou, D.; Nazari Soltan Ahmad, S.; Rashedi, J.; Dastmalchi, S.; Mesgari Abbasi, M. Empagliflozin alleviates renal inflammation and oxidative stress in streptozotocin-induced diabetic rats partly by repressing HMGB1-TLR4 receptor axis. Iran. J. Basic Med. Sci. 2019, 22, 384–390. [Google Scholar] [CrossRef]
- Kimura, Y.; Kuno, A.; Tanno, M.; Sato, T.; Ohno, K.; Shibata, S.; Nakata, K.; Sugawara, H.; Abe, K.; Igaki, Y.; et al. Canagliflozin, a sodium-glucose cotransporter 2 inhibitor, normalizes renal susceptibility to type 1 cardiorenal syndrome through reduction of renal oxidative stress in diabetic rats. J. Diabetes Investig. 2019, 10, 933–946. [Google Scholar] [CrossRef]
- Das, N.A.; Carpenter, A.J.; Belenchia, A.; Aroor, A.R.; Noda, M.; Siebenlist, U.; Chandrasekar, B.; DeMarco, V.G. Empagliflozin reduces high glucose-induced oxidative stress and miR-21-dependent TRAF3IP2 induction and RECK suppression, and inhibits human renal proximal tubular epithelial cell migration and epithelial-to-mesenchymal transition. Cell. Signal. 2020, 68, 109506. [Google Scholar] [CrossRef]
- Zaibi, N.; Li, P.; Xu, S.Z. Protective effects of dapagliflozin against oxidative stress-induced cell injury in human proximal tubular cells. PLoS ONE 2021, 16, e0247234. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Mona, M.M.; Abdel-Kareem, M.A.; Elsisy, R.A. SGLT2 inhibitor empagliflozin monotherapy alleviates renal oxidative stress in albino Wistar diabetic rats after myocardial infarction induction. Biomed. Pharmacother. 2021, 139, 111624. [Google Scholar] [CrossRef]
- Hudkins, K.L.; Li, X.; Holland, A.L.; Swaminathan, S.; Alpers, C.E. Regression of diabetic nephropathy by treatment with empagliflozin in BTBR ob/ob mice. Nephrol. Dial. Transplant. 2022, 37, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Ala, M.; Khoshdel, M.R.F.; Dehpour, A.R. Empagliflozin Enhances Autophagy, Mitochondrial Biogenesis, and Antioxidant Defense and Ameliorates Renal Ischemia/Reperfusion in Nondiabetic Rats. Oxid. Med. Cell. Longev. 2022, 2022, 1197061. [Google Scholar] [CrossRef] [PubMed]
- Malinska, H.; Huttl, M.; Markova, I.; Miklankova, D.; Hojna, S.; Papousek, F.; Silhavy, J.; Mlejnek, P.; Zicha, J.; Hrdlicka, J.; et al. Beneficial Effects of Empagliflozin Are Mediated by Reduced Renal Inflammation and Oxidative Stress in Spontaneously Hypertensive Rats Expressing Human C-Reactive Protein. Biomedicines 2022, 10, 2066. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Zhang, J.; Wu, D.; Shi, J.; Kuang, Z.; Ma, Y.; Xu, Q.; Chen, B.; Kan, C.; Sun, X.; et al. Empagliflozin Attenuates Obesity-Related Kidney Dysfunction and NLRP3 Inflammasome Activity through the HO-1-Adiponectin Axis. Front. Endocrinol. 2022, 13, 907984. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.J.; Liu, B.H.; Wan, S.J.; Cheng, Y.; Zhou, S.M.; Sun, Y.; Yao, X.M.; Hua, Q.; Meng, X.J.; Cheng, J.H.; et al. A SGLT2 Inhibitor Dapagliflozin Alleviates Diabetic Cardiomyopathy by Suppressing High Glucose-Induced Oxidative Stress in vivo and in vitro. Front. Pharmacol. 2021, 12, 708177. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.L.; Chu, P.M.; Cheng, H.C.; Huang, Y.T.; Chou, W.C.; Tsai, K.L.; Chan, S.H. Dapagliflozin Mitigates Doxorubicin-Caused Myocardium Damage by Regulating AKT-Mediated Oxidative Stress, Cardiac Remodeling, and Inflammation. Int. J. Mol. Sci. 2022, 23, 10146. [Google Scholar] [CrossRef] [PubMed]
- Bugga, P.; Mohammed, S.A.; Alam, M.J.; Katare, P.; Meghwani, H.; Maulik, S.K.; Arava, S.; Banerjee, S.K. Empagliflozin prohibits high-fructose diet-induced cardiac dysfunction in rats via attenuation of mitochondria-driven oxidative stress. Life Sci. 2022, 307, 120862. [Google Scholar] [CrossRef]
- Li, X.; Flynn, E.R.; do Carmo, J.M.; Wang, Z.; da Silva, A.A.; Mouton, A.J.; Omoto, A.C.M.; Hall, M.E.; Hall, J.E. Direct Cardiac Actions of Sodium-Glucose Cotransporter 2 Inhibition Improve Mitochondrial Function and Attenuate Oxidative Stress in Pressure Overload-Induced Heart Failure. Front. Cardiovasc. Med. 2022, 9, 859253. [Google Scholar] [CrossRef]
- Tsai, K.L.; Hsieh, P.L.; Chou, W.C.; Cheng, H.C.; Huang, Y.T.; Chan, S.H. Dapagliflozin attenuates hypoxia/reoxygenation-caused cardiac dysfunction and oxidative damage through modulation of AMPK. Cell Biosci. 2021, 11, 44. [Google Scholar] [CrossRef]
- Rosa, C.M.; Campos, D.H.S.; Reyes, D.R.A.; Damatto, F.C.; Kurosaki, L.Y.; Pagan, L.U.; Gomes, M.J.; Correa, C.R.; Fernandes, A.A.H.; Okoshi, M.P.; et al. Effects of the SGLT2 Inhibition on Cardiac Remodeling in Streptozotocin-Induced Diabetic Rats, a Model of Type 1 Diabetes Mellitus. Antioxidants 2022, 11, 982. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Liu, H.; Chen, Y.; Li, P.; Liu, L.; Li, J.; Ren, Y.; Huang, J.; Xiong, E.; et al. Empagliflozin Ameliorates Diabetic Cardiomyopathy via Attenuating Oxidative Stress and Improving Mitochondrial Function. Oxid. Med. Cell. Longev. 2022, 2022, 1122494. [Google Scholar] [CrossRef] [PubMed]
- Kolijn, D.; Pabel, S.; Tian, Y.; Lodi, M.; Herwig, M.; Carrizzo, A.; Zhazykbayeva, S.; Kovacs, A.; Fulop, G.A.; Falcao-Pires, I.; et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Galpha oxidation. Cardiovasc. Res. 2021, 117, 495–507. [Google Scholar] [CrossRef]
- El-Shafey, M.; El-Agawy, M.S.E.; Eldosoky, M.; Ebrahim, H.A.; Elsherbini, D.M.A.; El-Sherbiny, M.; Asseri, S.M.; Elsherbiny, N.M. Role of Dapagliflozin and Liraglutide on Diabetes-Induced Cardiomyopathy in Rats: Implication of Oxidative Stress, Inflammation, and Apoptosis. Front. Endocrinol. 2022, 13, 862394. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Wei, W.B.; Li, X.; Huo, J.Y.; Jiang, W.Y.; Wang, H.Y.; Qian, P.; Li, Z.Z.; Zhou, Y.B. The cardioprotective effect of the sodium-glucose cotransporter 2 inhibitor dapagliflozin in rats with isoproterenol-induced cardiomyopathy. Am. J. Transl. Res. 2021, 13, 10950–10961. [Google Scholar]
- Yurista, S.R.; Sillje, H.H.W.; Oberdorf-Maass, S.U.; Schouten, E.M.; Pavez Giani, M.G.; Hillebrands, J.L.; van Goor, H.; van Veldhuisen, D.J.; de Boer, R.A.; Westenbrink, B.D. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur. J. Heart Fail. 2019, 21, 862–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahadian, A.; Fukuda, D.; Salim, H.M.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Sata, M. Canagliflozin Prevents Diabetes-Induced Vascular Dysfunction in ApoE-Deficient Mice. J. Atheroscler. Thromb. 2020, 27, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Lambadiari, V.; Thymis, J.; Kouretas, D.; Skaperda, Z.; Tekos, F.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of a 12-Month Treatment with Glucagon-like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Oxidant and Antioxidant Biomarkers in Patients with Type 2 Diabetes. Antioxidants 2021, 10, 1379. [Google Scholar] [CrossRef]
- Nabrdalik-Lesniak, D.; Nabrdalik, K.; Sedlaczek, K.; Glowczynski, P.; Kwiendacz, H.; Sawczyn, T.; Hajzler, W.; Drozdz, K.; Hendel, M.; Irlik, K.; et al. Influence of SGLT2 Inhibitor Treatment on Urine Antioxidant Status in Type 2 Diabetic Patients: A Pilot Study. Oxid. Med. Cell. Longev. 2021, 2021, 5593589. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Kimura, K.; Peter, N.; Lai, V.; Tse, J.; Cham, L.; Perkins, B.A.; Soleymanlou, N.; Cherney, D.Z.I. Renal and Vascular Effects of Combined SGLT2 and Angiotensin-Converting Enzyme Inhibition. Circulation 2022, 146, 450–462. [Google Scholar] [CrossRef]
- Shimohata, H.; Iwaki, Y.; Yamashita, M.; Ohgi, K.; Maruyama, H.; Takayasu, M.; Hirayama, K.; Kobayashi, M. The effect of sodium-glucose cotransporter 2 inhibitor (tofogliflozin) on renal tubular damage in diabetic patients without albuminuria. Int. Urol. Nephrol. 2022, 54, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Ferreira, J.P.; Butler, J.; Filippatos, G.; Januzzi, J.L.; Sumin, M.; Zwick, M.; Saadati, M.; Pocock, S.J.; Sattar, N.; et al. Effect of Empagliflozin on Circulating Proteomics in Heart Failure: Mechanistic Insights from the EMPEROR Program. Eur. Heart J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Song, L. Glucagon-like peptide-1 (GLP-1) receptor agonists and cardiovascular events in patients with type 2 diabetes mellitus: A meta-analysis of double-blind, randomized, placebo-controlled clinical trials. BMC Endocr. Disord. 2022, 22, 125. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S.; Yu, A.L.; Lo, H.Y.; Lien, C.W.; Lee, J.K.; Chen, W.J. Major adverse cardiovascular and limb events in people with diabetes treated with GLP-1 receptor agonists vs SGLT2 inhibitors. Diabetologia 2022. [Google Scholar] [CrossRef] [PubMed]
- Fu, E.L.; Clase, C.M.; Janse, R.J.; Lindholm, B.; Dekker, F.W.; Jardine, M.J.; Carrero, J.J. Comparative effectiveness of SGLT2i versus GLP1-RA on cardiovascular outcomes in routine clinical practice. Int. J. Cardiol. 2022, 352, 172–179. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Guo, T.; Xiao, G.; Li, Q. Effect of glucagon-like peptide 1 receptor agonists on the renal protection in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2022, 48, 101366. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Longo, M.; Signoriello, S.; Maiorino, M.I.; Solerte, B.; Chiodini, P.; Esposito, K. The effect of DPP-4 inhibitors, GLP-1 receptor agonists and SGLT-2 inhibitors on cardiorenal outcomes: A network meta-analysis of 23 CVOTs. Cardiovasc. Diabetol. 2022, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Liljedahl, L.; Pedersen, M.H.; McGuire, J.N.; James, P. The impact of the glucagon-like peptide 1 receptor agonist liraglutide on the streptozotocin-induced diabetic mouse kidney proteome. Physiol. Rep. 2019, 7, e13994. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Latif, R.G.; Ahmed, A.F.; Heeba, G.H. Low-dose lixisenatide protects against early-onset nephropathy induced in diabetic rats. Life Sci. 2020, 263, 118592. [Google Scholar] [CrossRef]
- Shi, J.X.; Huang, Q. Glucagonlike peptide1 protects mouse podocytes against high glucoseinduced apoptosis, and suppresses reactive oxygen species production and proinflammatory cytokine secretion, through sirtuin 1 activation in vitro. Mol. Med. Rep. 2018, 18, 1789–1797. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.S.; Wu, Z.; Zhang, Z.; Xiong, Z.Y.; Chen, H.; Huang, Q.B. Glucagon-like peptide-1 inhibits the receptor for advanced glycation endproducts to prevent podocyte apoptosis induced by advanced oxidative protein products. Biochem. Biophys. Res. Commun. 2017, 482, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Baylan, U.; Korn, A.; Emmens, R.W.; Schalkwijk, C.G.; Niessen, H.W.M.; Krijnen, P.A.J.; Simsek, S. Liraglutide treatment attenuates inflammation markers in the cardiac, cerebral and renal microvasculature in streptozotocin-induced diabetic rats. Eur. J. Clin. Investig. 2022, 52, e13807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, C.; Zhu, Q.; Li, N.; Zhou, H. Liraglutide, a glucagon-like peptide-1 analog, inhibits high glucose-induced oxidative stress and apoptosis in neonatal rat cardiomyocytes. Exp. Ther. Med. 2019, 17, 3734–3740. [Google Scholar] [CrossRef]
- Nuamnaichati, N.; Parichatikanond, W.; Mangmool, S. Cardioprotective Effects of Glucagon-like Peptide-1 (9-36) Against Oxidative Injury in H9c2 Cardiomyoblasts: Potential Role of the PI3K/Akt/NOS Pathway. J. Cardiovasc. Pharmacol. 2022, 79, e50–e63. [Google Scholar] [CrossRef]
- Shiraki, A.; Oyama, J.; Komoda, H.; Asaka, M.; Komatsu, A.; Sakuma, M.; Kodama, K.; Sakamoto, Y.; Kotooka, N.; Hirase, T.; et al. The glucagon-like peptide 1 analog liraglutide reduces TNF-alpha-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis 2012, 221, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Qiao, Y.; Zhang, L.; Pan, Q. Protective Role of Glucagon-like Peptide-1 against High-Glucose-Induced Endothelial Oxidative Damage. Medicine 2015, 94, e2055. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, L.; Guo, Y.; Lin, F.; Chen, H.; Chen, W.; Chen, M. Effect of Glucagon-like Peptide-1 on High-Glucose-induced Oxidative Stress and Cell Apoptosis in Human Endothelial Cells and Its Underlying Mechanism. J. Cardiovasc. Pharmacol. 2015, 66, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Mari, A.; DeVries, J.H.; Urva, S.; Li, J.; Pratt, E.J.; Coskun, T.; Thomas, M.K.; Mather, K.J.; Haupt, A.; et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: A multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol. 2022, 10, 418–429. [Google Scholar] [CrossRef]
- Karagiannis, T.; Avgerinos, I.; Liakos, A.; Del Prato, S.; Matthews, D.R.; Tsapas, A.; Bekiari, E. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: A systematic review and meta-analysis. Diabetologia 2022, 65, 1251–1261. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Inagaki, N.; Takeuchi, M.; Oura, T.; Imaoka, T.; Seino, Y. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): A double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022, 10, 623–633. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, F.; Li, G.; Tao, Y. Novel dual glucagon-like peptide-1/glucose-dependent insulinotropic polypeptide receptor agonist attenuates diabetes and myocardial injury through inhibiting hyperglycemia, inflammation and oxidative stress in rodent animals. Bioengineered 2022, 13, 9184–9196. [Google Scholar] [CrossRef] [PubMed]
- Lachaux, M.; Barrera-Chimal, J.; Nicol, L.; Remy-Jouet, I.; Renet, S.; Dumesnil, A.; Wecker, D.; Richard, V.; Kolkhof, P.; Jaisser, F.; et al. Short- and long-term administration of the non-steroidal mineralocorticoid receptor antagonist finerenone opposes metabolic syndrome-related cardio-renal dysfunction. Diabetes Obes. Metab. 2018, 20, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Blazquez, R.; Somoza, B.; Gil-Ortega, M.; Martin Ramos, M.; Ramiro-Cortijo, D.; Vega-Martin, E.; Schulz, A.; Ruilope, L.M.; Kolkhof, P.; Kreutz, R.; et al. Finerenone Attenuates Endothelial Dysfunction and Albuminuria in a Chronic Kidney Disease Model by a Reduction in Oxidative Stress. Front. Pharmacol. 2018, 9, 1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.S.; Yoon, Y.M.; Go, G.; Lee, J.H.; Lee, S.H. Melatonin Protects Human Renal Proximal Tubule Epithelial Cells against High Glucose-Mediated Fibrosis via the Cellular Prion Protein-TGF-beta-Smad Signaling Axis. Int. J. Med. Sci. 2020, 17, 1235–1245. [Google Scholar] [CrossRef]
- Ebaid, H.; Bashandy, S.A.E.; Abdel-Mageed, A.M.; Al-Tamimi, J.; Hassan, I.; Alhazza, I.M. Folic acid and melatonin mitigate diabetic nephropathy in rats via inhibition of oxidative stress. Nutr. Metab. 2020, 17, 6. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Hu, F.; Cao, X.; Luo, L.; Tu, Q. Melatonin receptor protects cardiomyocyte against oxidative stress-induced apoptosis through the MAPK-ERK signaling pathway. J. Recept. Signal Transduct. 2020, 40, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, Y.B.; Tosun, V.; Guntekin, U. Melatonin protects against streptozotocin-induced diabetic cardiomyopathy through the mammalian target of rapamycin (mTOR) signaling pathway. Adv. Clin. Exp. Med. 2019, 28, 1171–1177. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Novials, A.; Ortega, E.; Canivell, S.; La Sala, L.; Pujadas, G.; Esposito, K.; Giugliano, D.; Genovese, S. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care 2013, 36, 2346–2350. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Guo, S.; Li, H.; Liu, X.Y. Effects of glucagon-like peptide-1 receptor agonists (GLP-1RAs) on podocytes, inflammation, and oxidative stress in patients with diabetic nephropathy (DN). Pak. J. Med. Sci. 2022, 38, 1170–1174. [Google Scholar] [CrossRef]
- Bray, J.J.H.; Foster-Davies, H.; Salem, A.; Hoole, A.L.; Obaid, D.R.; Halcox, J.P.J.; Stephens, J.W. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomised controlled trials. Diabetes Obes. Metab. 2021, 23, 1806–1822. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Pitt, B.; Kober, L.; Ponikowski, P.; Gheorghiade, M.; Filippatos, G.; Krum, H.; Nowack, C.; Kolkhof, P.; Kim, S.Y.; Zannad, F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur. Heart J. 2013, 34, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Gueret, A.; Harouki, N.; Favre, J.; Galmiche, G.; Nicol, L.; Henry, J.P.; Besnier, M.; Thuillez, C.; Richard, V.; Kolkhof, P.; et al. Vascular Smooth Muscle Mineralocorticoid Receptor Contributes to Coronary and Left Ventricular Dysfunction after Myocardial Infarction. Hypertension 2016, 67, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Chimal, J.; Andre-Gregoire, G.; Nguyen Dinh Cat, A.; Lechner, S.M.; Cau, J.; Prince, S.; Kolkhof, P.; Loirand, G.; Sauzeau, V.; Hauet, T.; et al. Benefit of Mineralocorticoid Receptor Antagonism in AKI: Role of Vascular Smooth Muscle Rac1. J. Am. Soc. Nephrol. 2017, 28, 1216–1226. [Google Scholar] [CrossRef] [Green Version]
- Theofilis, P.; Vordoni, A.; Kalaitzidis, R.G. The Role of Melatonin in Chronic Kidney Disease and Its Associated Risk Factors: A New Tool in Our Arsenal? Am. J. Nephrol. 2022, 53, 565–574. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.H.; Jeon, E.J.; Leem, J.; Park, K.K. Melatonin Prevents Transforming Growth Factor-beta1-Stimulated Transdifferentiation of Renal Interstitial Fibroblasts to Myofibroblasts by Suppressing Reactive Oxygen Species-Dependent Mechanisms. Antioxidants 2020, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Hajam, Y.A.; Rai, S.; Pandi-Perumal, S.R.; Brown, G.M.; Reiter, R.J.; Cardinali, D.P. Coadministration of Melatonin and Insulin Improves Diabetes-Induced Impairment of Rat Kidney Function. Neuroendocrinology 2021, 112, 807–822. [Google Scholar] [CrossRef]
- Arinno, A.; Maneechote, C.; Khuanjing, T.; Ongnok, B.; Prathumsap, N.; Chunchai, T.; Arunsak, B.; Kerdphoo, S.; Shinlapawittayatorn, K.; Chattipakorn, S.C.; et al. Cardioprotective effects of melatonin and metformin against doxorubicin-induced cardiotoxicity in rats are through preserving mitochondrial function and dynamics. Biochem. Pharmacol. 2021, 192, 114743. [Google Scholar] [CrossRef]
- Ciric Zdravkovic, S.; Kostic, T.; Marcetic, Z.P.; Sulovic, L.S.; Nedeljkovic, B.M.; Preljevic, A.; Toskic, D.; Sokolovic, D. Melatonin modulates acute cardiac muscle damage induced by carbon tetrachloride—Involvement of oxidative damage, glutathione, and arginine and nitric oxide metabolism. Can. J. Physiol. Pharmacol. 2021, 99, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Durdagi, G.; Pehlivan, D.Y.; Oyar, E.O.; Bahceci, S.A.; Ozbek, M. Effects of Melatonin and Adrenomedullin in Reducing the Cardiotoxic Effects of Doxorubicin in Rats. Cardiovasc. Toxicol. 2021, 21, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liang, S.; Zhang, J.; Du, Z.; Xu, Q.; Duan, J.; Sun, Z. Melatonin ameliorates PM2.5-induced cardiac perivascular fibrosis through regulating mitochondrial redox homeostasis. J. Pineal Res. 2021, 70, e12686. [Google Scholar] [CrossRef]
- Lan, H.; Su, Y.; Liu, Y.; Deng, C.; Wang, J.; Chen, T.; Jules, K.E.D.; Masau, J.F.; Li, H.; Wei, X. Melatonin protects circulatory death heart from ischemia/reperfusion injury via the JAK2/STAT3 signalling pathway. Life Sci. 2019, 228, 35–46. [Google Scholar] [CrossRef]
- Ma, X.; Wang, S.; Cheng, H.; Ouyang, H.; Ma, X. Melatonin Attenuates Ischemia/Reperfusion-Induced Oxidative Stress by Activating Mitochondrial Fusion in Cardiomyocytes. Oxid. Med. Cell. Longev. 2022, 2022, 7105181. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Fan, Z.; Zhang, S.; Qiao, R.; Liu, Y.; Yang, J.; Yang, L.; Wang, H. Cardioprotective effects of melatonin against myocardial ischaemia/reperfusion injury: Activation of AMPK/Nrf2 pathway. J. Cell. Mol. Med. 2021, 25, 6455–6459. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yang, Y.; Cui, B.; Lin, D.; Wang, Z.; Ma, J. Temporal effect of melatonin posttreatment on anoxia/reoxygenation injury in H9c2 cells. Cell Biol. Int. 2022, 46, 637–648. [Google Scholar] [CrossRef]
- Singhanat, K.; Apaijai, N.; Sumneang, N.; Maneechote, C.; Arunsak, B.; Chunchai, T.; Chattipakorn, S.C.; Chattipakorn, N. Therapeutic potential of a single-dose melatonin in the attenuation of cardiac ischemia/reperfusion injury in prediabetic obese rats. Cell. Mol. Life Sci. 2022, 79, 300. [Google Scholar] [CrossRef]
- Han, D.; Wang, Y.; Chen, J.; Zhang, J.; Yu, P.; Zhang, R.; Li, S.; Tao, B.; Wang, Y.; Qiu, Y.; et al. Activation of melatonin receptor 2 but not melatonin receptor 1 mediates melatonin-conferred cardioprotection against myocardial ischemia/reperfusion injury. J. Pineal Res. 2019, 67, e12571. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Bao, M.; Chen, R.; Li, H.; Lu, B.; Chen, M.; Huang, D.; Zhang, Y.; Gao, F.; et al. Melatonin Attenuates Diabetic Myocardial Microvascular Injury through Activating the AMPK/SIRT1 Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 8882130. [Google Scholar] [CrossRef]
- Yang, Y.; Du, J.; Xu, R.; Shen, Y.; Yang, D.; Li, D.; Hu, H.; Pei, H.; Yang, Y. Melatonin alleviates angiotensin-II-induced cardiac hypertrophy via activating MICU1 pathway. Aging 2020, 13, 493–515. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Barros, M.P.; Silva, C.M.D.; Borges, L.D.S.; Hatanaka, E.; Lambertucci, R.H. Melatonin improves the antioxidant capacity in cardiac tissue of Wistar rats after exhaustive exercise. Free Radic. Res. 2021, 55, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Ma, Y.; Xiang, A.; Sun, H.; Song, J.; Yang, W.; Li, X.; Xu, H. Melatonin protected against myocardial infarction injury in rats through a Sirt6-dependent antioxidant pathway. Adv. Clin. Exp. Med. 2022, 31, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Satari, M.; Bahmani, F.; Reiner, Z.; Soleimani, A.; Aghadavod, E.; Kheiripour, N.; Asemi, Z. Metabolic and Anti-inflammatory Response to Melatonin Administration in Patients with Diabetic Nephropathy. Iran. J. Kidney Dis. 2021, 1, 22–30. [Google Scholar]
- Ostadmohammadi, V.; Soleimani, A.; Bahmani, F.; Aghadavod, E.; Ramezani, R.; Reiter, R.J.; Mansournia, M.A.; Banikazemi, Z.; Soleimani, M.; Zaroudi, M.; et al. The Effects of Melatonin Supplementation on Parameters of Mental Health, Glycemic Control, Markers of Cardiometabolic Risk, and Oxidative Stress in Diabetic Hemodialysis Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Ren. Nutr. 2020, 30, 242–250. [Google Scholar] [CrossRef]
- Panah, F.; Ghorbanihaghjo, A.; Argani, H.; Haiaty, S.; Rashtchizadeh, N.; Hosseini, L.; Dastmalchi, S.; Rezaeian, R.; Alirezaei, A.; Jabarpour, M.; et al. The effect of oral melatonin on renal ischemia-reperfusion injury in transplant patients: A double-blind, randomized controlled trial. Transpl. Immunol. 2019, 57, 101241. [Google Scholar] [CrossRef]
- Hoseini, S.G.; Heshmat-Ghahdarijani, K.; Khosrawi, S.; Garakyaraghi, M.; Shafie, D.; Mansourian, M.; Roohafza, H.; Azizi, E.; Sadeghi, M. Melatonin supplementation improves N-terminal pro-B-type natriuretic peptide levels and quality of life in patients with heart failure with reduced ejection fraction: Results from MeHR trial, a randomized clinical trial. Clin. Cardiol. 2022, 45, 417–426. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Baez-Ferrer, N.; Reiter, R.J.; Avanzas, P.; Hernandez-Vaquero, D. Melatonin and Cardioprotection in Humans: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2021, 8, 635083. [Google Scholar] [CrossRef]
- Lv, T.; Yan, J.; Lou, Y.; Zhang, Z.; Ye, M.; Zhou, J.; Luo, F.; Bi, C.; Lin, H.; Zhang, J.; et al. Evaluation of Melatonin Therapy in Patients with Myocardial Ischemia-Reperfusion Injury: A Systematic Review and Meta-Analysis. Oxid. Med. Cell. Longev. 2022, 2022, 4610522. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.H.; Cho, J.H.; Lee, H.; Yim, H.W.; Yoon, K.H.; Kim, H.S. Discontinuation rate and reason for discontinuation after sodium-glucose cotransporter 2 inhibitor prescription in real clinical practice. J. Clin. Pharm. Ther. 2020, 45, 1271–1277. [Google Scholar] [CrossRef]
- Trujillo, J.M.; Nuffer, W.; Smith, B.A. GLP-1 receptor agonists: An updated review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018821997320. [Google Scholar] [CrossRef] [PubMed]
- Meili-Butz, S.; Niermann, T.; Fasler-Kan, E.; Barbosa, V.; Butz, N.; John, D.; Brink, M.; Buser, P.T.; Zaugg, C.E. Dimethyl fumarate, a small molecule drug for psoriasis, inhibits Nuclear Factor-kappaB and reduces myocardial infarct size in rats. Eur. J. Pharmacol. 2008, 586, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Sun, Q.; Zhao, H.; Tao, J.; Yan, D. The Effects of Dimethyl Fumarate on Atherosclerosis in the Apolipoprotein E-Deficient Mouse Model with Streptozotocin-Induced Hyperglycemia Mediated By the Nuclear Factor Erythroid 2-Related Factor 2/Antioxidant Response Element (Nrf2/ARE) Signaling Pathway. Med. Sci. Monit. 2019, 25, 7966–7975. [Google Scholar] [CrossRef] [PubMed]
- Ashari, S.; Naghsh, N.; Salari, Y.; Barghi, N.G.; Bagheri, A. Dimethyl Fumarate Attenuates Di-(2-Ethylhexyl) Phthalate-Induced Nephrotoxicity through the Nrf2/HO-1 and NF-kappaB Signaling Pathways. Inflammation 2022. [Google Scholar] [CrossRef]
- Zhen, X.; Jindong, L.; Yang, Z.; Yashi, R.; Wei, G.; Wei, J.; Wei, Z.; Sudong, L. Activation of Nrf2 Pathway by Dimethyl Fumarate Attenuates Renal Ischemia-Reperfusion Injury. Transplant. Proc. 2021, 53, 2133–2139. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, M.; Yi, S.; Tang, Y.; Luo, H.; Xiao, Q.; Xiao, J.; Li, Y. Dimethyl fumarate ameliorates endotoxin-induced acute kidney injury against macrophage oxidative stress. Ren. Fail. 2021, 43, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Takasu, C.; Vaziri, N.D.; Li, S.; Robles, L.; Vo, K.; Takasu, M.; Pham, C.; Liu, S.; Farzaneh, S.H.; Foster, C.E., 3rd; et al. Treatment with Dimethyl Fumarate Attenuates Calcineurin Inhibitor-induced Nephrotoxicity. Transplantation 2015, 99, 1144–1150. [Google Scholar] [CrossRef]
- Zeng, S.Y.; Yang, L.; Yan, Q.J.; Gao, L.; Lu, H.Q.; Yan, P.K. Nox1/4 dual inhibitor GKT137831 attenuates hypertensive cardiac remodelling associating with the inhibition of ADAM17-dependent proinflammatory cytokines-induced signalling pathways in the rats with abdominal artery constriction. Biomed. Pharmacother. 2019, 109, 1907–1914. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, N.; Zhang, Z.; Wang, F.; Xiao, J.; Ji, X. Setanaxib (GKT137831) Ameliorates Doxorubicin-Induced Cardiotoxicity by Inhibiting the NOX1/NOX4/Reactive Oxygen Species/MAPK Pathway. Front. Pharmacol. 2022, 13, 823975. [Google Scholar] [CrossRef]
- Zhao, Q.D.; Viswanadhapalli, S.; Williams, P.; Shi, Q.; Tan, C.; Yi, X.; Bhandari, B.; Abboud, H.E. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFkappaB signaling pathways. Circulation 2015, 131, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Gray, S.P.; Jha, J.C.; Kennedy, K.; van Bommel, E.; Chew, P.; Szyndralewiez, C.; Touyz, R.M.; Schmidt, H.; Cooper, M.E.; Jandeleit-Dahm, K.A.M. Combined NOX1/4 inhibition with GKT137831 in mice provides dose-dependent reno- and atheroprotection even in established micro- and macrovascular disease. Diabetologia 2017, 60, 927–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Liu, H.; Fang, Y.; Lin, P.; Lu, Z.; Zhang, P.; Jiao, X.; Teng, J.; Ding, X.; Dai, Y. Salvianolate ameliorates oxidative stress and podocyte injury through modulation of NOX4 activity in db/db mice. J. Cell. Mol. Med. 2021, 25, 1012–1023. [Google Scholar] [CrossRef]
- Gorin, Y.; Cavaglieri, R.C.; Khazim, K.; Lee, D.Y.; Bruno, F.; Thakur, S.; Fanti, P.; Szyndralewiez, C.; Barnes, J.L.; Block, K.; et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Renal. Physiol. 2015, 308, F1276–F1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, J.C.; Gray, S.P.; Barit, D.; Okabe, J.; El-Osta, A.; Namikoshi, T.; Thallas-Bonke, V.; Wingler, K.; Szyndralewiez, C.; Heitz, F.; et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1237–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marco, E.; Gray, S.P.; Chew, P.; Koulis, C.; Ziegler, A.; Szyndralewiez, C.; Touyz, R.M.; Schmidt, H.H.; Cooper, M.E.; Slattery, R.; et al. Pharmacological inhibition of NOX reduces atherosclerotic lesions, vascular ROS and immune-inflammatory responses in diabetic Apoe(-/-) mice. Diabetologia 2014, 57, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Jeong, S.; Lee, Y.; Jeon, C.; Kwon, G.; Kim, S.; Lee, D. H2O2-Activatable Antioxidant Polymeric Prodrug Nanoparticles for the Prevention of Renal Ischemia/Reperfusion Injury. Biomacromolecules 2022, 23, 3810–3821. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Park, M.; Kang, C.; Dilmen, S.; Kang, T.H.; Kang, D.G.; Ke, Q.; Lee, S.U.; Lee, D.; Kang, P.M. Hydrogen Peroxide-Responsive Nanoparticle Reduces Myocardial Ischemia/Reperfusion Injury. J. Am. Heart Assoc. 2016, 5, e003697. [Google Scholar] [CrossRef]
- Altshuler, P.J.; Schiazza, A.R.; Luo, L.; Helmers, M.R.; Chhay, B.; Han, J.J.; Hu, R.; Herbst, D.A.; Tsourkas, A.; Cheng, Z.; et al. Superoxide Dismutase-Loaded Nanoparticles Attenuate Myocardial Ischemia-Reperfusion Injury and Protect against Chronic Adverse Ventricular Remodeling. Adv. Ther. 2021, 4, 2100036. [Google Scholar] [CrossRef]
- Seshadri, G.; Sy, J.C.; Brown, M.; Dikalov, S.; Yang, S.C.; Murthy, N.; Davis, M.E. The delivery of superoxide dismutase encapsulated in polyketal microparticles to rat myocardium and protection from myocardial ischemia-reperfusion injury. Biomaterials 2010, 31, 1372–1379. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Mathew, A.P.; Uthaman, S.; Vasukutty, A.; Kim, I.J.; Suh, S.H.; Kim, C.S.; Ma, S.K.; Graham, S.A.; Kim, S.W.; et al. Inflammation-sensing catalase-mimicking nanozymes alleviate acute kidney injury via reversing local oxidative stress. J. Nanobiotechnol. 2022, 20, 205. [Google Scholar] [CrossRef]
| Study | Experimental Model | Disease Type | SGLT2 Inhibitor | SGLT2 Inhibitor Effect |
|---|---|---|---|---|
| Ashrafi Jigheh et al. [66] | Wistar rats | DM | Empagliflozin |
↓ renal MDA ↑ renal SOD and GPx |
| Kimura et al. [67] | OLETF rats | DM | Canagliflozin | ↓ renal MDA, 4HNE, Nox2, and Nox4 |
| Das et al. [68] | Proximal tubular epithelial cells | High glucose | Empagliflozin | ↓ O− 2 and H2O2 generation |
| Zaibi et al. [69] | Human proximal tubular cells | H2O2-induced injury | Dapagliflozin | ↓ cytosolic ROS production |
| Ahmed et al. [70] | Wistar rats | DM post-MI | Empagliflozin | ↓ renal Nox2 and Nox4 mRNA |
| Hudkins et al. [71] | BTBR ob/ob mice | DN | Empagliflozin |
↓ urinary markers of RNA/DNA damage ↓ carbonyl oxidation in situ |
| Ala et al. [72] | Wistar rats | Renal IR injury | Empagliflozin | ↓ renal MDA |
| Malinska et al. [73] | Spontaneously hypertensive rats | Inflammation | Empagliflozin |
↑ renal GPx, CAT, GSH ↓ renal CD, TBARS |
| Ye et al. [74] | C57BL/6J mice | Obesity | Empagliflozin | ↑ heme oxygenase-1 |
| Study | Experimental Model | Disease Type | SGLT2 Inhibitor | SGLT2 Inhibitor Effect |
|---|---|---|---|---|
| Xing et al. [75] | Sprague-Dawley rats Cardiac myoblasts H9C2 | DM | Dapagliflozin |
↓ myocardial MDA, Cu/Zn SOD ↓ cardiomyoblast H2O2, ↑ cardiomyoblast Cu/Zn-SOD expression and total SOD activity |
| Hsieh et al. [76] | Cardiac myoblast H9C2 | Doxorubicin-induced injury | Dapagliflozin |
↑ heme oxygenase-1 and NADPH quinone oxidoreductase ↑ SOD activity |
| Bugga et al. [77] | Sprague-Dawley rats | DM | Empagliflozin | ↓ total cellular and mitochondrial ROS |
| Li et al. [78] | C57Bl/6J | Pressure Overload-Induced HF | Empagliflozin | ↑ heme oxygenase-1, NRF-2, catalase ↓ O2-, H2O2 |
| Tsai et al. [79] | Cardiac myoblasts H9C2 Primary cardiomyocytes | Cardiac IR injury | Dapagliflozin |
↓ NADPH activity ↓ ROS formation |
| Rosa et al. [80] | Wistar rats | DM | Dapagliflozin |
↓ lipid hydroperoxide ↑ SOD, GPx |
| Wang et al. [81] | db/db mice Cardiac myoblasts H9C2 | DM | Empagliflozin |
↓ cardiac 4HNE and 3-nitrotyrosine ↓ cardiac total cellular and mitochondrial ROS ↓ cardiomyoblast total cellular and mitochondrial ROS |
| Kolijn et al. [82] | Human cardiomyocytes ZDF rats | HFpEF | Empagliflozin |
↓ H2O2, 3-nitrotyrosine ↑ glutathione |
| El-Shafey et al. [83] | Sprague-Dawley rats | DM | Dapagliflozin |
↓ myocardial MDA ↑ myocardial glutathione, catalase |
| Li et al. [84] | KK-Ay mice | DM | Empagliflozin |
↓ myocardial lipid hydroperoxide, MDA, Nox4 ↑ myocardial GPx, SOD |
| Wang et al. [85] | Sprague-Dawley rats | Isoproterenol-induced cardiomyopathy | Dapagliflozin |
↓ myocardial Nox2, MDA, ROS, NADPH activity, |
| Yurista et al. [86] | Sprague-Dawley rats | Post-MI HF | Empagliflozin | ↓ AOPP, Nox2 |
| Uthman et al. [65] | HUVECs HCAECs | TNFα-induced endothelial dysfunction | Empagliflozin | ↓ HUVEC and HCAEC ROS production |
| Rahadian et al. [87] | ApoE−/− mice | DM | Canagliflozin |
↓ aortic Nox2, p22phox ↓ urinary 8-hydroxydeoxyguanosine |
| Study | Experimental Model | Disease Type | Agent | Antioxidant Effect |
|---|---|---|---|---|
| Liljedahl et al. [98] | 129SV mice | DM | Liraglutide | ↑ renal catalase, GPx |
| Abdel-Latif et al. [99] | Wistar rats | DM | Lixisenatide |
↓ renal MDA and total Nox ↑ total antioxidant capacity |
| Baylan et al. [102] | Sprague-Dawley rats | DM | Liraglutide | ↓ atrial and ventricular Nox2 |
| Nuamnaichati et al. [104] | H9C2 cells | H2O2 | GLP1 analogue | ↑ GPx, catalase, heme oxygenase-1 |
| Zhang et al. [103] | Neonatal cardiomyocyte | DM + inflammation | Liraglutide |
↓ MDA ↑ SOD activity |
| Lachaux et al. [113] | Zucker fa/fa rats | Metabolic syndrome | Finerenone |
↓ myocardial ROS ↑ NO bioavailability |
| González-Blázquez et al. [114] | Munich Wistar Frömter rats | CKD | Finerenone |
↑ aortic Mn-SOD and Cu/Zn-SOD ↑ renal total SOD activity |
| Han et al. [115] | Human renal proximal tubule epithelial cells | DM | Melatonin | ↑ catalase and SOD activity |
| Ebaid et al. [116] | Albino rats | DN | Melatonin |
↓ renal MDA ↑ GSH, SOD, catalase |
| Li et al. [117] | H9C2 cells | H2O2 toxicity | Melatonin | ↑ GSH, GPx, SOD |
| Kandemir et al. [118] | Wistar rats | DM | Melatonin | ↑ cardiac SOD, catalase, GPx |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theofilis, P.; Vordoni, A.; Kalaitzidis, R.G. Oxidative Stress Management in Cardiorenal Diseases: Focus on Novel Antidiabetic Agents, Finerenone, and Melatonin. Life 2022, 12, 1663. https://doi.org/10.3390/life12101663
Theofilis P, Vordoni A, Kalaitzidis RG. Oxidative Stress Management in Cardiorenal Diseases: Focus on Novel Antidiabetic Agents, Finerenone, and Melatonin. Life. 2022; 12(10):1663. https://doi.org/10.3390/life12101663
Chicago/Turabian StyleTheofilis, Panagiotis, Aikaterini Vordoni, and Rigas G. Kalaitzidis. 2022. "Oxidative Stress Management in Cardiorenal Diseases: Focus on Novel Antidiabetic Agents, Finerenone, and Melatonin" Life 12, no. 10: 1663. https://doi.org/10.3390/life12101663
APA StyleTheofilis, P., Vordoni, A., & Kalaitzidis, R. G. (2022). Oxidative Stress Management in Cardiorenal Diseases: Focus on Novel Antidiabetic Agents, Finerenone, and Melatonin. Life, 12(10), 1663. https://doi.org/10.3390/life12101663







