Nystatin Effectiveness in Oral Candidiasis Treatment: A Systematic Review & Meta-Analysis of Clinical Trials
Abstract
:1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Database and Search Strategies
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
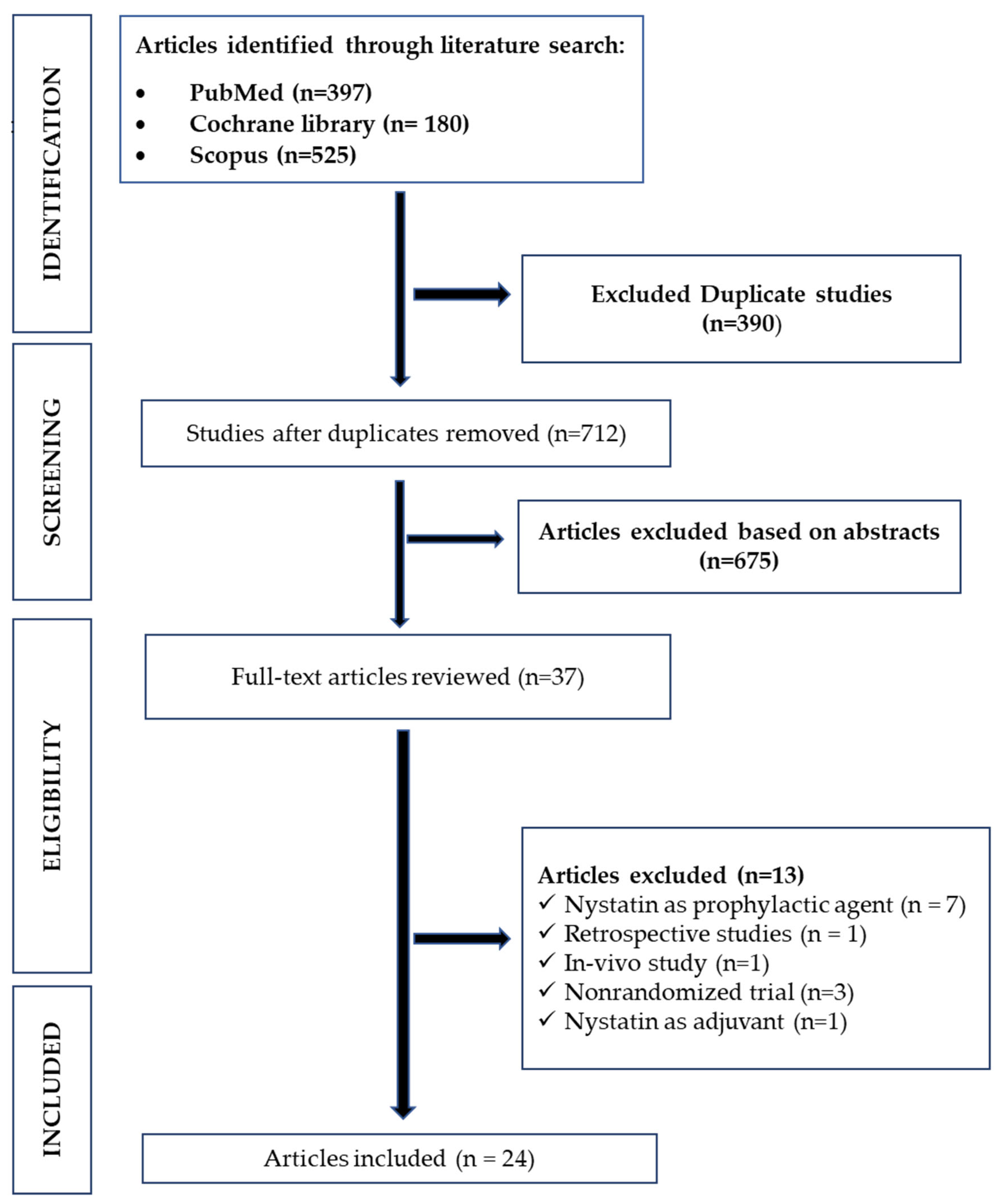
3.1. Databases Search Results
3.2. Characteristics of the Included Studies
3.3. Risk of Bias and Quality of the Included Studies
3.4. Potency Evaluation
3.5. Duration, Dosage, Formulations and Adverse Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuganbaev, T.; Yoshida, K.; Honda, K. The effects of oral microbiota on health. Science 2022, 376, 934–936. [Google Scholar] [CrossRef]
- Baker, J.L.; Bor, B.; Agnello, M.; Shi, W.; He, X. Ecology of the Oral Microbiome: Beyond Bacteria. Trends Microbiol. 2017, 25, 362–374. [Google Scholar] [CrossRef] [Green Version]
- Arkell, S.; Shinnick, A. Update on oral candidosis. Nurs. Times 2003, 99, 52–53. [Google Scholar] [PubMed]
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef]
- Sherman, R.G.; Prusinski, L.; Ravenel, M.C.; Joralmon, R.A. Oral Candidosis. Quintessence Int. 2001, 33, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, M.D.; Warnock, D.W. Fungal Infection: Diagnosis and Management; Blackwell Publishing: Oxford, UK, 2012. [Google Scholar]
- Bulad, K.; Taylor, R.L.; Verran, J.; McCord, J.F. Colonization and penetration of denture soft lining materials by Candida albicans. Dent. Mater. 2004, 20, 167–175. [Google Scholar] [CrossRef]
- Nowakowska-Toporowska, A.; Raszewski, Z.; Wieckiewicz, W. Color change of soft silicone relining materials after storage in artificial saliva. J. Prosthet. Dent. 2016, 115, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.D.; Jones, B.L. Therapeutic Guidelines in Systemic Fungal Infections, 3rd ed.; Remedica Publishing: London, UK, 2003. [Google Scholar]
- Pankhurst, C.L. Candidiasis (Oropharyngeal). BMJ Clin. Evid. 2013, 2013, 1304. [Google Scholar]
- Krishnan, P. Fungal infections of the oral mucosa. Indian J. Dent. Res. 2012, 23, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Taudorf, E.; Jemec, G.; Hay, R.; Saunte, D.M.L. Cutaneous candidiasis—An evidence-based review of topical and systemic treatments to inform clinical practice. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1863–1873. [Google Scholar] [CrossRef]
- Samaranayake, L.P.; Leung, W.K.; Jin, L. Oral mucosal fungal infections. Periodontology 2000 2009, 49, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Hoare, A.; Marsh, P.D.; Diaz, P.I. Ecological Therapeutic Opportunities for Oral Diseases. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Sakaguchi, H. Treatment and Prevention of Oral Candidiasis in Elderly Patients. Med. Mycol. J. 2017, 58, J43–J49. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.A.O.; Williams, D.W. Diagnosis and management of oral candidosis. Br. Dent. J. 2017, 223, 675–681. [Google Scholar] [CrossRef]
- Xiao, Y.; Yuan, P.; Sun, Y.; Xu, Y.; Deng, X.; Wang, X.; Liu, R.; Chen, Q.; Jiang, L. Comparison of topical antifungal agents for oral candidiasis treatment: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Lu, X.-L.; Mounmin, F.A. Diagnosis and Treatment of Esophageal Candidiasis: Current Updates. Can. J. Gastroenterol. Hepatol. 2019, 2019, 3585136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Černáková, L.; Rodrigues, C.F. Microbial interactions and immunity response in oral Candida species. Futur. Microbiol. 2020, 15, 1653–1677. [Google Scholar] [CrossRef]
- Akpan, A. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Quindos, G.; Gil-Alonso, S.; Marcos-Arias, C.; Sevillano, E.; Mateo, E.; Jauregizar, N.; Eraso, E. Therapeutic tools for oral candidiasis: Current and new antifungal drugs. Med. Oral Patol. Oral Y Cir. Buccal 2019, 24, e172–e180. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Huang, B.; Ding, Z. Efficacy of antifungal drugs in the treatment of oral candidiasis: A Bayesian network meta-analysis. J. Prosthet. Dent. 2021, 125, 257–265. [Google Scholar] [CrossRef]
- Mundula, T.; Ricci, F.; Barbetta, B.; Baccini, M.; Amedei, A. Effect of Probiotics on Oral Candidiasis: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, X.; Zhao, C.; Yan, Z.M.; Hua, H. Efficacy of Nystatin for the Treatment of Oral Candidiasis: A Systematic Review and Meta-Analysis. Drug Des. Devel. Ther. 2016, 10, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Sayáns, M.; Beiro-Fuentes, R.; Otero-Rey, E.M.; Chamorro-Petronacci, C.M.; Gándara-Vila, P.; Somoza-Martín, J.M.; García-García, A.; Blanco-Carrión, A. Efficacy of different formulations of nystatin in an experimental model of oral candidiasis in sialoadenectomized rats. J. Dent. Sci. 2021, 16, 123–130. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Afroozi, B.; Zomorodian, K.; Lavaee, F.; Shahrabadi, Z.Z.; Mardani, M. Comparison of the efficacy of indocyanine green-mediated photodynamic therapy and nystatin therapy in treatment of denture stomatitis. Photodiagnosis Photodyn. Ther. 2019, 27, 193–197. [Google Scholar] [CrossRef]
- Alrabiah, M.; Alsahhaf, A.; Alofi, R.S.; Al-Aali, K.A.; Abduljabbar, T.; Vohra, F. Efficacy of photodynamic therapy versus local nystatin in the treatment of denture stomatitis: A randomized clinical study. Photodiagnosis Photodyn. Ther. 2019, 28, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Carmello, J.C.; Alonso, G.C.; Mima, E.G.D.O.; Bagnato, V.S.; Pavarina, A.C. A randomized clinical trial evaluating Photodithazine-mediated Antimicrobial Photodynamic Therapy as a treatment for Denture stomatitis. Photodiagnosis Photodyn. Ther. 2020, 32, 102041. [Google Scholar] [CrossRef]
- Bakhshi, M.; Taheri, J.-B.; Basir Shabestari, S.; Tanik, A.; Pahlevan, R. Comparison of therapeutic effect of aqueous extract of garlic and nystatin mouthwash in denture stomatitis. Gerodontology 2012, 29, e680–e684. [Google Scholar] [CrossRef] [PubMed]
- Gonoudi, E.; Rezai, M.; Farrokhnia, T.; Goudarzi, M.; Sima, A. Comparison of Antifungal Efficacy of Zataria Multiflora and Nystatin for Treatment of Denture Stomatitis: A Randomized Clinical Trial. J. Dent. 2021, 22, 60–66. [Google Scholar] [CrossRef]
- Labban, N.; Al Taweel, S.M.; Alrabiah, M.A.; Alfouzan, A.F.; Alshiddi, I.F.; Assery, M.K. Efficacy of Rose Bengal and Curcumin mediated photodynamic therapy for the treatment of denture stomatitis in patients with habitual cigarette smoking: A randomized controlled clinical trial. Photodiagnosis Photodyn. Ther. 2021, 35, 102380. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Q.; Liu, C.; Lin, M.; Li, X.; Xiao, X.; Zhu, Z.; Gong, Q.; Zhou, H. Efficacy and safety of probiotics in the treatment ofCandida-associated stomatitis. Mycoses 2014, 57, 141–146. [Google Scholar] [CrossRef]
- de Araújo, M.R.C.; Maciel, P.P.; Castellano, L.R.C.; Bonan, P.R.F.; Alves, D.D.N.; de Medeiros, A.C.D.; de Castro, R.D. Efficacy of essential oil of cinnamon for the treatment of oral candidiasis: A randomized trial. Spéc. Care Dent. 2021, 41, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Mima, E.G.D.O.; Colombo, A.L.; Sanitá, P.V.; Jorge, J.H.; Massucato, E.M.S.; Vergani, C.E. Comparison of denture microwave disinfection and conventional antifungal therapy in the treatment of denture stomatitis: A randomized clinical study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 469–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nairn, R. Nystatin and amphotericin B in the treatment of denture-related candidiasis. Oral Surg. Oral Med. Oral Pathol. 1975, 40, 68–75. [Google Scholar] [CrossRef]
- Pinelli, L.A.P.; Montandon, A.A.B.; Corbi, S.C.T.; Moraes, T.A.; Fais, L.M.G. Ricinus communis treatment of denture stomatitis in institutionalised elderly. J. Oral Rehabil. 2013, 40, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.H.; Taylor, T.D.; Heid, D.W. Clinical evaluation of a nystatin pastille for treatment of denture-related oral candidiasis. J. Prosthet. Dent. 1989, 61, 699–703. [Google Scholar] [CrossRef]
- Mima, E.G.; Vergani, C.E.; Machado, A.L.; Massucato, E.M.S.; Colombo, A.L.; Bagnato, V.S.; Pavarina, A.C. Comparison of Photodynamic Therapy versus conventional antifungal therapy for the treatment of denture stomatitis: A randomized clinical trial. Clin. Microbiol. Infect. 2012, 18, E380–E388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, P.J.; Wingfield, H.J.; Cosgrove, R.F.; Hughes, B.O.; Turner-Warwick, M.E. Assessment of oral candidiasis in patients with respiratory disease and efficacy of a new nystatin formulation. BMJ Br. Med. J. 1986, 292, 1699–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanita, P.V.; Machado, A.L.; Pavarina, A.C.; Massucato, E.M.S.; Colombo, A.L.; Vergani, C.E. Microwave denture disinfection versus nystatin in treating patients with well-controlled type 2 diabetes and denture stomatitis: A randomized clinical trial. Int. J. Prosthodont. 2012, 25, 232–244. [Google Scholar]
- Flynn, P.M.; Cunningham, C.K.; Kerkering, T.; Jorge, A.R.S.; Peters, V.B.; Pitel, P.A.; Harris, J.; Gilbert, G.; Castagnaro, L.; Robinson, P. Oropharyngeal candidiasis in immunocompromised children: A randomized, multicenter study of orally administered fluconazole suspension versus nystatin. The Multicenter Fluconazole Study Group. J. Pediatr. 1995, 127, 322–328. [Google Scholar] [CrossRef]
- Goins, R.A.; Ascher, D.; Waecker, N.; Arnold, J.; Moorefield, E. Comparison of fluconazole and nystatin oral suspensions for treatment of oral candidiasis in infants. Pediatr. Infect. Dis. J. 2002, 21, 1165–1167. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, J.E.; Hahn, H. The Antimycotics Study Group Randomized comparison of two nystatin oral gels with miconazole oral gel for treatment of oral thrush in infants. Infection 1996, 24, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, J.E. Treatment of oropharyngeal candidiasis in immunocompetent infants: A randomized multicenter study of miconazole gel vs. nystatin suspension. The Antifungals Study Group. Pediatr. Infect. Dis. J. 1997, 16, 288–293. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Chen, H.; Patton, L.; Evans, S.; Lee, A.; Kumwenda, J.; Hakim, J.; Masheto, G.; Sawe, F.; Pho, M.T.; et al. Topical gentian violet compared with nystatin oral suspension for the treatment of oropharyngeal candidiasis in HIV-1-infected participants. AIDS 2017, 31, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Nyst, M.J.; Perriens, J.H.; Kimputu, L.; Lumbila, M.; Nelson, A.M.; Piot, P. Gentian Violet, Ketoconazole and Nystatin in Oropharynge-al and Esophageal Candidiasis in Zairian AIDS Patients. Ann. Soc. Belg. Med. Trop. 1992, 72, 45–52. [Google Scholar]
- Pons, V.; Greenspan, D.; Lozada-Nur, F.; McPhail, L.; Gallant, J.E.; Tunkel, A.; Johnson, C.C.; McCarty, J.; Panzer, H.; Levenstein, M.; et al. Oropharyngeal Candidiasis in Patients with AIDS: Randomized Comparison of Fluconazole Versus Nystatin Oral Suspensions. Clin. Infect. Dis. 1997, 24, 1204–1207. [Google Scholar] [CrossRef] [Green Version]
- Blomgren, J.; Berggren, U.; Jontell, M. Fluconazole versus nystatin in the treatment of oral candidosis. Acta Odontol. Scand. 1998, 56, 202–205. [Google Scholar] [CrossRef]
- Meunier, F.; Aoun, M.; Gerard, M. Therapy for Oropharyngeal Candidiasis in the Immunocompromised Host: A Randomized Double-Blind Study of Fluconazole vs. Ketoconazole. Rev. Infect. Dis. 1990, 12, S364–S368. [Google Scholar] [CrossRef]
- Chaubal, T.; Bapat, R. Oral Thrush. Am. J. Med. 2018, 131, e371–e372. [Google Scholar] [CrossRef]
- Hellstein, J.W.; Marek, C.L. Candidiasis: Red and White Manifestations in the Oral Cavity. Head Neck Pathol. 2019, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Firoozi, P.; Farshidfar, N.; Fekrazad, R. Efficacy of antimicrobial photodynamic therapy compared to nystatin therapy in reducing Candida colony count in patients with Candida-associated denture stomatitis: A systematic review and meta-analysis. Evid. -Based Dent. 2021, 23, 34862461. [Google Scholar] [CrossRef] [PubMed]
- Iversen, D.B.; Hellfritzsch, M.; Stage, T.B.; Aabenhus, R.M.; Lind, B.S.; Pottegård, A. Antimycotic Treatment of Oral Candidiasis in Warfarin Users. Am. J. Med. 2020, 134, e308–e312. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Yoshioka, M.; Ihara, F.; Nihira, T. Cryptic antifungal compounds active by synergism with polyene antibiotics. J. Biosci. Bioeng. 2016, 121, 394–398. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, D.K., Jr.; Calandra, T.F.; Edwards, J.E., Jr.; Filler, S.G.; Fisher, J.F.; Kullberg, B.J.; Ostrosky-Zeichner, L.; et al. Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 503–535. [Google Scholar] [CrossRef] [Green Version]
- WHO. Guidelines on the Treatment of Skin and Oral HIV-Associated Conditions in Children and Adults; World Health Organization: Geneva, Switzerlan, 2014.
- Haro-Reyes, T.; Díaz-Peralta, L.; Galván-Hernández, A.; Rodríguez-López, A.; Rodríguez-Fragoso, L.; Ortega-Blake, I. Polyene Antibiotics Physical Chemistry and Their Effect on Lipid Membranes; Impacting Biological Processes and Medical Applications. Membranes 2022, 12, 681. [Google Scholar] [CrossRef]






| Author/Country | Risk Factor | Nystatin Group | Control Group | Nystatin Group | Control Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (Mean) | Sex | N | Age (Mean) | Sex | N | Formulation | Dose | Frequency (Times/day) | Duration (Days) | Medication | Formulation | Dose | Frequency | Duration (Days) | ||
| Afroozi et al. 2019 [28], Iran | Denture | 67.4 y | - | 33 | 67.6 y | - | 33 | Sol | 100,000 IU | 3 times a day | 15 | PDT | - | - | 2 sessions | 15 |
| Alrabiah et al. 2019 [29], Saudi Arabia | Denture | - | - | 18 | - | - | 18 | Susp | 100,000 IU | 4 times a day | 14 | PDT | - | - | twice in one week | 30 |
| Alves et al. 2020 [30], Brazil | Denture | 69 y | - | 35 | 70 y | F = 19 M = 11 | 30 | Susp | 100,000 IU/mL) | 4 times a day | 15 | PDT | - | - | 6 sessions | 15 |
| Araújo et al. 2021 [35], Brazil | Denture | 57 y | - | 18 | - | - | 18 | Sol | 1,000,000 IU | 3 times a day | 15 | CZ | oral spray | - | 3 times a day | 15 |
| Bakhshi et al. 2012 [31], Iran | Denture | 73.52 y | - | 20 | - | - | 20 | Sol | 100,000 IU/ml | 3 times a day | 30 | GE | Sol | 40 mg/ml | 3 times a day | 30 |
| Gonoudi et al. 2021 [32], Iran | Denture | 60.93 y | - | 14 | 55.86 y | - | 14 | Susp | 100,000 IU | 4 times a day | 14 | ZM | Sol | 5 ml | 5 times a day | 14 |
| Johnson et al. 1989 [39], USA | Denture | - | - | 8 | - | - | 8 | Pas | 200,000 IU | 5 | 14 | Placebo | Pastilles | 5 | 14 | |
| - | - | 8 | - | - | 8 | Pas | 400,000 IU | 5 | 14 | Placebo | Pas | 5 | 14 | |||
| Labban et al. 2021 [33], Saudi Arabia | Denture | 56.9 y | - | 15 | 57.2 y | - | 15 | Susp | 100,000 IU/mL | 4 times a day | 15 | PDT | - | - | 6 sessions | 15 |
| Li et al. 2014 [34], China | Denture | 64.84 y | F = 24 M = 7 | 31 | 62.72 y | F = 29 M = 5 | 34 | Paste | 2% | 3 | 30 | NYT + Pb | Paste + Lozenges | 3 | 30 | |
| Mima et al. 2012 [40], Brazil | Denture | 62.45 y | - | 20 | 61.25 y | - | 20 | Susp | 100 000 IU | 4 times a day | 15 | PDT | - | - | 6 sessions | 15 |
| Nairn et al. 1975 [37], England | Denture | - | - | 13 | - | - | 18 | Pas | 500,000 IU | 4 | 30 | AMB | Lozenges | 10 mg | 4 | 30 |
| - | - | 13 | - | - | 15 | Pas | 500,000 IU | 4 | 30 | Placebo | ||||||
| Pinelli et al. 2013 [38], Brazil | Denture | 81.4 y | - | 10 | - | - | 10 | Sol | 100,000 IU | 4 times a day | 30 | RC | Sol | - | - | 30 |
| 81.4 y | - | 10 | - | - | 10 | Sol | 100,000 IU | 4 times a day | 30 | MIC | Gel | 4 times a day | 30 | |||
| Silva et al. 2012 [36], Brazil | Denture | 62.5 y | - | 20 | 59.5 y | - | 20 | Susp | 100,000 IU/ml | 4 | 14 | DM | Irr | Once per week | 14 | |
| 62.5 y | - | 20 | 56.8 y | - | 20 | Susp | 100,000 IU/ml | 4 | 14 | DM | Irr | 3 times per week | 14 | |||
| Sanita et al. 2012 [42], Brazil | Denture in diabetic patients | 62.6 y | - | 10 | 62.2 y | - | 10 | Susp | 100,000 IU/m | 4 | 14 | DM | Irr | 3 times per week | 14 | |
| Thompson et al. 1986 [41], England | Respiratory disease | 59 y | - | 18 | - | - | 18 | Pas | 100,000 IU | 4 | 7 | NYT | Susp | 100 000 units | 4 | 7 |
| Goins et al. 2002 [44], USA | Infants | 1–12 mon | - | 28 | 1–12 mon | - | 17 | Susp | 100,000 IU | 4 | 10 | FLC | Susp | 1 per day | 7 | |
| Hoppe 1997 [46], Multicenter study | Infants | 130 days | F = 0 M = 77 | 85 | 132 days | F = 0 M = 95 | 98 | Susp | 100,000 IU | 4 | 12 | MIC | Gel | 4 | 12 | |
| Hoppe et al. 1996 [45], Multicenter study | Infants | 5 months | - | 35 | 5 mon | - | 27 | Gel | 250,000 IU | 4 | 14 | MIC | Gel | 4 | 14 | |
| 5 months | - | 35 | 5 mon | - | 27 | Gel | 100,000 IU | 4 | 14 | MIC | Gel | 4 | 14 | |||
| Flynn et al. 1995 [43], USA | Infants Children | 6 months–13 y | - | 88 | 6 mon–13 y | - | 94 | Susp | 400,000 IU | 4 | 14 | FLC | Susp | 14 | ||
| Meunier et al. 1990 [51], Belgium | Cancer patients | - | F = 10 M = 14 | 24 | - | F = 8 M = 10 | 18 | Susp + Pas | 1000,000 IU + 100,000 IU | 3 | 10 to 12 | KCZ | Tab | 10 to 12 | ||
| Mukherjee et al. 2017 [47], Multicenter study | HIV | - | F = 66 M = 45 | 111 | - | F = 62 M = 48 | 110 | Susp | 500,000 IU | 4 | 14 | GV | Sol | 14 | ||
| Pons et al. 1997 [49], USA | HIV, AIDS | 38 y | - | 84 | 38 y | - | 83 | Susp | 500,000 IU | 4 | 14 | FLC | Susp | 14 | ||
| Nyst et al. 1992 [48], Zaire | AIDS | 35.4 y | - | 47 | 34.5 y | - | 49 | Susp | 200,000 IU | 4 | 14 | GV | Susp | 14 | ||
| 35.4 y | - | 47 | 34.5 y | - | 45 | Susp | 200,000 IU | 4 | 14 | KCZ | Troche | 14 | ||||
| Blomgren et al. 1998 [50], Sweden | Multigroup patients | 60.7 y | - | 33 | 58.4 y | - | 34 | Sol | 100,000 IU | 4 | 21 | FLC | Cap | 7 | ||
| Authors | Risk Factors | Clinical Cure Rates | Mycological Cure Rates | ||
|---|---|---|---|---|---|
| Nystatin | Controls | Nystatin | Controls | ||
| Afroozi et al. 2019 [28] | Denture | 89.30% | 53.60% | - | - |
| Alrabiah et al. 2019 [29] | Denture | - | - | - | - |
| Alves et al. 2020 [30] | Denture | 54.20% | 53.30% | - | - |
| Bakhshi et al. 2012 [31] | Denture | - | - | - | - |
| Araújo et al. 2021 [35] | Denture | 89% | 61% | 83% | 33% |
| Gonoudi et al. 2021 [32] | Denture | - | - | - | - |
| Johnson et al. 1989 [39] | Denture | 28.60% | 0 | 57.10% | 0 |
| 14.30% | 0 | 71.40% | 0 | ||
| Labban et al. 2021 [33] | Denture | - | - | - | - |
| Li et al. 2014 [34] | Denture | - | - | 30.77% | 20% |
| Mima et al. 2012 [40] | Denture | 53% | 45% | - | - |
| Nairn et al. 1975 [37] | Denture | 76.90% | 88.80% | 40% | 6.25% |
| 76.90% | 40% | 40% | 20% | ||
| Pinelli et al. 2013 [38] | Denture | - | - | - | - |
| Silva et al. 2012 [36] | Denture | 18.75% | 23.53% | - | - |
| 18.75% | 22.22% | - | - | ||
| Sanita et al. 2012 [42] | Denture in diabetic patients | 20% | 25% | - | - |
| Thompson et al. 1986 [41] | Respiratory disease and dentures | 87% | 80% | - | - |
| Goins et al. 2002 [44] | Infants | 28.60% | 100% | 5.60% | 73.30% |
| Hoppe et al. 1996 [45] | Infants | 42.80% | 85.10% | 20% | 29.60% |
| 48.50% | 85.10% | 3.00% | 29.60% | ||
| Hoppe 1997 [46] | Infants | 54.10% | 99% | 8.20% | 54.10% |
| Flynn et al. 1995 [43] | Infants, Children | 51% | 91% | 11% | 76% |
| Meunier et al. 1990 [51] | Cancer patients | 72% | 87% | 24% | 61% |
| Mukherjee et al. 2017 [47] | HIV | 67.80% | 68.50% | - | - |
| Pons et al. 1997 [49] | HIV, AIDS | 52% | 87% | 6% | 60% |
| Nyst et al. 1992 [48] | AIDS | 9% | 42% | 13% | 62% |
| 9% | 43% | 13% | 57% | ||
| Blomgren et al. 1998 [50] | Multigroup patients | 16.70% | 30% | - | - |
| Risk Factor | Formulation | Dose | Frequency (Times/Day) | Duration (Days) | Clinical Cure Rates (%) | Mycological Cure Rates (%) |
|---|---|---|---|---|---|---|
| Denture | Susp | 100,000 IU | 4 | 15 | 54.2 | |
| Denture | Sol | 100,000 IU | 3 | 15–30 | 89.3 | |
| Denture | Pas | 200,000–400,000 IU | 5 | 14 | 28.6–14.3 | 57.10–71.4 |
| Denture | Pas | 500,000 IU | 4 | 30 | 76.9 | 40 |
| Denture | Paste | 2% | 3 | 30 | 30.77 | |
| Denture in diabetic patients | Susp | 100,000 IU | 4 | 14 | 20 | - |
| Respiratory disease | Pas | 100,000 IU | 4 | 7 | 87 | - |
| Infants and children | Susp | 100,000–400,000 IU | 4 | 10 to 14 | 28.6–54.1 | 5.6 – 11 |
| Infants and children | Gel | 250,000 IU | 4 | 14 days | 42.8/48.5 | 20/3.0 |
| Cancer | Susp + Pas | 1,000,000 IU + 100,000 IU | 3 | 10 to 12 | 87.5 | 66 |
| HIV/AIDS | Susp | 100,000–500,000 IU | 4 | 14 | 9–67.8 | 6–13 |
| Multigroup | Susp | 100,000 IU | 4 | 21 | 16.7 | - |
| Author | Risk Factor | Adverse Effects in Nystatin Group | Adverse Effects in Control Group |
|---|---|---|---|
| Bakhshi et al. 2012 [31] | Denture | nausea in 6, vomiting in 1, diarrhea in 5, anorexia in 1, burning in 1 | itching in 1 |
| Nairn et al. 1975 [37] | Denture | unpleasant taste in eight patients | unpleasant taste in five patients |
| Hoppe et al. 1996 [45] | Infants | vomiting in one patient | vomiting in two patients |
| Flynn et al. 1995 [43] | Infants, Children | three patients (vomiting, nausea, diarrhea, anorexia, abdominal pain), one patient (rash, headache) | six patients (vomiting, nausea, diarrhea, anorexia, abdominal pain), one patient (rash, headache) |
| Pons et al. 1997 [49] | HIV, AIDS | nausea, vomiting and diarrhea | nausea in one patient, and elevated liver enzyme concentrations in two patients |
| Nyst et al. 1992 [48] | AIDS | - | irritation and small superficial oral ulcers in two patients |
| Blomgren et al. 1998 [50] | Multigroup patients | nausea in one patient | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, A.; Misra, S.R.; Panda, S.; Sokolowski, G.; Mishra, L.; Das, R.; Lapinska, B. Nystatin Effectiveness in Oral Candidiasis Treatment: A Systematic Review & Meta-Analysis of Clinical Trials. Life 2022, 12, 1677. https://doi.org/10.3390/life12111677
Rai A, Misra SR, Panda S, Sokolowski G, Mishra L, Das R, Lapinska B. Nystatin Effectiveness in Oral Candidiasis Treatment: A Systematic Review & Meta-Analysis of Clinical Trials. Life. 2022; 12(11):1677. https://doi.org/10.3390/life12111677
Chicago/Turabian StyleRai, Anamika, Satya Ranjan Misra, Saurav Panda, Grzegorz Sokolowski, Lora Mishra, Rupsa Das, and Barbara Lapinska. 2022. "Nystatin Effectiveness in Oral Candidiasis Treatment: A Systematic Review & Meta-Analysis of Clinical Trials" Life 12, no. 11: 1677. https://doi.org/10.3390/life12111677
APA StyleRai, A., Misra, S. R., Panda, S., Sokolowski, G., Mishra, L., Das, R., & Lapinska, B. (2022). Nystatin Effectiveness in Oral Candidiasis Treatment: A Systematic Review & Meta-Analysis of Clinical Trials. Life, 12(11), 1677. https://doi.org/10.3390/life12111677






