Antibiofilm Activity of LL-37 Peptide and D-Amino Acids Associated with Antibiotics Used in Regenerative Endodontics on an Ex Vivo Multispecies Biofilm Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biofilm Preparation
2.2. Biofilm Treatments
2.3. Bacterial Quantification
2.4. Statistical Analysis
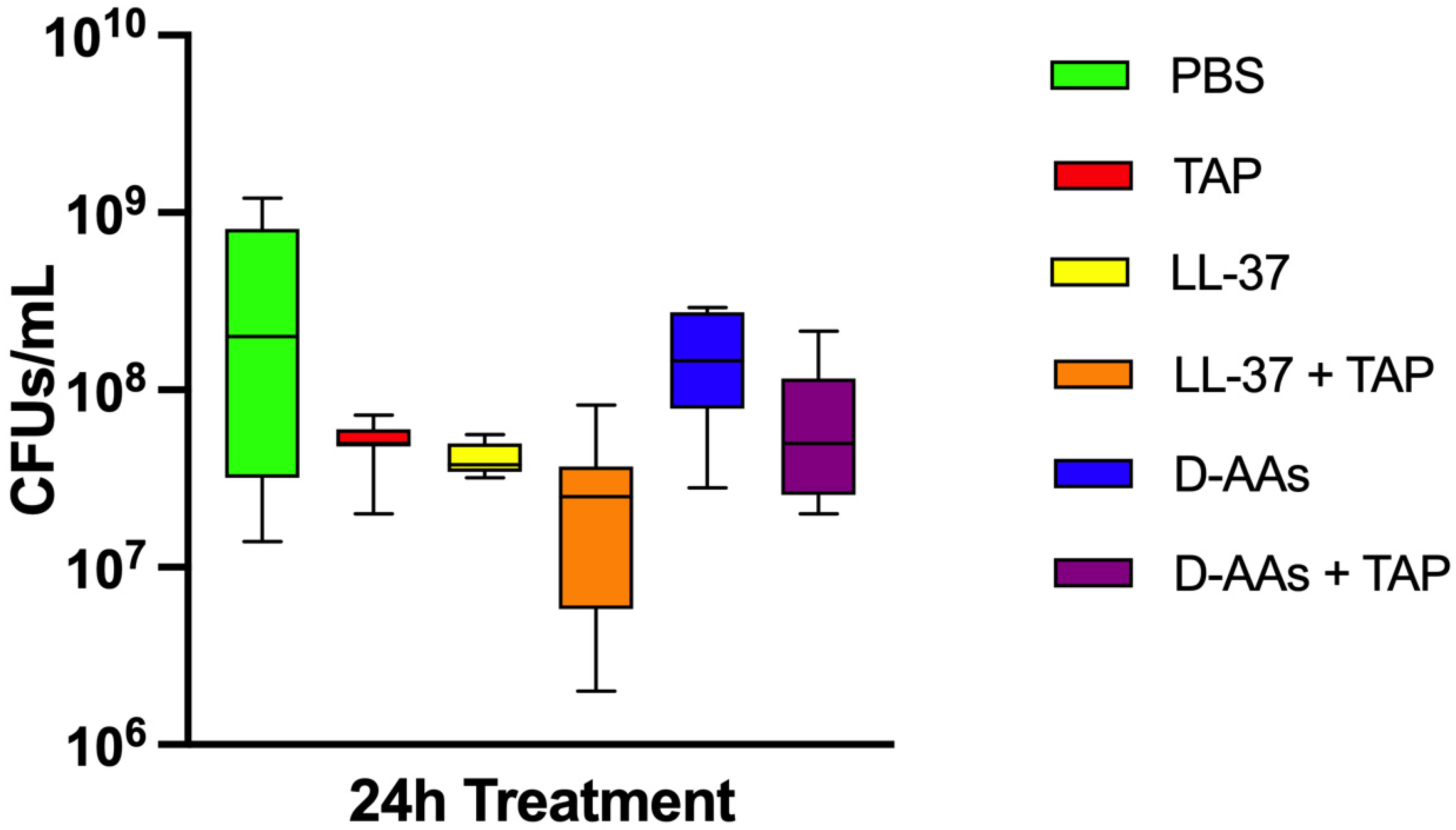
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hargreaves, K.M.; Diogenes, A.; Teixeira, F.B. Treatment options: Biological basis of regenerative endodontic procedures. J. Endod. 2013, 39 (Suppl. 3), S30–S43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.K.; Lim, G.S.; Singh, M.; Fial, A.V. Quantitative Assessment of Root Development after Regenerative Endodontic Therapy: A Systematic Review and Meta-Analysis. J. Endod. 2020, 46, 1856–1866.e2. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Nosrat, A.; Kim, J.R.; Price, J.B.; Wang, P.; Bair, E.; Xu, H.H.; Fouad, A.F. Effect of Residual Bacteria on the Outcome of Pulp Regeneration In Vivo. J. Dent. Res. 2017, 96, 100–106. [Google Scholar] [CrossRef]
- Fouad, A.F. Microbial Factors and Antimicrobial Strategies in Dental Pulp Regeneration. J. Endod. 2017, 43 (Suppl. 9), S46–S50. [Google Scholar] [CrossRef]
- De-Jesus-Soares, A.; Prado, M.C.; Nardello, L.C.L.; Pereira, A.C.; Cerqueira-Neto, A.; Nagata, J.Y.; Martinez, E.F.; Frozoni, M.; Gomes, B.P.; Pinheiro, E.T. Clinical and Molecular Microbiological Evaluation of Regenerative Endodontic Procedures in Immature Permanent Teeth. J. Endod. 2020, 46, 1448–1454. [Google Scholar] [CrossRef]
- American Association of Endodontists. AAE Clinical Considerations for a Regenerative Procedure (New, Revised 6-18-16); American Association of Endodontists: Chicago, IL, USA, 2016; pp. 1–6. [Google Scholar]
- Ribeiro, J.S.; Münchow, E.A.; Ferreira Bordini, E.A.; de Oliveira da Rosa, W.L.; Bottino, M.C. Antimicrobial Therapeutics in Regenerative Endodontics: A Scoping Review. J. Endod. 2020, 46 (Suppl. 9), S115–S127. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [Green Version]
- Ridyard, K.E.; Overhage, J. The Potential of Human Peptide LL-37 as an Antimicrobial and Anti-Biofilm Agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
- Luo, Y.; McLean, D.T.; Linden, G.J.; McAuley, D.F.; McMullan, R.; Lundy, F.T. The Naturally Occurring Host Defense Peptide, LL-37, and Its Truncated Mimetics KE-18 and KR-12 Have Selected Biocidal and Antibiofilm Activities Against Candida albicans, Staphylococcus aureus, and Escherichia coli In Vitro. Front. Microbiol. 2017, 8, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Dietz, M.J.; Li, B. Antimicrobial peptide LL-37 is bactericidal against Staphylococcus aureus biofilms. PLoS ONE 2019, 14, e0216676. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Murakami, T.; Suzuki, K.; Tamura, H.; Kuwahara-Arai, K.; Iba, T.; Nagaoka, I. Antimicrobial cathelicidin peptide LL-37 inhibits the LPS/ATP-induced pyroptosis of macrophages by dual mechanism. PLoS ONE 2014, 9, e85765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Int. J. Mol. Sci. 2020, 21, 5973. [Google Scholar] [CrossRef] [PubMed]
- Nireeksha; Varma, S.R.; Damdoum, M.; Alsaegh, M.A.; Hegde, M.N.; Kumari, S.N.; Ramamurthy, S.; Narayanan, J.; Imran, E.; Shabbir, J.; et al. Immunomodulatory Expression of Cathelicidins Peptides in Pulp Inflammation and Regeneration: An Update. Curr. Issues Mol. Biol. 2021, 43, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Caiaffa, K.S.; Dos Santos, V.R.; Abuna, G.F.; Santos-Filho, N.A.; Cilli, E.M.; Sakai, V.T.; Cintra, L.T.A.; Duque, C. Cytocompatibility and Synergy of EGCG and Cationic Peptides Against Bacteria Related to Endodontic Infections, in Planktonic and Biofilm Conditions. Probiotics Antimicrob. Proteins 2021, 13, 1808–1819. [Google Scholar] [CrossRef]
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. D-amino acids trigger biofilm disassembly. Science 2010, 328, 627–629. [Google Scholar] [CrossRef] [Green Version]
- Kolodkin-Gal, I. Beyond the wall: Can D-amino acids and small molecule inhibitors eliminate infections? Future Med. Chem. 2017, 9, 843–846. [Google Scholar] [CrossRef]
- Tong, Z.; Zhang, L.; Ling, J.; Jian, Y.; Huang, L.; Deng, D. An In Vitro study on the effect of free amino acids alone or in combination with nisin on biofilms as well as on planktonic bacteria of Streptococcus mutans. PLoS ONE 2014, 9, e99513. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.J., Jr.; Akers, K.S.; Romano, D.R.; Woodbury, R.L.; Hardy, S.K.; Murray, C.K.; Wenke, J.C. D-amino acids enhance the activity of antimicrobials against biofilms of clinical wound isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 4353–4361. [Google Scholar] [CrossRef]
- Rosen, E.; Tsesis, I.; Elbahary, S.; Storzi, N.; Kolodkin-Gal, I. Eradication of Enterococcus faecalis Biofilms on Human Dentin. Front. Microbiol. 2016, 7, 2055. [Google Scholar] [CrossRef] [PubMed]
- Zilm, P.S.; Butnejski, V.; Rossi-Fedele, G.; Kidd, S.P.; Edwards, S.; Vasilev, K. D-amino acids reduce Enterococcus faecalis biofilms In Vitro and in the presence of antimicrobials used for root canal treatment. PLoS ONE 2017, 12, e0170670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warraich, A.A.; Mohammed, A.R.; Perrie, Y.; Hussain, M.; Gibson, H.; Rahman, A. Evaluation of anti-biofilm activity of acidic amino acids and synergy with ciprofloxacin on Staphylococcus aureus biofilms. Sci. Rep. 2020, 10, 9021. [Google Scholar] [CrossRef] [PubMed]
- Lukic, D.; Karygianni, L.; Flury, M.; Attin, T.; Thurnheer, T. Endodontic-Like Oral Biofilms as Models for Multispecies Interactions in Endodontic Diseases. Microorganisms 2020, 8, 674. [Google Scholar] [CrossRef]
- Pinheiro, E.T.; Karygianni, L.; Attin, T.; Thurnheer, T. Antibacterial Effect of Sodium Hypochlorite and EDTA in Combination with High-Purity Nisin on an Endodontic-like Biofilm Model. Antibiotics 2021, 10, 1141. [Google Scholar] [CrossRef]
- Guggenheim, B.; Giertsen, E.; Schüpbach, P.; Shapiro, S. Validation of an In Vitro biofilm model of supragingival plaque. J. Dent. Res. 2001, 80, 363–370. [Google Scholar] [CrossRef]
- Gmür, R.; Guggenheim, B. Antigenic heterogeneity of Bacteroides intermedius as recognized by monoclonal antibodies. Infect. Immun. 1983, 42, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.Y.; Rajasekaran, G.; Shin, S.Y. LL-37-derived short antimicrobial peptide KR-12-a5 and its d-amino acid substituted analogs with cell selectivity, anti-biofilm activity, synergistic effect with conventional antibiotics, and anti-inflammatory activity. Eur. J. Med. Chem. 2017, 136, 428. [Google Scholar] [CrossRef]
- Latham, J.; Fong, H.; Jewett, A.; Johnson, J.D.; Paranjpe, A. Disinfection Efficacy of Current Regenerative Endodontic Protocols in Simulated Necrotic Immature Permanent Teeth. J. Endod. 2016, 42, 1218–1225. [Google Scholar] [CrossRef]
- Lin, L.M.; Shimizu, E.; Gibbs, J.L.; Loghin, S.; Ricucci, D. Histologic and histobacteriologic observations of failed revascularization/revitalization therapy: A case report. J. Endod. 2014, 40, 291–295. [Google Scholar] [CrossRef]
- Wimley, W.C.; Hristova, K. Antimicrobial Peptides: Successes, challenges and unanswered. Quest. Membr. Biol. 2011, 239, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majewska, M.; Zamlynny, V.; Pieta, I.S.; Nowakowski, R.; Pieta, P. Interaction of LL-37 human cathelicidin peptide with a model microbial-like lipid membrane. Bioelectrochemistry 2021, 141, 107842. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef] [PubMed]
- Stojicic, S.; Shen, Y.; Haapasalo, M. Effect of the source of biofilm bacteria, level of biofilm maturation, and type of disinfecting agent on the susceptibility of biofilm bacteria to antibacterial agents. J. Endod. 2013, 39, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Siqueira, J.F., Jr. Biofilms and apical periodontitis: Study of prevalence and association with clinical and histopathologic findings. J. Endod. 2010, 36, 1277–1288. [Google Scholar] [CrossRef]
- Manoil, D.; Al-Manei, K.; Belibasakis, G.N. A Systematic Review of the Root Canal Microbiota Associated with Apical Periodontitis: Lessons from Next-Generation Sequencing. Proteom. Clin. Appl. 2020, 14, e1900060. [Google Scholar] [CrossRef] [Green Version]
- Swimberghe, R.C.D.; Coenye, T.; de Moor, R.J.G.; Meire, M.A. Biofilm model systems for root canal disinfection: A literature review. Int. Endod. J. 2019, 52, 604–628. [Google Scholar] [CrossRef] [Green Version]
- Guggenheim, B.; Gmür, R.; Galicia, J.C.; Stathopoulou, P.G.; Benakanakere, M.R.; Meier, A.; Thurnheer, T.; Kinane, D.F. In Vitro modeling of host-parasite interactions: The ‘subgingival’ biofilm challenge of primary human epithelial cells. BMC Microbiol. 2009, 9, 280. [Google Scholar] [CrossRef] [Green Version]
- Thurnheer, T.; Bostanci, N.; Belibasakis, G.N. Microbial dynamics during conversion from supragingival to subgingival biofilms in an In Vitro model. Mol. Oral Microbiol. 2016, 31, 125–135. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.C.C.; Aguiar, A.P.S.; Araujo, L.M.P.; Dantas, L.O.; Mayer, M.P.A.; Karygianni, L.; Thurnheer, T.; Pinheiro, E.T. Antibiofilm Activity of LL-37 Peptide and D-Amino Acids Associated with Antibiotics Used in Regenerative Endodontics on an Ex Vivo Multispecies Biofilm Model. Life 2022, 12, 1686. https://doi.org/10.3390/life12111686
Pereira ACC, Aguiar APS, Araujo LMP, Dantas LO, Mayer MPA, Karygianni L, Thurnheer T, Pinheiro ET. Antibiofilm Activity of LL-37 Peptide and D-Amino Acids Associated with Antibiotics Used in Regenerative Endodontics on an Ex Vivo Multispecies Biofilm Model. Life. 2022; 12(11):1686. https://doi.org/10.3390/life12111686
Chicago/Turabian StylePereira, Ana C. C., Alana P. S. Aguiar, Leticia M. P. Araujo, Larissa O. Dantas, Marcia P. A. Mayer, Lamprini Karygianni, Thomas Thurnheer, and Ericka T. Pinheiro. 2022. "Antibiofilm Activity of LL-37 Peptide and D-Amino Acids Associated with Antibiotics Used in Regenerative Endodontics on an Ex Vivo Multispecies Biofilm Model" Life 12, no. 11: 1686. https://doi.org/10.3390/life12111686
APA StylePereira, A. C. C., Aguiar, A. P. S., Araujo, L. M. P., Dantas, L. O., Mayer, M. P. A., Karygianni, L., Thurnheer, T., & Pinheiro, E. T. (2022). Antibiofilm Activity of LL-37 Peptide and D-Amino Acids Associated with Antibiotics Used in Regenerative Endodontics on an Ex Vivo Multispecies Biofilm Model. Life, 12(11), 1686. https://doi.org/10.3390/life12111686






