Isolation and Characterization of Primary DMD Pig Muscle Cells as an In Vitro Model for Preclinical Research on Duchenne Muscular Dystrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
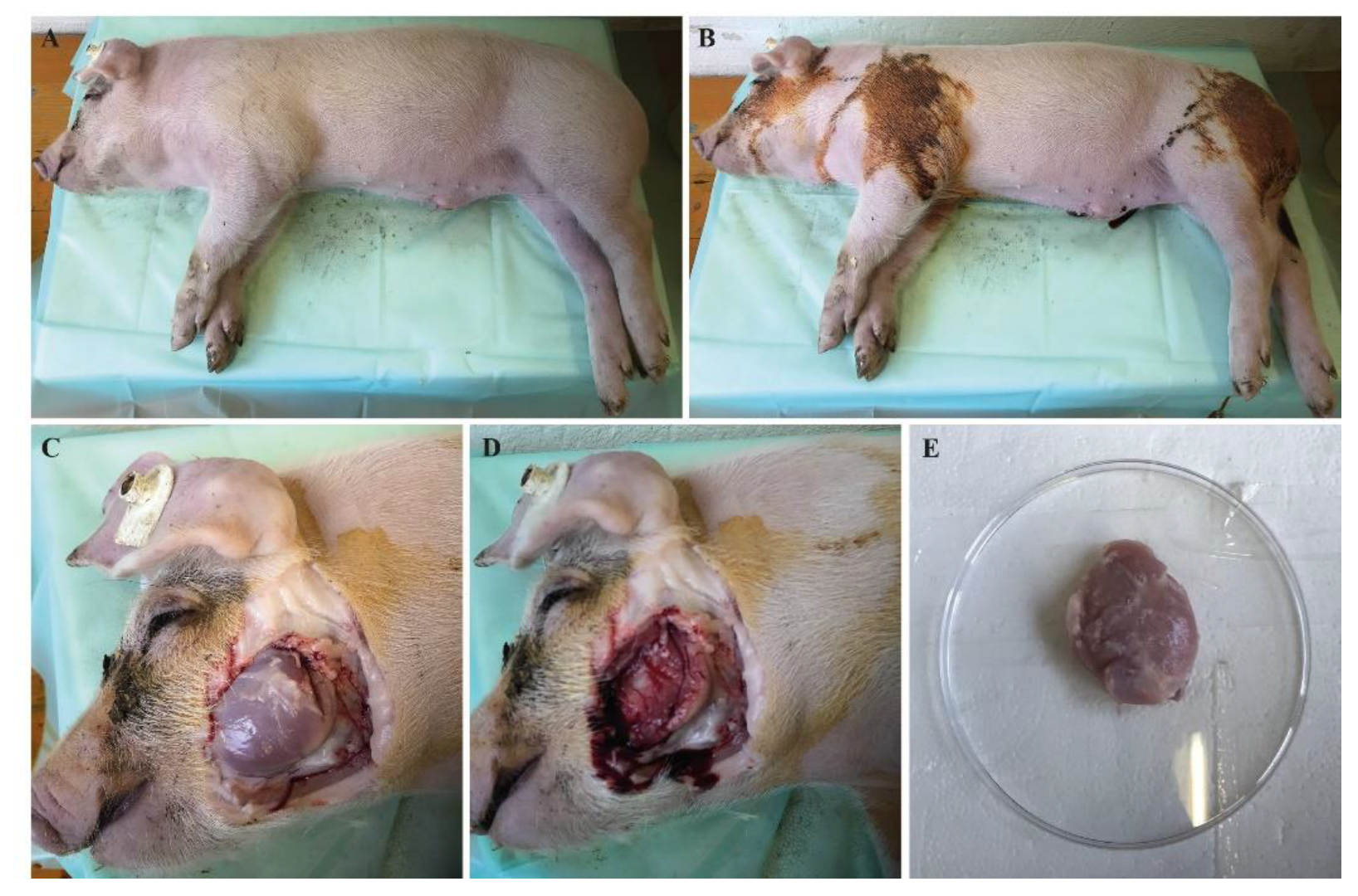
2.2. Isolation of Different Muscle Groups
2.3. Isolation of Satellite Cells
- Using a laminar flow cabinet, solution A was removed from the sample.
- Muscle tissue was separated from connective and fat tissue using a sterile scalpel.
- Then, remaining muscle was weighed, a minimum of 3 g, but if possible, 5–20 g of muscle were used for each primary culture.
- The piece of muscle was cut into very small pieces.
- The cut muscle pieces were magnetically stirred for 20 min at 37 °C in a 500 mL Schott® bottle in 25 mL of Hank’s balanced salt solution (HBSS) containing 0.2% collagenase I, 0.01% DNase I, and 0.025% trypsin.
- Following, the bottle was chilled in an ice bath for 2 min.
- The supernatant was transferred into fresh tubes and mixed with an equal amount (1:1) of growth medium (α-MEM, 10% FCS, 1% Glutamax, 0.2% amphotericin B, 0.2% gentamicin). For the remaining muscle piece, the steps from the magnetic stirring to the transfer of the supernatant were repeated two times.
- This mix was filtered through a 100 µm micro strainer and then centrifuged (800× g, 10 min, 4 °C).
- The supernatant was discarded and the pellet was resuspended in 15 mL of growth medium (4 °C).
- The suspension was then filtered through a 70 µm micro strainer and centrifuged (800× g, 10 min, 4 °C).
- The supernatant was discarded and the pellet resuspended in 12 mL of PBS.
- The suspension was slowly and carefully given on top of a step gradient [(50-mL tube containing 3 mL of a 60% Percoll-solution (Easycoll and PBS, lower layer) and 35 mL of a 20% Percoll-solution (upper layer)].
- This 50 mL tube was centrifuged (4800× g, 45 min, 4 °C, no brake)
- The boundary layer between the 20% and the 60% Percoll-solution was taken out by a sterile Pasteur pipette (approx. 8–12 mL), transferred into a new sterile tube, and mixed with 15 mL of growth medium. This suspension was centrifuged (500× g, 10 min, 4 °C), the supernatant was discarded, and the pellet resuspended in 10 mL growth medium.
- Depending on the size of the pellet, approx. 1 mL cell-pellet was transferred with 18 mL of additional growth medium into one 75 cm2 cell culture bottle.
- The newly harvested satellite cells were grown in growth medium in a 37 °C incubator with 5% CO2 for 24 h. The growth medium was changed after one day.
- A mycoplasma infection test was made regularly with the cells, using the MycoAlert™ Mycoplasma Detection Kit (LT07-118, Lonza, Basel, Switzerland).
2.4. Cell Growth, Storage, and Differentiation
2.5. Western Blot
2.6. Immunofluorescence Staining
3. Results
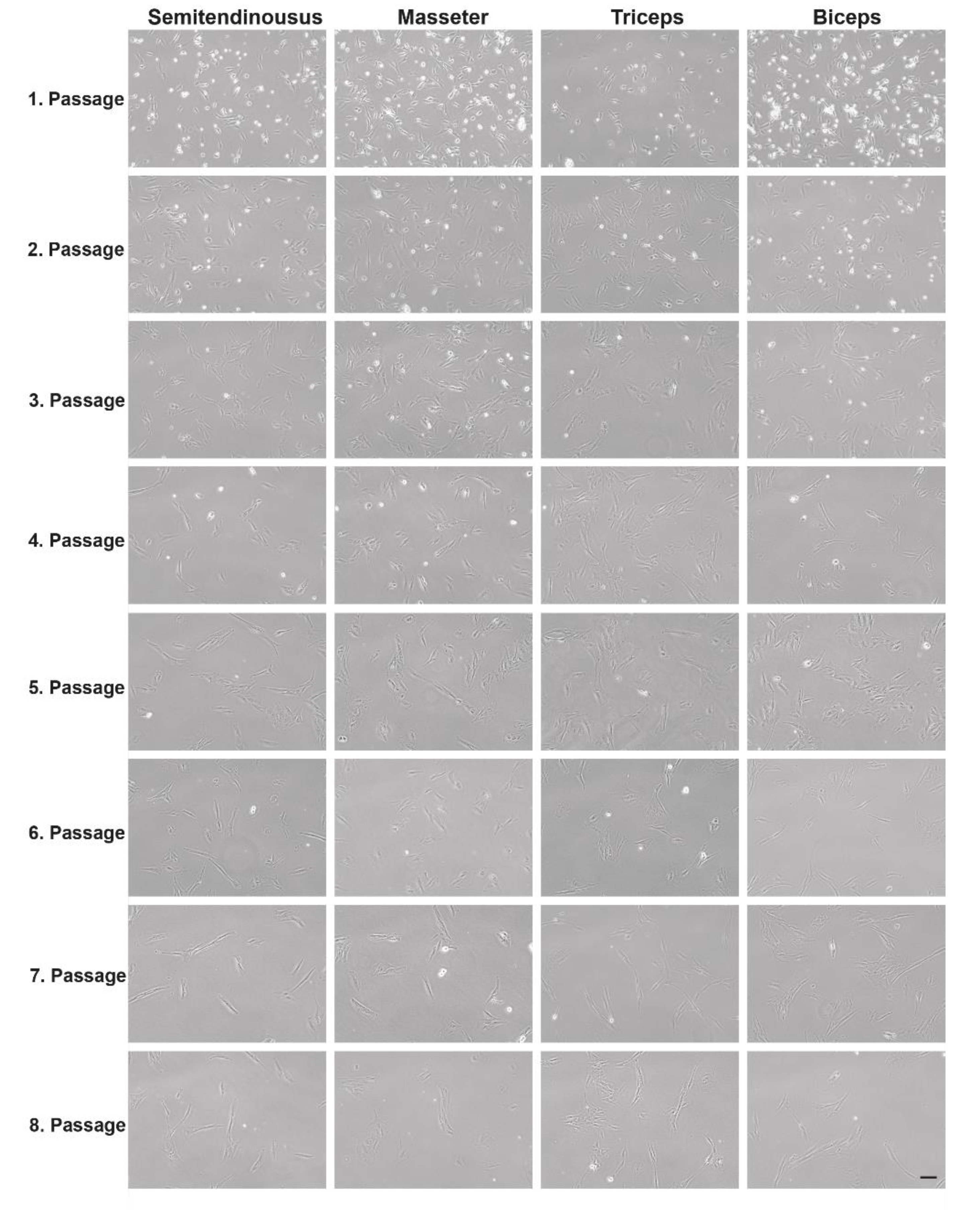
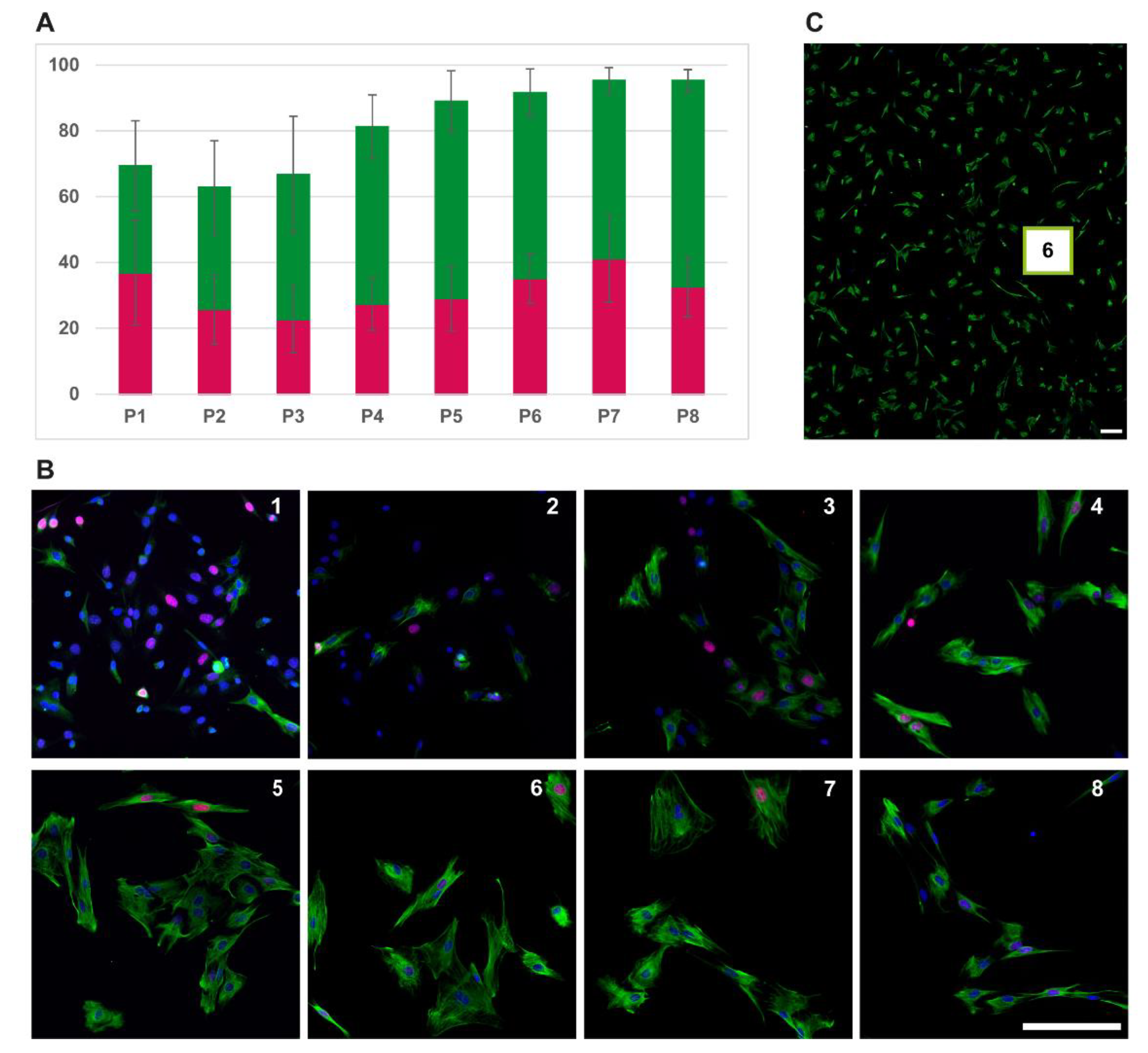
3.1. Characterization of Cell Growth and Myogenic Content
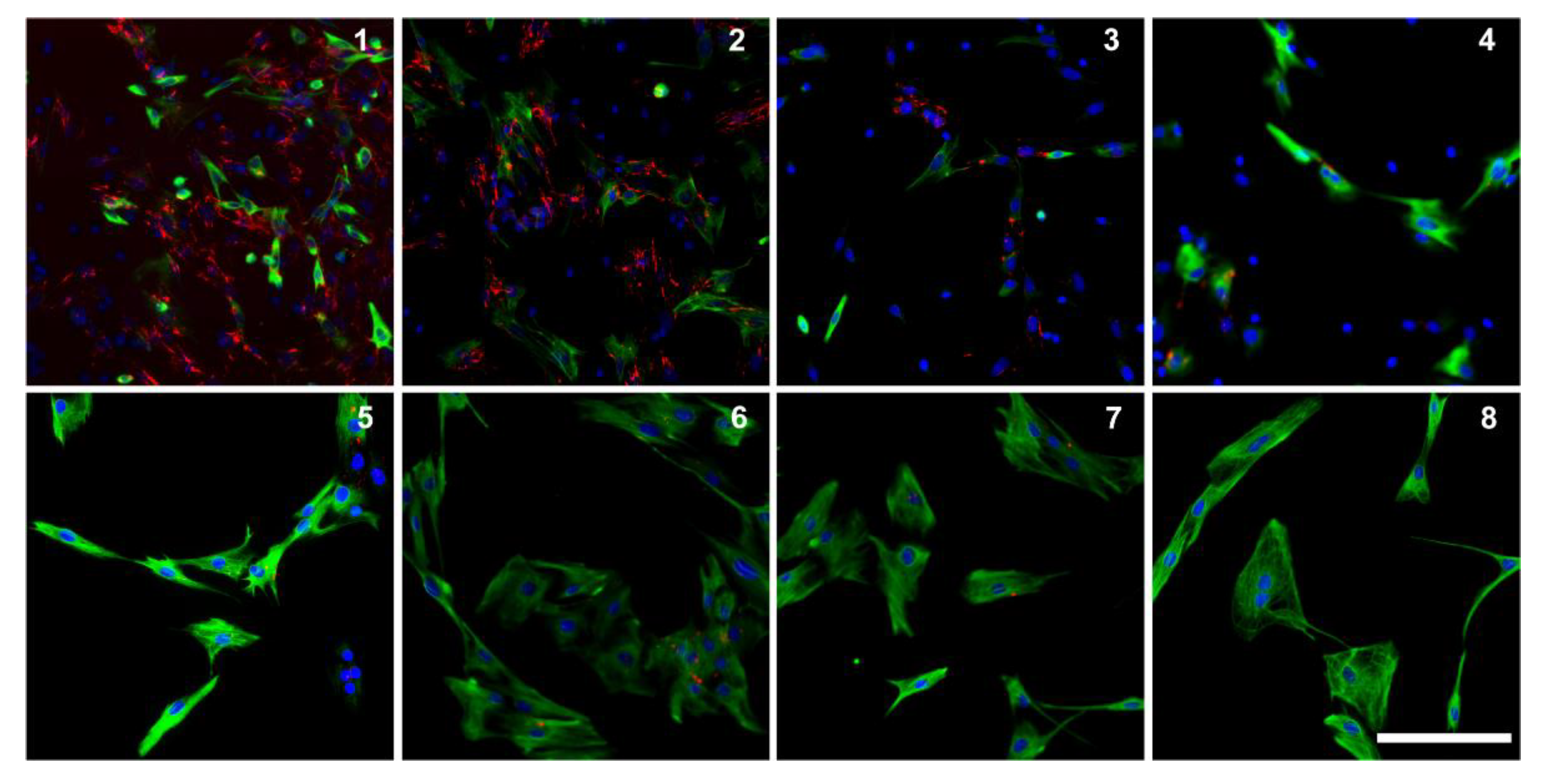
3.2. Differentiation into Myotubes
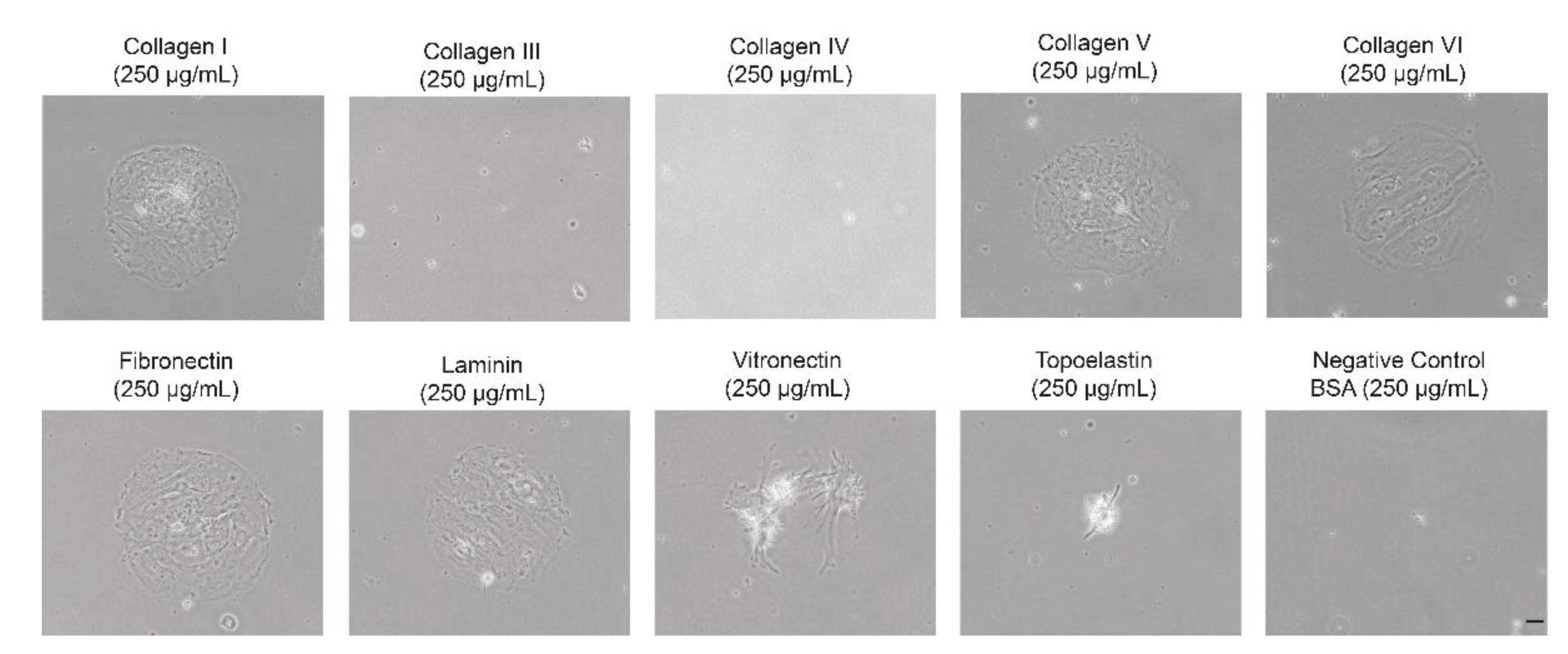
3.3. Coating of Dishes
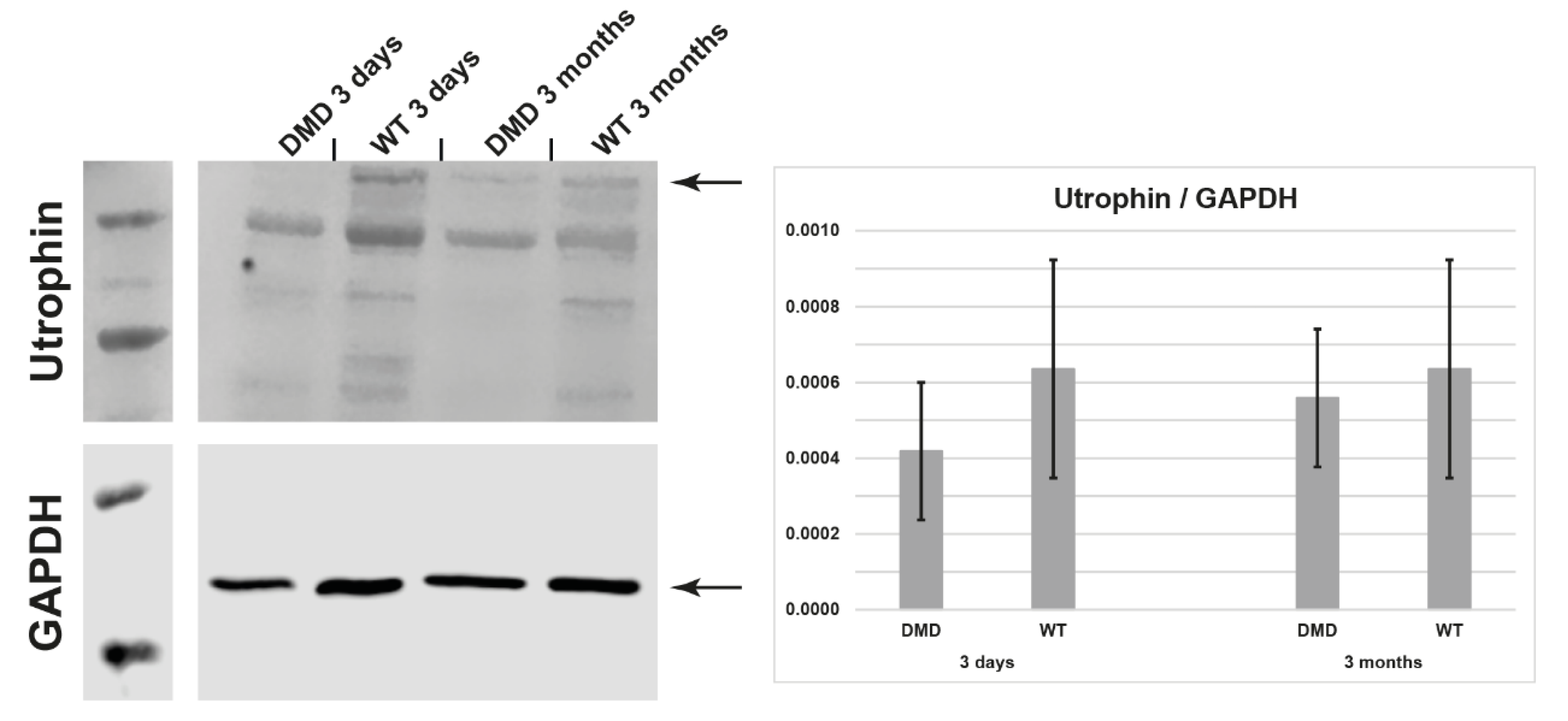
3.4. Utrophin Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, L.E.; Clemens, P.R.; Hadjiyannakis, S.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018, 17, 251–267. [Google Scholar] [CrossRef]
- Walter, M.C.; Reilich, P. Recent developments in Duchenne muscular dystrophy: Facts and numbers. J. Cachexia Sarcopenia Muscle 2017, 8, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Kupatt, C.; Windisch, A.; Moretti, A.; Wolf, E.; Wurst, W.; Walter, M.C. Genome editing for Duchenne muscular dystrophy: A glimpse of the future? Gene Ther. 2021, 28, 542–548. [Google Scholar] [CrossRef]
- Fortunato, F.; Rossi, R.; Falzarano, M.; Ferlini, A. Innovative Therapeutic Approaches for Duchenne Muscular Dystrophy. J. Clin. Med. 2021, 10, 820. [Google Scholar] [CrossRef]
- Pramono, Z.A.; Takeshima, Y.; Alimsardjono, H.; Ishii, A.; Takeda, S.I.; Matsuo, M. Induction of exon skipping of the dystrophin transcript in lymphoblastoid cells by transfecting an antisense oligodeoxynucleotide complementary to an exon recognition sequence. Biochem. Biophys. Res. Commun. 1996, 226, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; Barohn, R.J.; Bertini, E.; Chabrol, B.; Comi, G.P.; Darras, B.T.; Finkel, R.S.; Flanigan, K.M.; Goemans, N.; Iannaccone, S.T.; et al. Meta-analyses of ataluren randomized controlled trials in nonsense mutation Duchenne muscular dystrophy. J. Comp. Eff. Res. 2020, 9, 973–984. [Google Scholar] [CrossRef]
- Ramos, J.N.; Hollinger, K.; Bengtsson, N.E.; Allen, J.M.; Hauschka, S.D.; Chamberlain, J.S. Development of Novel Micro-dystrophins with Enhanced Functionality. Mol. Ther. 2019, 27, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Amoasii, L.; Mireault, A.A.; McAnally, J.R.; Li, H.; Sanchez-Ortiz, E.; Bhattacharyya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 2016, 351, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Amoasii, L.; Long, C.; Li, H.; Mireault, A.A.; Shelton, J.M.; Sanchez-Ortiz, E.; McAnally, J.R.; Bhattacharyya, S.; Schmidt, F.; Grimm, D.; et al. Single-cut genome editing restores dystrophin expression in a new mouse model of muscular dystrophy. Sci. Transl. Med. 2017, 9, eaan8081. [Google Scholar] [CrossRef]
- Babbs, A.; Chatzopoulou, M.; Edwards, B.; Squire, S.E.; Wilkinson, I.V.; Wynne, G.M.; Russell, A.J.; Davies, K.E. From diagnosis to therapy in Duchenne muscular dystrophy. Biochem. Soc. Trans. 2020, 48, 813–821. [Google Scholar] [CrossRef]
- Stirm, M.; Fonteyne, L.M.; Shashikadze, B.; Stöckl, J.B.; Kurome, M.; Keßler, B.; Zakhartchenko, V.; Kemter, E.; Blum, H.; Arnold, G.J.; et al. Pig models for Duchenne muscular dystrophy—From disease mechanisms to validation of new diagnostic and therapeutic concepts. Neuromuscul. Disord. 2022, 32, 543–556. [Google Scholar] [CrossRef]
- Klymiuk, N.; Blutke, A.; Graf, A.; Krause, S.; Burkhardt, K.; Wuensch, A.; Krebs, S.; Kessler, B.; Zakhartchenko, V.; Kurome, M.; et al. Dystrophin-deficient pigs provide new insights into the hierarchy of physiological derangements of dystrophic muscle. Hum. Mol. Genet. 2013, 22, 4368–4382. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Conway, K.M.; Fapo, O.; Street, N.; Mathews, K.D.; Mann, J.R.; Romitti, P.A.; Soim, A.; Westfield, C.; Fox, D.J.; et al. Time to diagnosis of Duchenne muscular dystrophy remains unchanged: Findings from the Muscular Dystrophy Surveillance, Tracking, and Research Network, 2000–2015. Muscle Nerve 2022, 66, 193–197. [Google Scholar] [CrossRef]
- Nguyen, Q.; Yokota, T. Immortalized Muscle Cell Model to Test the Exon Skipping Efficacy for Duchenne Muscular Dystrophy. J. Pers. Med. 2017, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Soblechero-Martín, P.; Albiasu-Arteta, E.; Anton-Martinez, A.; de la Puente-Ovejero, L.; Garcia-Jimenez, I.; González-Iglesias, G.; Larrañaga-Aiestaran, I.; López-Martínez, A.; Poyatos-García, J.; Ruiz-Del-Yerro, E.; et al. Duchenne muscular dystrophy cell culture models created by CRISPR/Cas9 gene editing and their application in drug screening. Sci. Rep. 2021, 11, 18188. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, H.; Sato, M.; Aoki, Y. Exon Skipping in Directly Reprogrammed Myotubes Obtained from Human Urine-Derived Cells. J. Vis. Exp. 2020, 7, e60840. [Google Scholar] [CrossRef]
- Kodaka, Y.; Rabu, G.; Asakura, A. Skeletal Muscle Cell Induction from Pluripotent Stem Cells. Stem Cells Int. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Sugihara, H.; Teramoto, N.; Nakamura, K.; Shiga, T.; Shirakawa, T.; Matsuo, M.; Ogasawara, M.; Nishino, I.; Matsuwaki, T.; Nishihara, M.; et al. Cellular senescence-mediated exacerbation of Duchenne muscular dystrophy. Sci. Rep. 2020, 10, 16385. [Google Scholar] [CrossRef]
- Stirm, M.; Fonteyne, L.M.; Shashikadze, B.; Lindner, M.; Chirivi, M.; Lange, A.; Kaufhold, C.; Mayer, C.; Medugorac, I.; Kessler, B.; et al. A scalable, clinically severe pig model for Duchenne muscular dystrophy. Dis. Model. Mech. 2021, 14, dmm049285. [Google Scholar] [CrossRef]
- Metzger, K.; Tuchscherer, A.; Palin, M.-F.; Ponsuksili, S.; Kalbe, C. Establishment and validation of cell pools using primary muscle cells derived from satellite cells of pig skeletal muscle. Vitr. Cell. Dev. Biol.-Anim. 2019, 56, 193–199. [Google Scholar] [CrossRef]
- Mau, M.; Oksbjerg, N.; Rehfeldt, C. Establishment and conditions for growth and differentiation of a myoblast cell line derived from the semimembranosus muscle of newborn piglets. Vitr. Cell. Dev. Biol.-Anim. 2007, 44, 504–518. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Srsen, V.; Fant, X.; Heald, R.; Rabouille, C.; Merdes, A. Centrosome proteins form an insoluble perinuclear matrix during muscle cell differentiation. BMC Cell Biol. 2009, 10, 28. [Google Scholar] [CrossRef]
- Isaacs, W.B.; Fulton, A.B. Cotranslational assembly of myosin heavy chain in developing cultured skeletal muscle. Proc. Natl. Acad. Sci. USA 1987, 84, 6174–6178. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, J.M.; Potter, A.C.; Phelps, S.R.; Fisher, R.; Trickett, J.I.; Davies, K.E. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature 1996, 384, 349–353. [Google Scholar] [CrossRef]
- Zaynitdinova, M.I.; Lavrov, A.V.; Smirnikhina, S.A. Animal models for researching approaches to therapy of Duchenne muscular dystrophy. Transgenic Res. 2021, 30, 709–725. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Subramaniyan, S.A.; Park, J.; Kang, D.; Khan, M.; Belal, S.A.; Lee, S.C.; Shim, K. Modulatory effects of cell–cell interactions between porcine skeletal muscle satellite cells and fibroblasts on the expression of myogenesis-related genes. J. Appl. Anim. Res. 2022, 50, 259–268. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donandt, T.; Hintze, S.; Krause, S.; Wolf, E.; Schoser, B.; Walter, M.C.; Meinke, P. Isolation and Characterization of Primary DMD Pig Muscle Cells as an In Vitro Model for Preclinical Research on Duchenne Muscular Dystrophy. Life 2022, 12, 1668. https://doi.org/10.3390/life12101668
Donandt T, Hintze S, Krause S, Wolf E, Schoser B, Walter MC, Meinke P. Isolation and Characterization of Primary DMD Pig Muscle Cells as an In Vitro Model for Preclinical Research on Duchenne Muscular Dystrophy. Life. 2022; 12(10):1668. https://doi.org/10.3390/life12101668
Chicago/Turabian StyleDonandt, Tina, Stefan Hintze, Sabine Krause, Eckhard Wolf, Benedikt Schoser, Maggie C. Walter, and Peter Meinke. 2022. "Isolation and Characterization of Primary DMD Pig Muscle Cells as an In Vitro Model for Preclinical Research on Duchenne Muscular Dystrophy" Life 12, no. 10: 1668. https://doi.org/10.3390/life12101668
APA StyleDonandt, T., Hintze, S., Krause, S., Wolf, E., Schoser, B., Walter, M. C., & Meinke, P. (2022). Isolation and Characterization of Primary DMD Pig Muscle Cells as an In Vitro Model for Preclinical Research on Duchenne Muscular Dystrophy. Life, 12(10), 1668. https://doi.org/10.3390/life12101668







