Cerebello-Thalamo-Cortical MR Spectroscopy in Patients with Essential Tremor Undergoing MRgFUS Thalamotomy: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI Examination and Procedural Parameters
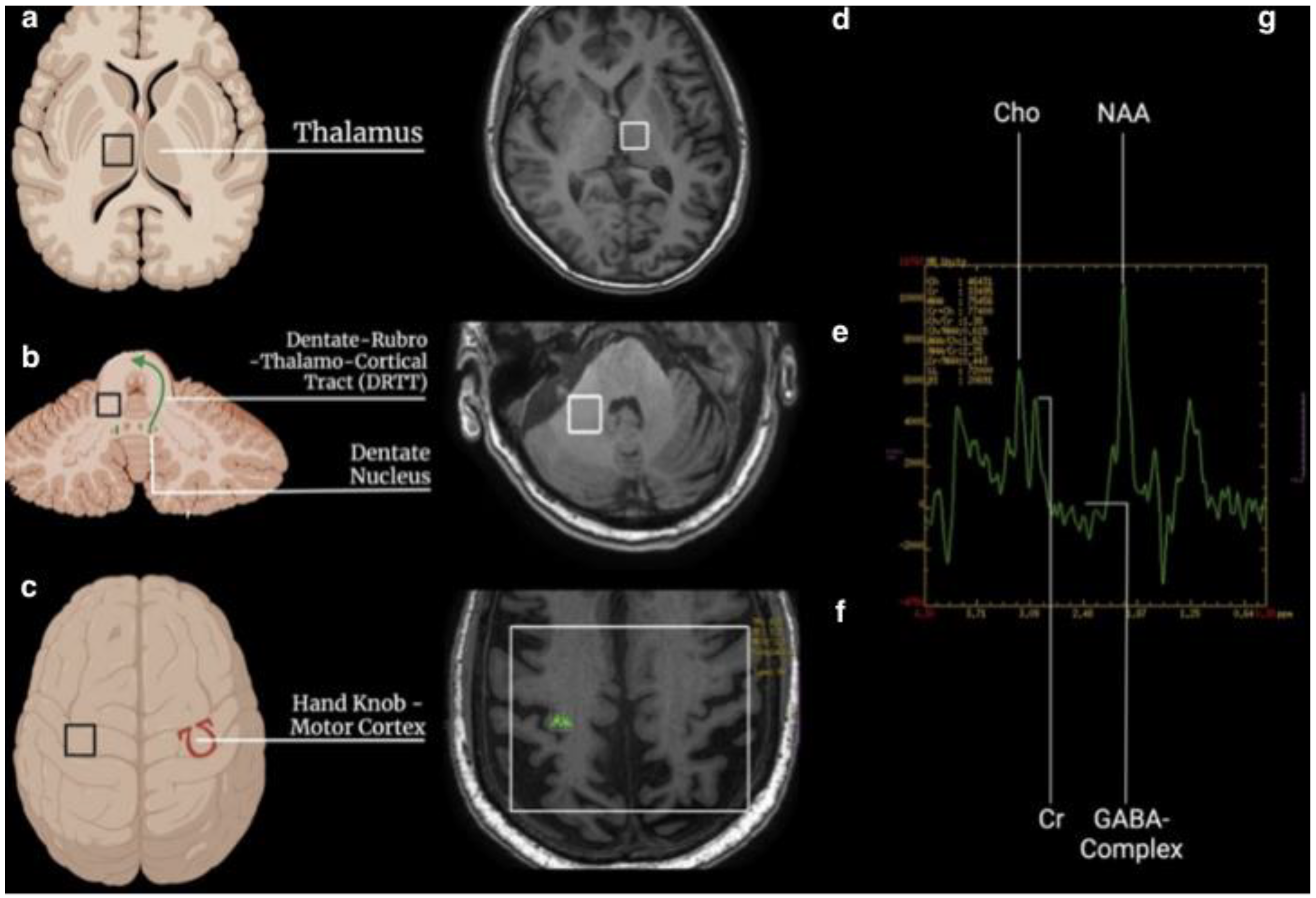
- two single-voxel (SV) sequences (TR 1700, TE 30, TM, voxel size 15 × 15 × 15, water suppression Hz, acquisition duration 4 m) with the 1st ROI placed to the contralateral T and the 2nd in the homolateral DN to the treated side.
- one multivoxel (SE) sequence (TR 1700, TE 30, TM, including the primary motor cortex 120 × 120, water suppression Hz, acquisition duration 6 m) with 2 ROIs placed on the hand knob area in the primary motor cortex bilaterally (Figure 1).
2.3. Statistical Analysis
3. Results
3.1. Thalamus
3.2. Cerebellum (Dentate Nucleus)
3.3. Motor Cortex (Hand Knob)
3.4. Correlation and Simple Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruno, F.; Catalucci, A.; Arrigoni, F.; Sucapane, P.; Cerone, D.; Cerrone, P.; Ricci, A.; Marini, C.; Masciocchi, C. An experience-based review of HIFU in functional interventional neuroradiology: Transcranial MRgFUS thalamotomy for treatment of tremor. Radiol. Med. 2020, 125, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Catalucci, A.; Arrigoni, F.; Gagliardi, A.; Campanozzi, E.; Corridore, A.; Tommasino, E.; Pagliei, V.; Pertici, L.; Palumbo, P.; et al. Comprehensive Evaluation of Factors Affecting Tremor Relapse after MRgFUS Thalamotomy: A Case-Control Study. Brain Sci. 2021, 11, 1183. [Google Scholar] [CrossRef] [PubMed]
- Jameel, A.; Bain, P.; Nandi, D.; Jones, B.; Gedroyc, W. Device profile of exAblate Neuro 4000, the leading system for brain magnetic resonance guided focused ultrasound technology: An overview of its safety and efficacy in the treatment of medically refractory essential tremor. Expert Rev. Med. Devices 2021, 18, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Sinai, A.; Nassar, M.; Eran, A.; Constantinescu, M.; Zaaroor, M.; Sprecher, E.; Schlesinger, I. Magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: A 5-year single-center experience. J. Neurosurg. 2019, 1–8. [Google Scholar] [CrossRef]
- Pineda-Pardo, J.A.; Urso, D.; Martinez-Fernandez, R.; Rodriguez-Rojas, R.; Del-Alamo, M.; Millar Vernetti, P.; Manez-Miro, J.; Hernandez-Fernandez, F.; de Luis-Pastor, E.; Vela-Desojo, L.; et al. Transcranial Magnetic Resonance-Guided Focused Ultrasound Thalamotomy in Essential Tremor: A Comprehensive Lesion Characterization. Neurosurgery 2020, 87, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pandey, S. Treatment of essential tremor: Current status. Postgrad. Med. J. 2020, 96, 84–93. [Google Scholar] [CrossRef]
- Lennon, J.C.; Hassan, I. Magnetic resonance-guided focused ultrasound for Parkinson’s disease since ExAblate, 2016–2019: A systematic review. Neurol. Sci. 2021, 42, 553–563. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, S.H.; Chang, K.W.; Kim, Y.; Gao, J.; Kovalevsky, M.; Rachmilevitch, I.; Zadicario, E.; Chang, W.S.; Jung, H.H.; et al. Technical and operative factors affecting magnetic resonance imaging-guided focused ultrasound thalamotomy for essential tremor: Experience from 250 treatments. J. Neurosurg. 2021, 135, 1780–1788. [Google Scholar] [CrossRef]
- Su, J.H.; Choi, E.Y.; Tourdias, T.; Saranathan, M.; Halpern, C.H.; Henderson, J.M.; Pauly, K.B.; Ghanouni, P.; Rutt, B.K. Improved Vim targeting for focused ultrasound ablation treatment of essential tremor: A probabilistic and patient-specific approach. Hum. Brain Mapp. 2020, 41, 4769–4788. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ito, H.; Fukutake, S.; Odo, T.; Kamei, T.; Yamaguchi, T.; Taira, T. Factors Associated with Heating Efficiency in Transcranial Focused Ultrasound Therapy. Neurol. Med. Chir. 2020, 60, 594–599. [Google Scholar] [CrossRef]
- Boutet, A.; Gwun, D.; Gramer, R.; Ranjan, M.; Elias, G.J.B.; Tilden, D.; Huang, Y.; Li, S.X.; Davidson, B.; Lu, H.; et al. The relevance of skull density ratio in selecting candidates for transcranial MR-guided focused ultrasound. J. Neurosurg. 2019, 132, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.D.; Collins, D.L.; Bertrand, G.; Peters, T.M.; Pike, G.B.; Sadikot, A.F. Optimal location of thalamotomy lesions for tremor associated with Parkinson disease: A probabilistic analysis based on postoperative magnetic resonance imaging and an integrated digital atlas. J. Neurosurg. 2002, 96, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lipsman, N.; Schwartz, M.L.; Krishna, V.; Sammartino, F.; Lozano, A.M.; Hynynen, K. Predicting lesion size by accumulated thermal dose in MR-guided focused ultrasound for essential tremor. Med. Phys. 2018, 45, 4704–4710. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Catalucci, A.; Varrassi, M.; Arrigoni, F.; Sucapane, P.; Cerone, D.; Pistoia, F.; Torlone, S.; Tommasino, E.; De Santis, L.; et al. Comparative evaluation of tractography-based direct targeting and atlas-based indirect targeting of the ventral intermediate (Vim) nucleus in MRgFUS thalamotomy. Sci. Rep. 2021, 11, 13538. [Google Scholar] [CrossRef]
- Tommasino, E.; Bruno, F.; Catalucci, A.; Varrassi, M.; Sucapane, P.; Cerone, D.; Pistoia, F.; Di Cesare, E.; Barile, A.; Ricci, A.; et al. Prognostic value of brain tissues’ volumes in patients with essential tremor treated with MRgFUS thalamotomy. J. Clin. Neurosci. 2021, 92, 33–38. [Google Scholar] [CrossRef]
- Zur, G.; Lesman-Segev, O.H.; Schlesinger, I.; Goldsher, D.; Sinai, A.; Zaaroor, M.; Assaf, Y.; Eran, A.; Kahn, I. Tremor Relief and Structural Integrity after MRI-guided Focused US Thalamotomy in Tremor Disorders. Radiology 2020, 294, 676–685. [Google Scholar] [CrossRef]
- Stanziano, M.; Andreasi, N.G.; Messina, G.; Rinaldo, S.; Palermo, S.; Verri, M.; Demichelis, G.; Medina, J.P.; Ghielmetti, F.; Bonvegna, S.; et al. Resting State Functional Connectivity Signatures of MRgFUS Vim Thalamotomy in Parkinson’s Disease: A Preliminary Study. Front. Neurol. 2021, 12, 786734. [Google Scholar] [CrossRef]
- Tognarelli, J.M.; Dawood, M.; Shariff, M.I.; Grover, V.P.; Crossey, M.M.; Cox, I.J.; Taylor-Robinson, S.D.; McPhail, M.J. Magnetic Resonance Spectroscopy: Principles and Techniques: Lessons for Clinicians. J. Clin. Exp. Hepatol. 2015, 5, 320–328. [Google Scholar] [CrossRef]
- Clarke, C.E.; Lowry, M.; Horsman, A. Unchanged basal ganglia N-acetylaspartate and glutamate in idiopathic Parkinson’s disease measured by proton magnetic resonance spectroscopy. Mov. Disord. 1997, 12, 297–301. [Google Scholar] [CrossRef]
- Lucetti, C.; Del Dotto, P.; Gambaccini, G.; Ceravolo, R.; Logi, C.; Berti, C.; Rossi, G.; Bianchi, M.C.; Tosetti, M.; Murri, L.; et al. Influences of dopaminergic treatment on motor cortex in Parkinson disease: A MRI/MRS study. Mov. Disord. 2007, 22, 2170–2175. [Google Scholar] [CrossRef]
- Llumiguano, C.; Kovacs, N.; Usprung, Z.; Schwarcz, A.; Doczi, T.P.; Balas, I. 1H-MRS experiences after bilateral DBS of the STN in Parkinson’s disease. Park. Relat. Disord. 2008, 14, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D.; Shungu, D.C.; Chan, S.; Mao, X.; Jurewicz, E.C.; Watner, D. Metabolic abnormality in the cerebellum in patients with essential tremor: A proton magnetic resonance spectroscopic imaging study. Neurosci. Lett. 2002, 333, 17–20. [Google Scholar] [CrossRef]
- Van Nuland, A.J.; Helmich, R.C.; Dirkx, M.F.; Zach, H.; Toni, I.; Cools, R.; Ouden, H.E.M.D. Effects of dopamine on reinforcement learning in Parkinson’s disease depend on motor phenotype. Brain 2020, 143, 3422–3434. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Shah, A.; Bhalsing, K.S.; Ingalhalikar, M.; Saini, J.; Pal, P.K. Clinical correlates of abnormal subcortical volumes in Essential Tremor. J. Neural Transm. 2019, 126, 569–576. [Google Scholar] [CrossRef] [PubMed]
| Thalamus | ||||||
|---|---|---|---|---|---|---|
| NAA/Cr Ratio | Cho/Cr Ratio | GABA/Cr Ratio | ||||
| Pretreatment | 6 M Follow-up | Pretreatment | 6 M Follow-up | Pretreatment | 6 M Follow-up | |
| Min. | 1.43 | 1.46 | 0.81 | 0.99 * | 0.58 | 0.40 |
| Max. | 1.81 | 2.80 | 1.16 | 1.40 | 0.93 | 0.66 |
| Median | 1.69 | 1.90 | 0.91 | 1.14 | 0.66 | 0.54 |
| CI 95% | 1.51–1.76 | 1.49–1.90 | 0.83–1.02 | 1.03–1.36 * | 0.45–0.96 | 0.35–0.74 |
| Dentate Nucleus (Cerebellum) | ||||
|---|---|---|---|---|
| NAA/Cr Ratio | Cho/Cr Ratio | |||
| Pretreatment | 6 M Follow-up | Pretreatment | 6 M Follow-up | |
| Min. | 1.12 | 1.30 * | 0.69 | 0.80 |
| Max. | 2.11 | 2.62 * | 1.21 | 1.44 |
| Median | 1.53 | 2.15 * | 1.01 | 1.16 |
| Motor Cortex | ||||||
|---|---|---|---|---|---|---|
| NAA/Cr Ratio | Cho/Cr Ratio | NAA/Cho Ratio | ||||
| Pretreatment | 6 M Follow-up | Pretreatment | 6 M Follow-up | Pretreatment | 6 M Follow-up | |
| Min. | 2.00 | 1.70 | 2.050 | 1.70 | 2.11 | 1.21 |
| Max. | 17.33 | 3.77 | 3.35 | 2.99 | 9.11 | 4.63 |
| Median | 2.96 | 2.28 | 2.96 | 2.12 | 3.44 | 2.92 * |
| CI 95% | 0.87–7.52 | 1.88–2.77 | 2.42–3.24 | 1.83–2.63 | 2.46–5.47 | 1.69–3.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, F.; Tommasino, E.; Catalucci, A.; Piccin, V.; Innocenzi, A.; Carugno, M.E.; Colarieti, F.; Pertici, L.; Di Gioia, A.; D’Alessandro, C.; et al. Cerebello-Thalamo-Cortical MR Spectroscopy in Patients with Essential Tremor Undergoing MRgFUS Thalamotomy: A Pilot Study. Life 2022, 12, 1741. https://doi.org/10.3390/life12111741
Bruno F, Tommasino E, Catalucci A, Piccin V, Innocenzi A, Carugno ME, Colarieti F, Pertici L, Di Gioia A, D’Alessandro C, et al. Cerebello-Thalamo-Cortical MR Spectroscopy in Patients with Essential Tremor Undergoing MRgFUS Thalamotomy: A Pilot Study. Life. 2022; 12(11):1741. https://doi.org/10.3390/life12111741
Chicago/Turabian StyleBruno, Federico, Emanuele Tommasino, Alessia Catalucci, Veronica Piccin, Antonio Innocenzi, Maria Ester Carugno, Filippo Colarieti, Leonardo Pertici, Antonio Di Gioia, Claudia D’Alessandro, and et al. 2022. "Cerebello-Thalamo-Cortical MR Spectroscopy in Patients with Essential Tremor Undergoing MRgFUS Thalamotomy: A Pilot Study" Life 12, no. 11: 1741. https://doi.org/10.3390/life12111741
APA StyleBruno, F., Tommasino, E., Catalucci, A., Piccin, V., Innocenzi, A., Carugno, M. E., Colarieti, F., Pertici, L., Di Gioia, A., D’Alessandro, C., Fagotti, C., Sucapane, P., Pistoia, F., Palumbo, P., Arrigoni, F., Di Cesare, E., Marini, C., Barile, A., Splendiani, A., & Masciocchi, C. (2022). Cerebello-Thalamo-Cortical MR Spectroscopy in Patients with Essential Tremor Undergoing MRgFUS Thalamotomy: A Pilot Study. Life, 12(11), 1741. https://doi.org/10.3390/life12111741