Middle-Aged Lpaatδ-Deficient Mice Have Altered Metabolic Measures
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Body Weight and Food Intake Measurements
2.3. Locomotion and Indirect Calorimetry
2.4. Statistics
3. Results
3.1. Body Weight and Food Intake
3.2. Locomotion
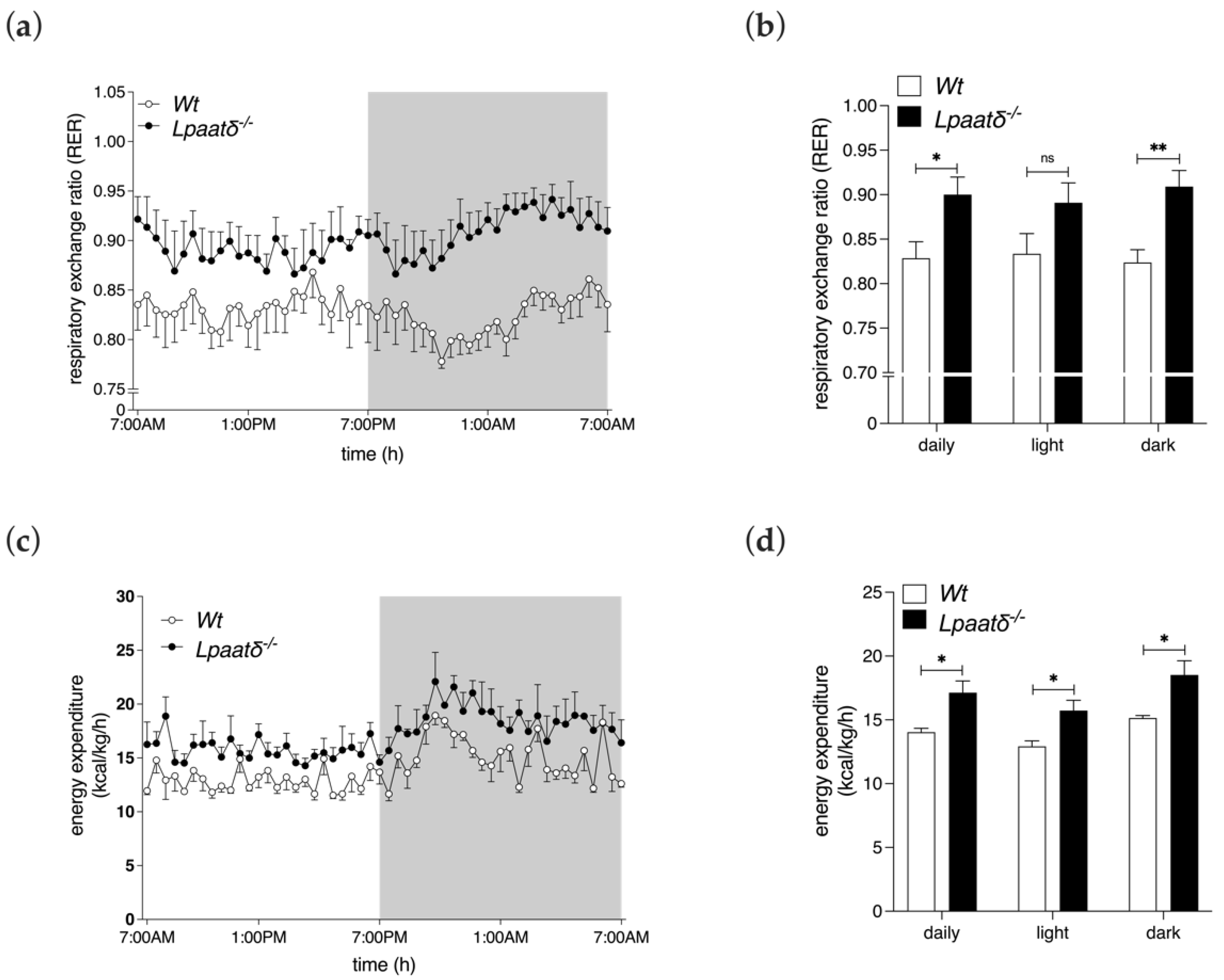
3.3. Metabolic Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takeuchi, K.; Reue, K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1195–E1209. [Google Scholar] [CrossRef]
- Kennedy, E.P.; Weiss, S.B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J. Biol. Chem. 1956, 222, 193–214. [Google Scholar] [CrossRef]
- Bradley, R.M.; Duncan, R.E. The lysophosphatidic acid acyltransferases (acylglycerophosphate acyltransferases) family: One reaction, five enzymes, many roles. Curr. Opin. Lipidol. 2018, 29, 110–115. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Tunison, K.; Dalal, J.S.; Nagamma, S.S.; Hamra, F.K.; Sankella, S.; Shao, X.; Auchus, R.J.; Garg, A. Metabolic, Reproductive, and Neurologic Abnormalities in Agpat1-Null Mice. Endocrinology 2017, 158, 3954–3973. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Sukumaran, S.; Cortés, V.A.; Tunison, K.; Mizrachi, D.; Sankella, S.; Gerard, R.D.; Horton, J.D.; Garg, A. Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: Biochemical characterization and inability to rescue hepatic steatosis in Agpat2(-/-) gene lipodystrophic mice. J. Biol. Chem. 2011, 286, 37676–37691. [Google Scholar] [CrossRef]
- Cortes, V.A.; Curtis, D.E.; Sukumaran, S.; Shao, X.; Parameswara, V.; Rashid, S.; Smith, A.R.; Ren, J.; Esser, V.; Hammer, R.E.; et al. Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell Metab. 2009, 9, 165–176. [Google Scholar] [CrossRef]
- Bradley, R.M.; Bloemberg, D.; Aristizabal Henao, J.J.; Hashemi, A.; Mitchell, A.S.; Fajardo, V.A.; Bellissimo, C.; Mardian, E.B.; Bombardier, E.; Pare, M.F.; et al. Lpaatdelta/Agpat4 deficiency impairs maximal force contractility in soleus and alters fibre type in extensor digitorum longus muscle. Biochim Biophys Acta Mol. Cell Biol. Lipids 2018, 1863, 700–711. [Google Scholar] [CrossRef]
- Bradley, R.M.; Mardian, E.B.; Bloemberg, D.; Aristizabal Henao, J.J.; Mitchell, A.S.; Marvyn, P.M.; Moes, K.A.; Stark, K.D.; Quadrilatero, J.; Duncan, R.E. Mice Deficient in lysophosphatidic acid acyltransferase delta (Lpaatdelta)/acylglycerophosphate acyltransferase 4 (Agpat4) Have Impaired Learning and Memory. Mol. Cell. Biol. 2017, 37, e00245-17. [Google Scholar] [CrossRef]
- Mardian, E.B.; Bradley, R.M.; Aristizabal Henao, J.J.; Marvyn, P.M.; Moes, K.A.; Bombardier, E.; Tupling, A.R.; Stark, K.D.; Duncan, R.E. Agpat4/Lpaatdelta deficiency highlights the molecular heterogeneity of epididymal and perirenal white adipose depots. J. Lipid Res. 2017, 58, 2037–2050. [Google Scholar] [CrossRef]
- Bradley, R.M.; Marvyn, P.M.; Aristizabal Henao, J.J.; Mardian, E.B.; George, S.; Aucoin, M.G.; Stark, K.D.; Duncan, R.E. Acylglycerophosphate acyltransferase 4 (AGPAT4) is a mitochondrial lysophosphatidic acid acyltransferase that regulates brain phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol levels. Biochim. Et Biophys. Acta 2015, 1851, 1566–1576. [Google Scholar] [CrossRef]
- Eto, M.; Shindou, H.; Shimizu, T. A novel lysophosphatidic acid acyltransferase enzyme (LPAAT4) with a possible role for incorporating docosahexaenoic acid into brain glycerophospholipids. BioChem. Biophys. Res. Commun. 2014, 443, 718–724. [Google Scholar] [CrossRef]
- Pagliuso, A.; Valente, C.; Giordano, L.L.; Filograna, A.; Li, G.; Circolo, D.; Turacchio, G.; Marzullo, V.M.; Mandrich, L.; Zhukovsky, M.A.; et al. Golgi membrane fission requires the CtBP1-S/BARS-induced activation of lysophosphatidic acid acyltransferase δ. Nat. Commun. 2016, 7, 12148. [Google Scholar] [CrossRef]
- Bradley, R.M.; Mardian, E.B.; Marvyn, P.M.; Vasefi, M.S.; Beazely, M.A.; Mielke, J.G.; Duncan, R.E. Data on acylglycerophosphate acyltransferase 4 (AGPAT4) during murine embryogenesis and in embryo-derived cultured primary neurons and glia. Data Brief 2016, 6, 28–32. [Google Scholar] [CrossRef]
- Chan, J.Z.; Fernandes, M.F.; Hashemi, A.; Grewal, R.S.; Mardian, E.B.; Bradley, R.M.; Duncan, R.E. Age-associated increase in anxiety-like behavior in Lpaatδ/Agpat4 knockout mice. Curr. Res. Behav. Sci. 2021, 2, 100042. [Google Scholar] [CrossRef]
- Perez-Atencio, L.; Garcia-Aracil, N.; Fernandez, E.; Barrio, L.C.; Barios, J.A. A four-state Markov model of sleep-wakefulness dynamics along light/dark cycle in mice. PLoS ONE 2018, 13, e0189931. [Google Scholar] [CrossRef]
- Even, P.C.; Nadkarni, N.A. Indirect calorimetry in laboratory mice and rats: Principles, practical considerations, interpretation and perspectives. Am J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R459–R476. [Google Scholar] [CrossRef]
- Yang, C.Y.; Frohman, M.A. Mitochondria: Signaling with phosphatidic acid. Int. J. BioChem. Cell Biol. 2012, 44, 1346–1350. [Google Scholar] [CrossRef]
- Mukai, S.; Mizokami, A.; Otani, T.; Sano, T.; Matsuda, M.; Chishaki, S.; Gao, J.; Kawakubo-Yasukochi, T.; Tang, R.; Kanematsu, T.; et al. Adipocyte-specific GPRC6A ablation promotes diet-induced obesity by inhibiting lipolysis. J. Biol. Chem. 2021, 296, 100274. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Zhang, X.; Xie, X.; Saarinen, A.; Lu, X.; Yang, X.; Liu, J. Defective adipose lipolysis and altered global energy metabolism in mice with adipose overexpression of the lipolytic inhibitor G0/G1 switch gene 2 (G0S2). J. Biol. Chem. 2014, 289, 1905–1916. [Google Scholar] [CrossRef]
- Rolfe, D.F.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef]
- Gonzalez-Baro, M.R.; Coleman, R.A. Mitochondrial acyltransferases and glycerophospholipid metabolism. Biochim Biophys Acta Mol. Cell Biol. Lipids 2017, 1862, 49–55. [Google Scholar] [CrossRef]
- Kameoka, S.; Adachi, Y.; Okamoto, K.; Iijima, M.; Sesaki, H. Phosphatidic Acid and Cardiolipin Coordinate Mitochondrial Dynamics. Trends Cell Biol. 2018, 28, 67–76. [Google Scholar] [CrossRef]
- Pollard, A.K.; Ortori, C.A.; Stöger, R.; Barrett, D.A.; Chakrabarti, L. Mouse mitochondrial lipid composition is defined by age in brain and muscle. Aging 2017, 9, 986–998. [Google Scholar] [CrossRef]
- Zhukovsky, M.A.; Filograna, A.; Luini, A.; Corda, D.; Valente, C. Phosphatidic acid in membrane rearrangements. FEBS Lett. 2019, 593, 2428–2451. [Google Scholar] [CrossRef]
- Choi, S.Y.; Huang, P.; Jenkins, G.M.; Chan, D.C.; Schiller, J.; Frohman, M.A. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat Cell Biol. 2006, 8, 1255–1262. [Google Scholar] [CrossRef]
- Huang, H.; Gao, Q.; Peng, X.; Choi, S.Y.; Sarma, K.; Ren, H.; Morris, A.J.; Frohman, M.A. piRNA-associated germline nuage formation and spermatogenesis require MitoPLD profusogenic mitochondrial-surface lipid signaling. Dev. Cell 2011, 20, 376–387. [Google Scholar] [CrossRef]
- Baba, T.; Kashiwagi, Y.; Arimitsu, N.; Kogure, T.; Edo, A.; Maruyama, T.; Nakao, K.; Nakanishi, H.; Kinoshita, M.; Frohman, M.A.; et al. Phosphatidic acid (PA)-preferring phospholipase A1 regulates mitochondrial dynamics. J. Biol. Chem. 2014, 289, 11497–11511. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, F.; Kerbl-Knapp, J.; Korbelius, M.; Kuentzel, K.B.; Vujić, N.; Akhmetshina, A.; Hörl, G.; Paar, M.; Steyrer, E.; et al. Phosphatidylethanolamine N-Methyltransferase Knockout Modulates Metabolic Changes in Aging Mice. Biomolecules 2022, 12, 1270. [Google Scholar] [CrossRef]
- Bessenyei, B.; Márka, M.; Urbán, L.; Zeher, M.; Semsei, I. Single nucleotide polymorphisms: Aging and diseases. Biogerontology 2004, 5, 291–303. [Google Scholar] [CrossRef]
- Di Bona, D.; Vasto, S.; Capurso, C.; Christiansen, L.; Deiana, L.; Franceschi, C.; Hurme, M.; Mocchegiani, E.; Rea, M.; Lio, D.; et al. Effect of interleukin-6 polymorphisms on human longevity: A systematic review and meta-analysis. Ageing Res. Rev. 2009, 8, 36–42. [Google Scholar] [CrossRef]
- Sugawara, J.; Tomoto, T.; Noda, N.; Matsukura, S.; Tsukagoshi, K.; Hayashi, K.; Hieda, M.; Maeda, S. Effects of endothelin-related gene polymorphisms and aerobic exercise habit on age-related arterial stiffening: A 10-yr longitudinal study. J. Appl. Physiol. 2018, 124, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Hong, S.H.; Lee, S.J.; Hong, S.P.; Cho, C. Transcriptome Analysis of Testicular Aging in Mice. Cells 2021, 10, 2895. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczewski, M.V.; Fernandes, M.F.; Grewal, R.S.; Duncan, R.E. Middle-Aged Lpaatδ-Deficient Mice Have Altered Metabolic Measures. Life 2022, 12, 1717. https://doi.org/10.3390/life12111717
Tomczewski MV, Fernandes MF, Grewal RS, Duncan RE. Middle-Aged Lpaatδ-Deficient Mice Have Altered Metabolic Measures. Life. 2022; 12(11):1717. https://doi.org/10.3390/life12111717
Chicago/Turabian StyleTomczewski, Michelle Victoria, Maria Fernanda Fernandes, Rajan Singh Grewal, and Robin Elaine Duncan. 2022. "Middle-Aged Lpaatδ-Deficient Mice Have Altered Metabolic Measures" Life 12, no. 11: 1717. https://doi.org/10.3390/life12111717
APA StyleTomczewski, M. V., Fernandes, M. F., Grewal, R. S., & Duncan, R. E. (2022). Middle-Aged Lpaatδ-Deficient Mice Have Altered Metabolic Measures. Life, 12(11), 1717. https://doi.org/10.3390/life12111717






