Simple Summary
In recent years, consumers’ concern over the use of synthetic antioxidants and antibiotics in food is on the rise, prompting extensive research for alternatives of natural origin. Three essential oils from aromatic plants used in Greek traditional medicine were tested for their antioxidant, anti-inflammatory, and antimicrobial activity in order to determine their applicability as feed additives. The in vitro results showed that plants originating from the western part of Greece, the area of Epirus, possess potent anticoccidial, antimicrobial, anti-inflammatory, and antioxidant activity.
Abstract
Origanum vulgare subsp. hirtum, Thymus vulgaris, and Salvia fructicosa are aromatic plants commonly found in Mediterranean countries and are traditionally used in Greece as a remedy for humans, since they are well known as potent antibacterial, antioxidant, and anti-inflammatory agents. Essential oils (EOs) derived from plants cultivated in the mountainous region of Epirus, Greece, were investigated for their inhibitory activity against key microorganisms with relevance to avian health, while also assessing their antioxidant and anti-inflammatory activity. The total phenolic content (TPC) of the EOs was estimated according to the Folin–Ciocalteu method, while the antioxidant capacity was tested through the EOs’ ability to scavenge free radicals by means of the DPPH, ABTS, and FRAP assays. Antibacterial and anti-inflammatory effects were examined by the agar disc diffusion method and the lipoxygenase (LOX) inhibition test, respectively. Furthermore, the EOs’ ability to inhibit the invasion of sporozoites of Eimeria tenella (Wisconsin strain) along with any toxic effects were assayed in Madin–Darby bovine kidney (MDBK) cells. The antioxidant activity of the EOs was observed in descending order: oregano > thyme > sage. The antimicrobial effects of thyme and oregano were equivalent and higher than that of sage, while the anti-inflammatory effect of thyme was higher compared to both sage and oregano. The intracellular invasion of sporozoites was evaluated by the detection of E. tenella DNA by qPCR from cell monolayers harvested at 2 and 24 h post-infection. Parasite invasion was inhibited by the addition of oregano essential oil at the concentration of 100 μg/mL by 83% or 93% after 2 or 24 h, respectively, and was higher compared to the addition of thyme and sage, which had similar effects, but at a less intensive level. The cytotoxic assessment of all three essential oils revealed that they had no effect on MDBK cells compared to dimethyl sulfoxide (DMSO), used as the control substance. The supplementation of oregano, thyme, and sage essential oils had a potent antioxidant, anti-inflammatory, antimicrobial, and anticoccidial in vitro effect that is comparable to synthetic substances or approved drugs, justifying the need for further evaluation by in vivo studies in broilers reared in the absence of antimicrobial and anticoccidial drugs or synthetic antioxidant and/or anti-inflammatory compounds.
1. Introduction
Calls to screen natural compounds to discover solutions for the control of avian pathogens and to preserve oxidative stability in meat products have increased in response to the overuse of antibiotics [1]. Plant-derived secondary metabolites of importance include phenolic acids, flavonoids, terpenes, and volatile compounds. Essential oils, belonging to the latter group, along with some of their constituents such as carvacrol, eugenol, or thymol have become well known for their antioxidant and/or antibacterial activity and several phenolic compounds have been investigated for their ability to suppress microbial growth [2,3,4].
Oregano, thyme, and sage have frequently been exploited for preservative, culinary, and medicinal functions throughout history [2,5,6]. Greek oregano (Oregano vulgare subsp. hirtum; family Lamiaceae) offers multiple medicinal qualities including anti-inflammatory, antioxidant, analgesic, hepato-, gastro-, and neuroprotective properties, while its essential oil (ΕO) contains a high concentration of active ingredients, acquiring a high ranking as one of the best quality herbs in the world with significant commercial value [7,8]. The antimicrobial activity of oregano essential oil (OEO) has been thoroughly investigated and attributed to its high content in carvacrol (up to 90%), thymol, γ-terpinene, and p-cymene, influenced by the area of cultivation, harvesting season, and species [9,10]. Moreover, it has been observed to possess excellent antimicrobial and antioxidant capacity [1,11]. Thus, it is potent not only against Gram-negative bacteria such as Escherichia coli, Salmonella spp., or Proteus, but also Gram-positive bacteria, including lactic acid bacteria such as Lactobacillus, Leuconostoc, Pediococcus, Lactococcus, and Streptococcus. The antibacterial action of EOs stems from their ability to inhibit bacterial growth by altering cell membrane permeability and reducing bacterial toxin production, as they are viscous lipophilic liquids [12,13,14,15]. Additionally, OEO may limit the oxidation processes of raw meat and other food preparations because of its antioxidant properties [16,17]. Both antioxidant and antimicrobial effects may delay food from becoming off-flavor, rotten, or deteriorating due to the production of reactive oxygen species (ROS) and the proliferation of harmful microorganisms [18,19,20,21]. Likewise, many studies support the notion that OEO can reduce lipid peroxidation in meat, liver, and serum when added to broiler chicken feed [22,23]. In a recent study, OEO was shown to be useful to treat inflammation and promote wound healing [24]. Although there is a lot of information regarding the antibacterial activity of OEO, limited evidence exists on its efficacy and/or effectiveness against parasites, including a small number of in vitro studies that revealed the potential of OEO against parasite activity [25]. OEO was shown to inhibit Cryptosporidium parvum infectivity in HCT-8 cells without modulating sporozoites invasion [25] and exerted an antiparasitic capacity against protozoa such as Plasmodium falciparum, trypomastigote forms of Trypanosoma spp. and amastigotes of Leishmania donovani [26]. Interestingly, both carvacrol, the main phenolic monoterpenoid in OEO, and thymol in thyme essential oil exhibited the same antiprotozoal potency. They have also been tested against coccidia in vivo and in vitro [27,28].
Thymus vulgaris is recognized as a high-yielding source of essential oil, providing a minimum of 12 mL/kg, and is widely used for pharmaceutical and culinary purposes. Thyme essential oil (TEO) is a rich source of a wide range of aromatic bioactive components such as thymol and carvacrol, with a noted role as an antioxidative and antimicrobial agent [29]. TEO components include thymol, γ-terpinene, p-cymene, linalool, geraniol, and carvacrol, all of which possess antimicrobial properties as shown in various studies [29,30,31]. TEO has been screened against several common food-related bacteria including Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella typhimurium, Escherichia coli, Klebsiella pneumoniae, and Enterococcus faecalis, and has been observed to exhibit strong antimicrobial properties under in vitro conditions [32]. TEO, alone or in combination with other EOs, has also been reported to act against Gram-negative and/or positive bacteria in food-related preparations [33]. For these purposes, EOs can be directly added to the food surface and some edible films used in food preparations may serve as carriers [34,35]. Regarding TEO’s antiparasitic action, previous studies have revealed contradictory results, with the proliferation of Trypanosoma brucei being compromised when TEO was added in HL-60 cells, but not exhibiting any antiparasitic potency against Leishmania spp. [36].
Another essential oil of interest is derived from Salvia fructicosa (family Lamiaceae), commonly known as sage, which is abundant in southern Europe. Sage has been widely used as a food herb and medicine since ancient times. Numerous studies corroborate its beneficial biological qualities, including antioxidant, anti-inflammatory, and antimicrobial properties [37,38]. Different chemical components have been shown to be responsible for these activities [39,40]. Due to its high content of phenolics (such as polyphenols and flavonoids) and terpenoids, sage EO (SEO) exhibits antioxidant activity [40,41]. The most important components in SEO include linalool and terpinene. In vitro studies have proven that sage extracts positively affect and protect cultured cells from inductive oxidative stress. SEO was reported to reduce DNA damage related to inflammation and its components inhibited both lipoxygenase and acetylcholinesterase enzymes that are related to inflammatory and other chronic illnesses [41]. Moreover, sage was observed to protect HepG cells [42].
The purpose of the present study was to focus on the evaluation of the in vitro antioxidant, antimicrobial, anti-inflammatory, and antiparasitic activity of the essential oils obtained from Greek oregano, thyme, and sage plants.
2. Materials and Methods
2.1. Plant Sources and Essential Oil Extraction
Plant material of native Greek Origanum vulgare subsp. hirtum L. (oregano, IPEN (International Plant Exchange Network) accession number GR-1-80 BBGK- 03,2107), Salvia fructicosa (sage, IPEN accession number GR-1-80 BBGK- 04,2411) and from the cultivar Thymus vulgaris L. var Varico 3 (thyme) are ex situ maintained at the collection of the Balkan Botanic Garden of Kroussia 84 (41°05′44.3′′ N 23°06′33.7′′ E) of the Institute of Plant Breeding and Genetic Resources, Hellenic Agricultural Organization—DEMETER, in Greece (Figure 1). From these mother plants, young plants were produced asexually by cuttings and were provided to the company “Aromata Epirus”, (Palaiohori, Filiates Thesprotia, Epirus, Greece) where they were cultivated in the fields in autumn. After 1.5 years, when the plants were in full blossom, the leaves and flowers were collected and dried and the dried material was transferred to the Laboratory of Pharmacognosy, School of Pharmacy, Aristotle University of Thessaloniki, where it was submitted to hydrodistillation for 2 h using a modified Clevenger-type apparatus with a water-cooled oil receiver to reduce hydrodistillation overheating artifacts. The volatiles were trapped in 5 mL gas chromatography-grade n-hexane, according to the standard procedure described in European Pharmacopeia, dried over anhydrous sodium sulfate, and kept in closed, air-tight Pyrex containers at −4 °C until use in the in vitro trials. The volatile constituents of the essential oils were analyzed by gas chromatography–mass spectrometry (GC-MS) analysis, using a Shimadzu GC-2010-GCMS-QP2010, as previously described [30]. Authentic compounds (Fluka, Sigma-Aldrich, Taufkirchen, Germany) were used for co-chromatography comparison.
Figure 1.
Photos of oregano, (A), Salvia, (B), Thyme, (C), and the map area BBGK in Greece, (D).
2.2. Determination of EOs’ Active Compounds
The composition of the volatile constituents obtained by hydrodistillation was established by gas chromatography–mass spectrometry (GC–MS) analysis. The analyses of the essential oils were performed on a Shimadzu GC-2010-GC/MS-QP2010 system operating at 70 eV. This was equipped with a split/splitless injector (230 °C) and an HP INNOWAX capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm). The temperature program ranged from 50 °C (20 min) to 260 °C, at a rate of 3 °C/min. Helium was used as a carrier gas at a flow rate of 1.0 mL/min. The injection volume of each sample was 1.0 μL. The relative percentage amounts were calculated from total ion chromatograms (TIC) by the computer. Arithmetic indices for all compounds were determined according to van den Dool and Katz [43], using n-alkanes as standards. The identification of the components was based on the comparison of their mass spectra with those of NIST21 and NIST107 and their retention indices with literature data [44,45]. Essential oils were also subjected to co-chromatography with authentic compounds (Fluka, Sigma) and their essential oil yield was expressed in mL 100 g−1 d.w.
2.3. Determination of Total Phenolic Content of EOs
The total phenolic content (TPC) of the EOs was determined using the Folin–Ciocalteu method [46], with slight modifications: 5 mg of each EO was diluted in 1 mL of absolute ethanol (EtOH), and 20 μL of each ethanolic solution was added to a test tube containing 2500 μL deionized water and 400 μL Folin–Ciocalteu phenol reagent (F9252, Sigma-Aldrich, Taufkirchen, Germany). The mixtures were kept in the dark at room temperature for 8 min. Then, 500 μL of Na2CO3 7% solution was added to the tubes and the mixture was incubated in the dark at room temperature for 45 min. Each sample absorbance was measured at λ = 750 nm, using a UV-Vis spectrophotometer (UV-1700 PharmaSpec, Shimadzu, Kyoto, Japan). The total phenolic content was calculated by means of a standard curve, using standard solutions of gradually increasing concentrations (0–1.5 mg mL −1, R2 = 0.947) of gallic acid. The results were expressed as mg of gallic acid equivalents per L of EO (mg GAE L−1), from the mean of three measurements ± standard error for each sample.
2.4. Antioxidant Assays
Several in vitro assays were employed for the assessment of the antioxidant activity of the examined EOs, namely: (i) interaction with the free stable radical DPPH (1,1-diphenyl-2-picrylhydrazyl), (ii) ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) radical cation decolorization assay, and (iii) FRAP (ferric-reducing antioxidant power). In all of the aforementioned assays, the EO samples were diluted in absolute EtOH at a concentration of 5 mg mL−1 (for the DPPH assay) or 1 mg mL−1 (for the FRAP and the ABTS assays). In all assays, a UV-1700 PharmaSpec spectrophotometer (Shimadzu, Kyoto, Japan) was used for the absorbance measurement. All experiments were carried out in triplicate and the results were expressed as mean ± standard error.
2.4.1. Interaction with DPPH
The DPPH assay was performed by incubating 20 μL of ethanol-diluted EOs (5 mg mL−1) with DPPH (D211400, Sigma-Aldrich, Taufkirchen, Germany) methanolic solution (0.1 mM) in the dark, at room temperature. Each sample absorbance was measured at λ = 517 nm at two time points, t1 = 20 min and t2 = 60 min, and the percentage of radical-scavenging activity (%RSA) was calculated as follows:
where Ab is the absorbance of the blank sample (containing EtOH instead of the EO samples) and As is the absorbance of each sample [38]. Trolox was used as a reference compound.
%RSA = [(Ab − As)/Ab] × 100
2.4.2. ABTS Radical Cation Decolorization Assay
For the preparation of the ABTS+ solution, 38.4 mg of ABTS (A1888, Sigma-Aldrich, Taufkirchen, Germany) was dissolved in 10 mL of water, along with 6.6 mg of K2S2O8 (potassium persulfate) (7 mM and 2.45 mM, respectively) and stored in the dark for 16 h at room temperature. The next day, the radical solution was diluted with ethanol to an absorbance of 0.70 ± 0.02 at λ = 234 nm. The protocol of Zhen et al. [47] was followed, with slight modifications: 30 μL of EO sample (diluted in EtOH) was added to 2970 μL of ABTS+˙ solution and the mixture was left to stand in the dark for 6 min at room temperature. The percentage of discoloration (and, hence, the reducing activity of the samples) was calculated in the same manner as in the DPPH assay (see Equation (1)). The blank sample consisted of 100% EtOH, and Trolox was used as a reference compound. The results were expressed in the same way as the DPPH assay, i.e., %RSA ± standard error.
2.4.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
The ferric-reducing antioxidant power (FRAP) of the samples was determined according to the protocol of Benzie and Devaki [48]: 2900 μL of the FRAP solution, heated at 37 °C, was added to a test tube containing 100 μL of EO sample (or pure EtOH for the blank sample) and the mixture was vortexed and left in the dark for 30 min at room temperature. At the end of the incubation time, the samples’ absorption was measured at 593 nm, and their FRAP values were expressed as μmol ascorbic acid L−1 (μmol AsA L−1) based on a calibration curve (0–2,000 μmol AsA L−1, R2 = 0.996). The FRAP solution consisted of acetate buffer (300 mM, pH 3.6), FeCl3 (20 mM in ultrapure water), and TPTZ (2,4,6-tripyridyl-S-triazine, A17201.06, Alfa Aesar, Sigma-Aldrich, Taufkirchen, Germany) (10 mM in 40 mM HCl) at a ratio of 10:1:1, freshly prepared before each assay.
2.5. Anti-Inflammatory Assay: Soybean Lipoxygenase Inhibition
Each sample’s ability to inhibit soybean lipoxygenase in vitro was used as a measurement of their anti-inflammatory potential by means of the FOX test, an assay based on the formation of a deep red-brown complex between xylenol orange and Fe3+ in acidic conditions, which are, in turn, formed by the hydroperoxides resulting from the oxidation of linoleic acid by soybean lipoxygenase. For this purpose, the protocol of Ondua et al. [49] was followed, with reagent volumes adjusted for a spectrophotometer: the EO samples were first diluted in dimethyl sulfoxide (DMSO) and subsequently in Tris–HCl buffer (50 mM, pH 7.4) at a final concentration of 0.5 mg mL−1. Quercetin was used as a positive control (final concentration 1 mg/mL). Then, 200 μL of the EOs (or quercetin) was added to test tubes and mixed with Tris–HCl buffer (200 μL) and 400 μL of LOX enzyme (L7395-15MU, Sigma-Aldrich, Taufkirchen, Germany) dissolved in ice-cold Tris–HCl buffer (final concentration 0.2 U mL−1). The mixture was incubated at room temperature for 5 min, and afterwards, 400 μL of linoleic acid (i.e., the enzyme’s substrate) dissolved in Tris–HCl (final concentration 140 μM) was added to the assay mixture, which was then left to stand in the dark for 20 min at room temperature. Finally, 1,000 μL of freshly prepared FOX reagent (xylenol orange (100 μM), FeSO4 (100 μM), and H2SO4 (30 mM) in MeOH(aq) 90%) were added to the tubes and the mixture was incubated for 30 min. The negative control contained a mixture of DMSO/Tris–HCl buffer instead of EOs, whereas in the blank samples, linoleic acid was added to the assigned tubes at the end of the third incubation period, just before the absorbance measurement, at 560 nm. Each EO sample had its own negative control and blank. Each sample’s inhibitory activity was calculated according to the following formula:
%LOX inhibition = [(Ac − As)/Ac] × 100
(Ac = absorbance of the negative control and As = absorbance of EO sample absorbance of the corresponding blank). The results are expressed as the mean of the three measurements ± standard error for every EO.
2.6. Antimicrobial Capacity
2.6.1. Antibacterial Activity with Disk Diffusion Method
All of the reference bacterial strains that were used for the experiment were purchased from American Type Culture Collection (ATCC). The antibacterial activity was examined by the agar disc diffusion method (Clinical and Laboratory Standards Institute CLSI 2012, doc M02-A11). The three essential oils were provided to the Laboratory of Animal Production, Nutrition, and Biotechnology, Department of Agriculture, School of Agriculture, University of Ioannina, to assess the antibacterial properties of the EOs test samples. Four ATCC bacterial strains were used: S. aureus ATCC25923, E. coli ATCC 25922, E. coli ATCC 35,218 and Lactobacillus fermentum ATCC 9338, obtained from the microbial collection of the Laboratory of Food Hygiene. Pure EOs were diluted to 50%, 20%, and 5% concentration in DMSO 5% (v/v). Forty-eight hours before the study, the bacterial strains were revitalized and checked for purity. Bacterial suspensions of each bacterial ATCC strain in saline solution of 0.5 McFarland (density of approximately 1.5 × 108 CFU mL−1) turbidity were streaked onto Mueller–Hinton agar (MHA) with a sterile swab. A sterile filter disk (diameter 6 mm, Whatman paper N. 1) was impregnated with EOs (15 µL/disk). The inoculated MHAs were incubated aerobically at 37 °C for 18–24 h for S. aureus ATCC25923, E. coli ATCC 25,922, and E. coli ATCC 35,218 and anaerobically at 37 °C for 48 h for Lactobacillus fermentum ATCC 9338. The antimicrobial activity was evaluated by measuring the zones of growth inhibition. Penicillin G (10 µg, Oxoid, Basingstoke, UK) was used as the positive control for Gram-positive bacteria, while enrofloxacin (5 µg, Oxoid, Basingstoke, UK) was used as the positive control for Gram-negative bacteria. The experiment was performed in triplicate.
2.6.2. Determination of MIC
The minimum inhibitory concentration (MIC) was determined using the broth microdilution method according to the Clinical and Laboratory Standards Institute doc M07Ed11, with modifications. Here, 200 µL of each pure EO was pipetted in the first well of rows A–F of a 96-well plate, and then 100 µL of double-strength Müller–Hinton broth (MHB) containing 5% DMSO was dispensed into wells (2 to 12) of each column. Following this, 100 µL of each EO from the first column was sequentially mixed in MHB in the neighboring column, achieving two-fold serial dilutions. The remaining 100 µL from the last dilution mix was discarded. Forty-eight hours before the study, the bacterial strains were revitalized on Columbia blood agar (Oxoid Limited, Basingstoke, UK) and checked for purity. A standard inoculum of 0.5 McFarland units (density of approximately 1.5 × 108 CFU mL−1) from each tested organism was prepared in sterile saline. The bacterial suspension was diluted 1/10 in saline solution and finally 100 µL was added to each well of each column over the EOs, which were mixed in the previous stage in MHB containing 5% DMSO. Thus, the EO concentration (v/v) in the final volume in wells 1–12 was 50%, 25%, 12.5%, 6.25%, 3.125%, 1.562%, 0.781%, 0.39%, 0.195%, 0.097%, 0.048%, and 0.024%, with each well containing approximatively 1.5 × 106 CFU. Positive and negative control wells were prepared for each plate, in the last row (100 µL Müller–Hinton broth and 100 µL of standardized bacterial inoculum for positive control in well H11, and 200 µL Müller–Hinton broth without essential oils for negative control in well H12). The plates were placed for incubation accordingly. The MIC was interpreted in the last well of each row where no visible bacterial growth was noticed (bacterial growth inhibition) and interpreted as v/v percentage of stock solution.
2.6.3. Determination of MBC
The minimum bactericidal concentrations (MBC) were determined from the last three wells of each row that showed no bacterial growth after plate incubation. For this, 15 µL from the corresponding wells was spot-inoculated on blood agar plates, which were labeled with the corresponding well’s coordinates. The plates were incubated overnight at 35 °C, and colony development was followed in each spot-inoculation place. The MBC was noted for the position where no bacterial colonies developed and interpreted as v/v percentage of stock solution.
2.7. Antiparasitic Capacity of EOs
2.7.1. Essential Oils
Stocks of oregano, thyme, and sage essential oils were supplied to the Department of Pathobiology and Population Sciences, Royal Veterinary College, University of London and prepared to a final concentration of 1 mg mL−1 in DMSO.
2.7.2. Cell Culture
Madin–Darby bovine kidney (MDBK) (Sigma-Aldrich, Taufkirchen, Germany) was maintained at 37 °C–5% CO2 in Advanced DMEM (Gibco, Leicestershire, UK) supplemented with 2% fetal bovine serum (FBS; Sigma, Suffolk, UK) and 100 U penicillin/streptomycin (Fisher, Leicestershire, UK). Monolayers of MDBK cells were prepared in 24-well plates at 0.3 × 106 cells/well and seeded ~3 h prior to infections.
2.7.3. Parasites
Sporozoites of the Eimeria tenella Wisconsin strain were used to perform the infections. Oocyst excystation and sporozoite purification were performed as described previously [50].
2.7.4. Cytotoxicity Test
Each EO was also tested for cytotoxic effects in MDBK cells using 100 μL mL−1 per well. Morphological changes on the cell line were observed up to 24 h after exposure and compared to the control groups (DMEM/DMSO).
2.7.5. Pretreatment and Infection
Sporozoites of E. tenella (0.5 × 106/well) were pretreated for 1 h at 41 °C–5% CO2 with essential oils of thyme and sage at different concentrations (100, 50, 20, and 5μg mL−1). DMSO (10 μL mL−1) and robenidine (5 μg mL−1) were used as untreated and inhibited controls for E. tenella invasion, respectively. After pretreatment, sporozoites were added to monolayers of MDBK cells (41 °C–5% CO2, two wells/time point/group). At 2 h and 24 h hours post infection (hpi), the infected monolayers were washed in phosphate-buffered saline (0.5 mL/well) and the cells were dissociated using 0.35 mL of RTL buffer provided with a DNeasy Blood & Tissue Kit (Qiagen, Manchester, UK) and stored at −20 °C. Two biological replicates were performed.
The proportion of sporozoite invasion was calculated normalizing samples with the DMSO group to evaluate the inhibition level following the method adapted to Thabet et al. [51].
2.7.6. Isolation of Nucleic Acids and Real Time Quantitative PCR
Genomic DNA was isolated using a DNeasy Blood & Tissue Kit (Qiagen, Manchester, United Kingdom) according to the manufacturer’s instructions. The DNA was eluted in a final volume of 165 μL per sample. Real-time quantitative PCR (qPCR) was performed in a CFX96 Touch® Real-Time PCR Detection System (Bio-Rad, Hertfordshire, UK) according to Marugán–Hernández et al. [52]. The quantification of E. tenella per sample used gDNA and primers targeting the Eimeria genus 5S rDNA. Each qPCR plate used a mix of 19 μL/well (10 μL of Evan Green, 0.5 μl of 10 μM 5S Primer and 8.5 μL molecular grade water) and 1 μL of DNA. A standard curve with serial dilutions of sporozoite DNA was also tested per plate using concentrations from 1 × 107 to 1 × 101 g DNA E. tenella genomes. All groups and the standard curve were evaluated testing three technical replicates per sample.
2.8. Statistical Analysis
The experimental data for antioxidant, anti-inflammatory, and antimicrobial activity were subjected to analysis of variance (ANOVA) using the statistical package SPSS version 20.0 for Windows (SPSS, Inc., Chicago, IL, USA). As the bacterial numbers were not normally distributed, they were log10 transformed to create a normal distribution prior to analysis. Tukey’s post hoc test (p < 0.05) was performed to assess any significant differences between the experimental treatments. All data obtained from the antiparasitic studies were analyzed using the Bio-Rad CFX Manager software (Bio-Rad). The quantification of the number of parasites was performed considering the standard deviation (SD) of Cq values for replicates, excluding SD > 0.05. The average starting quantity (SQ) values per sample were used to plot graphics. Statistical analysis was done by GraphPad (GraphPad Prism 8, CA, San Diego, USA). The Shapiro–Wilk test was used to access data normality. Differences and comparisons among groups were performed by one-way ANOVA or Kruskal–Wallis test, followed by Dunn’s multiple comparisons test. Two levels of comparison (time and dosage) were tested using normalized data and the mixed-effects model REML.
3. Results
3.1. Yield of Crude Extracts and Fractions
The hydrodistillation of oregano fresh material yielded 5.49% essential oil. The hydro-distillation of thyme fresh material yielded 4.15% essential oil, whereas the sage fresh material yielded 4.66% essential oil.
3.2. Chemical Composition of Tested EOs
Supplementary Figures S1–S3 provide the chromatograms for oregano, salvia and thyme essential oil, respectively. Table 1, Table 2 and Table 3 provide the detailed composition of OEO, TEO, and SEO, respectively.

Table 1.
Chemical composition of oregano essential oil (OEO).

Table 2.
Chemical composition of thyme essential oil (TEO).

Table 3.
Chemical composition of sage essential oil (SEO).
3.3. Total Phenolic Content (TPC) of EOs
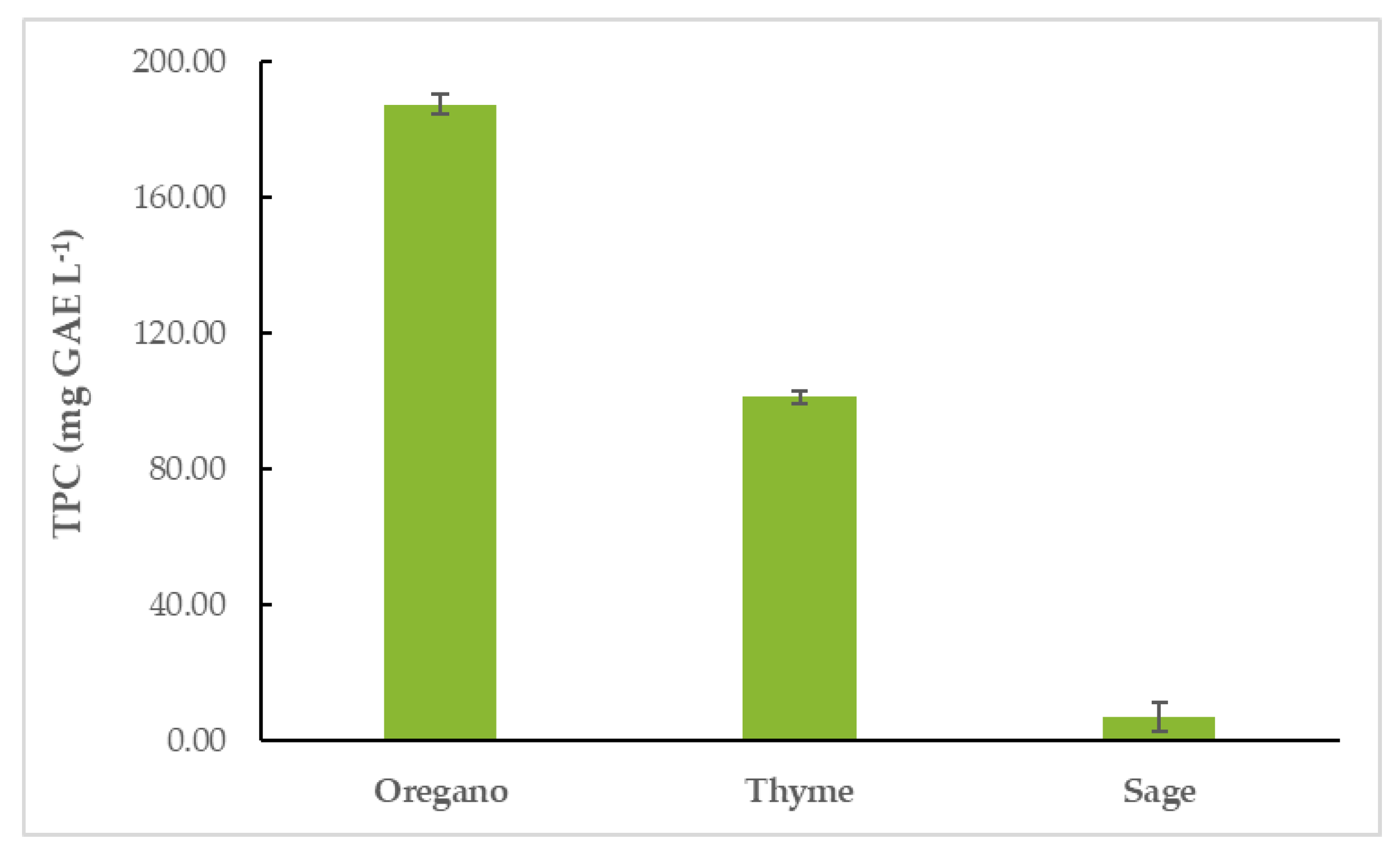
Oregano essential oil (OEO) had the highest TPC (187.64 ± 2.73 mg GAE L−1), followed by thyme (TEO) (101.36 ± 1.70 mg GAE L−1) and sage EO (SEO) (7.00 ± 4.19 mg GAE L−1). The results are shown in Figure 2 and Table S1.
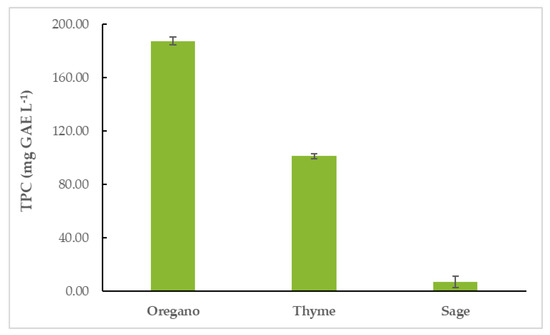
Figure 2.
Total phenolic content (TPC) of EOs.
3.4. Antioxidant Activity of EOs
3.4.1. Interaction with DPPH and ABTS
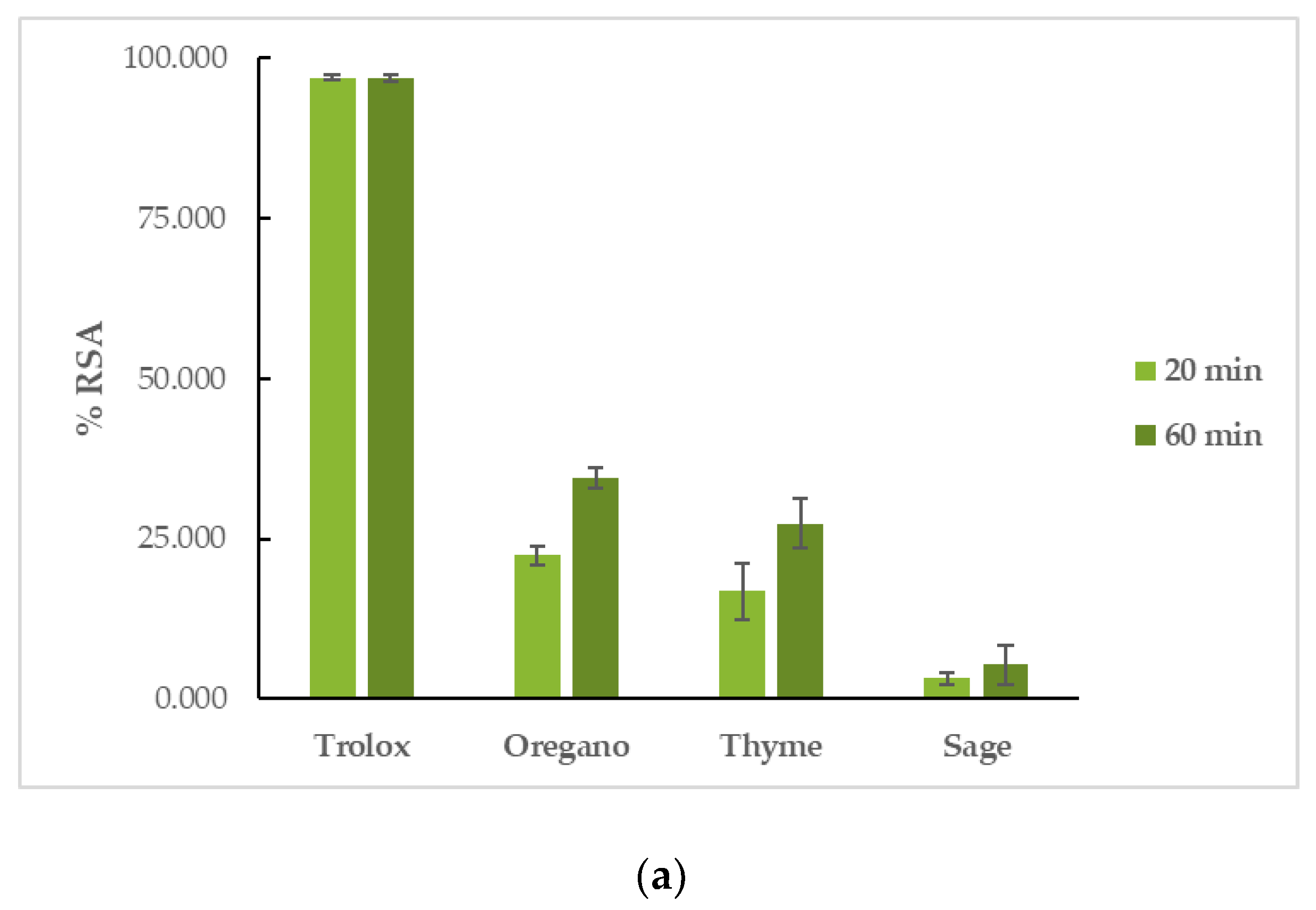
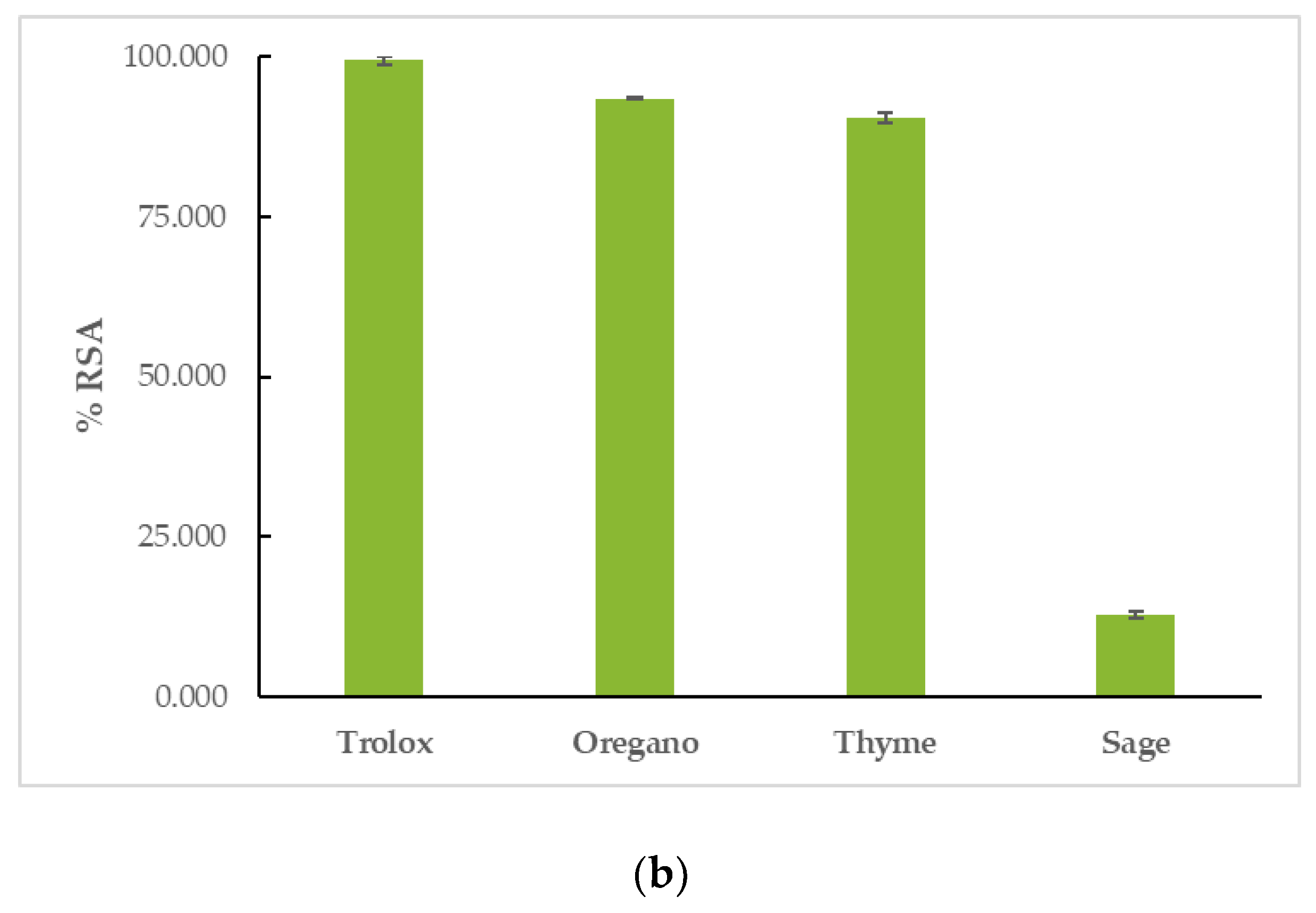
OEO had the highest reducing activity in both assays, followed by TEO, and finally, by SEO. More specifically, in the DPPH assay, OEO had a %RSA20min = 22.46 ± 1.51% and %RSA60min = 34.57 ± 1.55%, TEO had a %RSA20min = 16.83 ± 4.46% and %RSA60min = 27.38 ± 3.90%, and SEO had a %RSA20min = 3.20 ± 0.92% and %RSA60min = 5.31 ± 3.05%. The reaction proved to be time-dependent for the tested EOs, as their capacity to interact with the DPPH free radical increased with time. Trolox had a %RSA20min = 97.00 ± 0.46% and %RSA60min = 96.80 ± 0.53%. In the ABTS assay, OEO’s %RSA was 77.16 ± 0.51%, TEO’s %RSA was 73.38 ± 2.19%, and SEO’s %RSA was 7.24 ± 0.53%. Trolox had a %RSA = 99.41 ± 0.59%. The results can be seen in Figure 3 and Table S2.
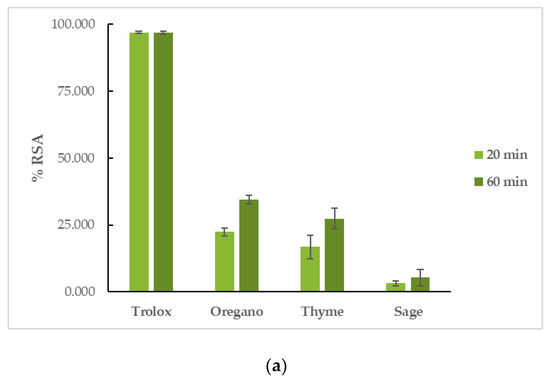
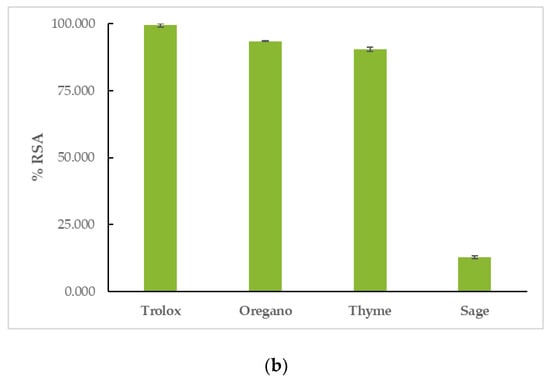
Figure 3.
Antioxidant activity of EOs: interaction with (a) DPPH and (b) ABTS free radicals.
3.4.2. FRAP Assay
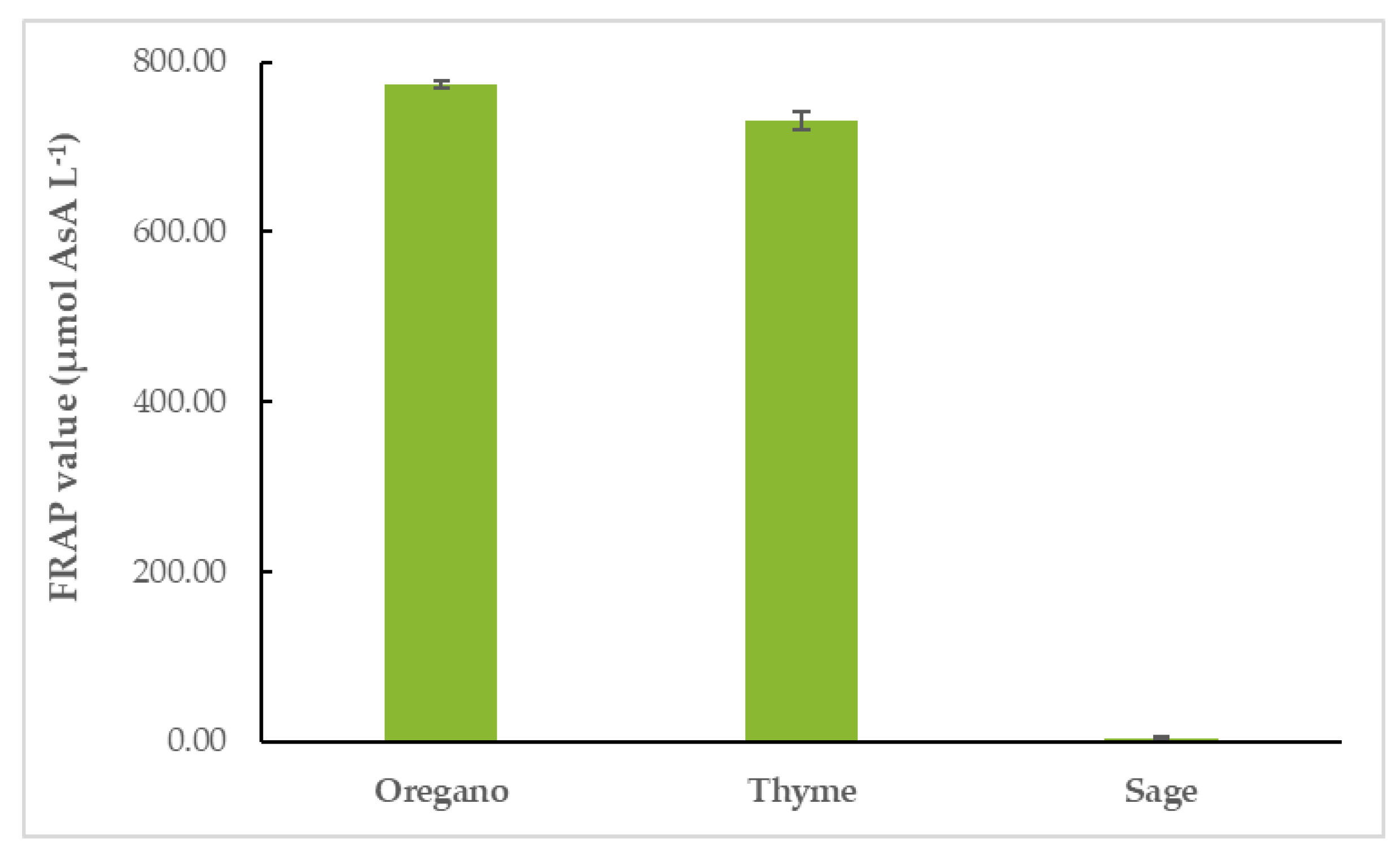
OEO had the highest FRAP value (774.04 ± 4.60 μmol AsA L−1), followed closely by TEO (731.29 ± 10.69 μmol AsA L−1). SEO’s FRAP value was the lowest of the three (4.30 ± 2.40 μmol AsA L−1), as shown in Figure 4 and Table S3.
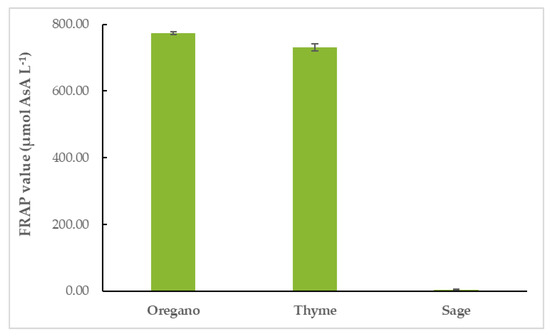
Figure 4.
Antioxidant activity of EOs: FRAP values of EOs.
3.5. Anti-Inflammatory (LOX-Inhibitory) Activity of EOs
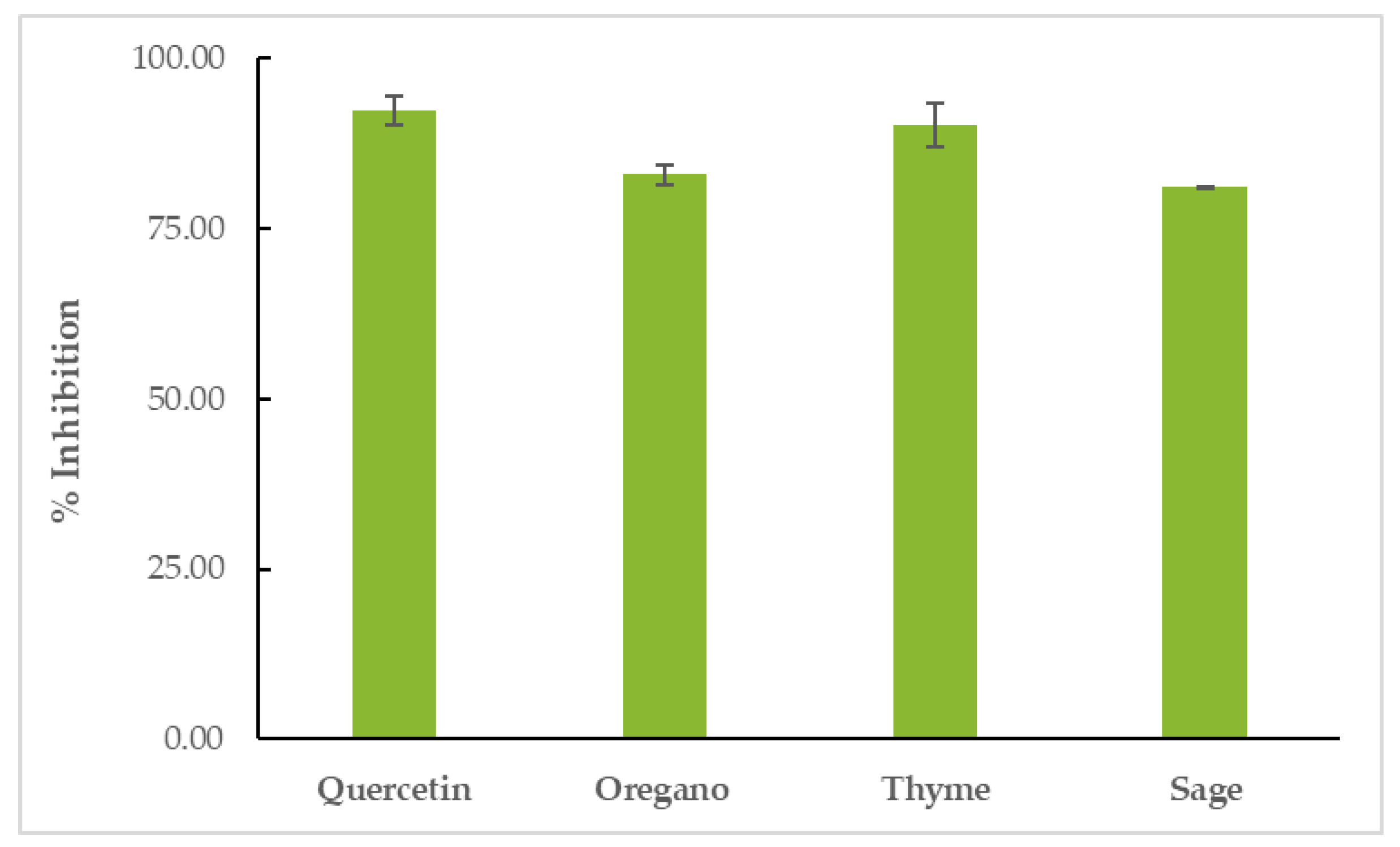
All EOs showed inhibitory activity against soybean lipoxygenase in vitro. TEO proved to be the most effective inhibitor of the enzyme (% LOX inhibition = 90.24 ± 3.24%), surpassed only by the positive control, quercetin (% LOX inhibition = 92.39 ± 2.21%). OEO’s inhibitory activity was 82.92 ± 1.40% and SEO’s inhibitory activity was 81.07 ± 0.24, as shown in Figure 5 and Table S4.
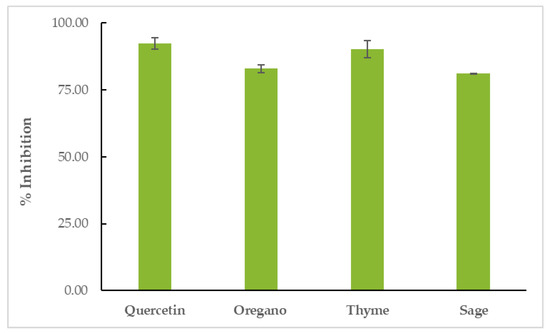
Figure 5.
Inhibition of soybean LOX by EOs.
3.6. Antibacterial Capacity
The antibacterial activity was estimated using disk diffusion and minimum inhibitory concentration (MIC) assays. Briefly, the disk diffusion test was used to determine the susceptibility of ATCC bacterial strains to each of the investigated EOs. The effectiveness of each EO was based on the production of an inhibition zone, while an ineffective EO may not affect bacterial growth at all. MICs were defined as the lowest concentration of each EO that inhibited the visible growth of a microorganism after overnight incubation.
3.6.1. Disk Diffusion Assay
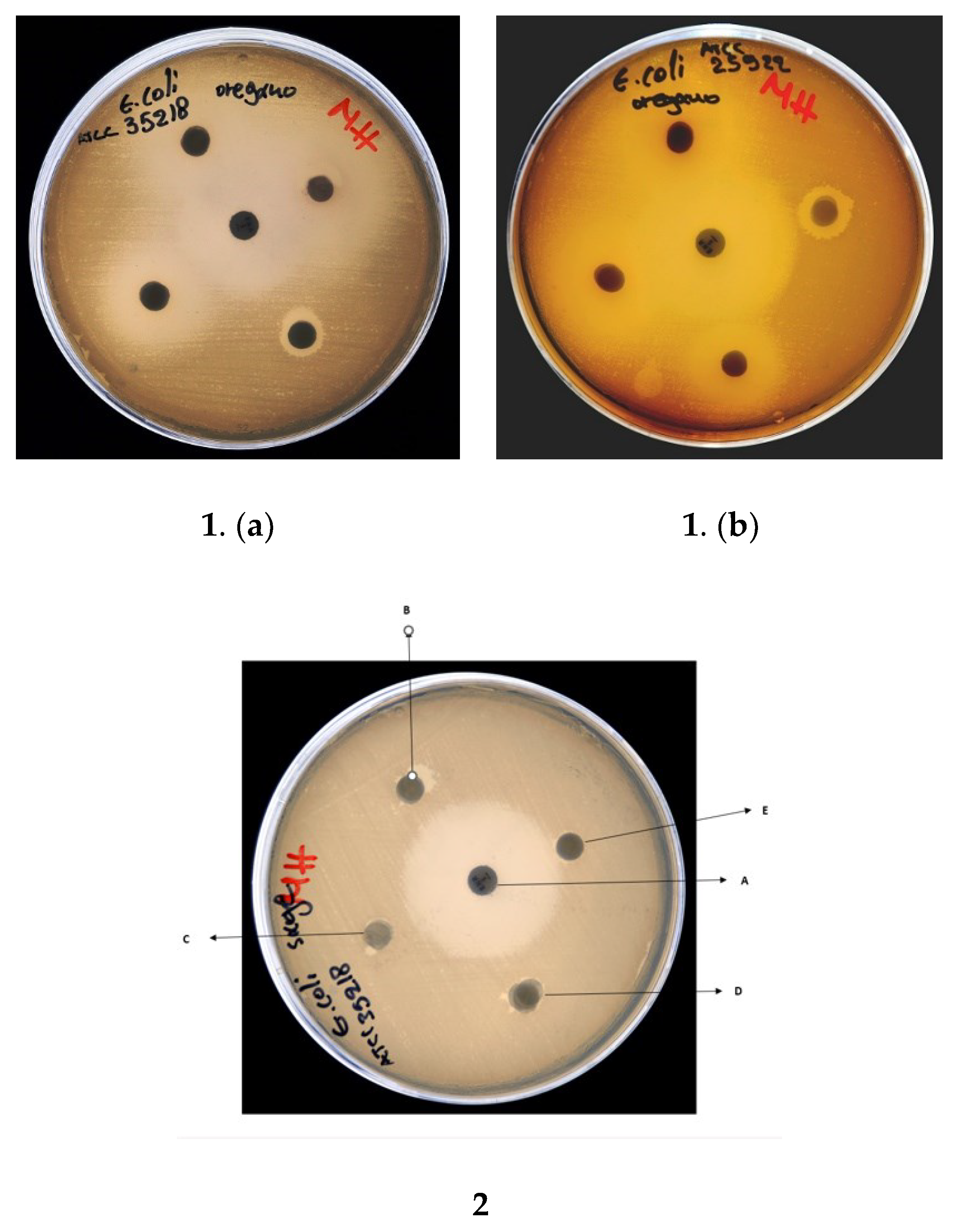
The results of the disk diffusion assays are shown in Table 4 and Figure 6. TEO exhibited the highest inhibitory activity among the three tested EOs, in some examples comparable to the control antibiotics. TEO contained a high carvacrol content. Sage, representing a low carvacrol concentration of 0.69%, showed the lowest inhibitory activity. In particular: (a) for the S. aureus ATCC 29,213 strain, TEO exhibited strong inhibition equal to OEO and control-1, (b) for the E. coli ATCC 25,922 strain, TEO had slightly stronger inhibition than ORE and less than Control-2, (c) for E. coli ATCC 35,218, TEO caused the same level of inhibition as Control-2 and slightly stronger than ORE, and (d) for Lactobacillus fermentum ATCC9398, TEO had the same level of inhibition compared to ORE and Control-1. In all cases, the inhibition of bacterial growth of EOs acted in a dose-dependent manner.

Table 4.
Antibacterial activity of EOs against bacterial ATCC strains by paper disk diffusion method.
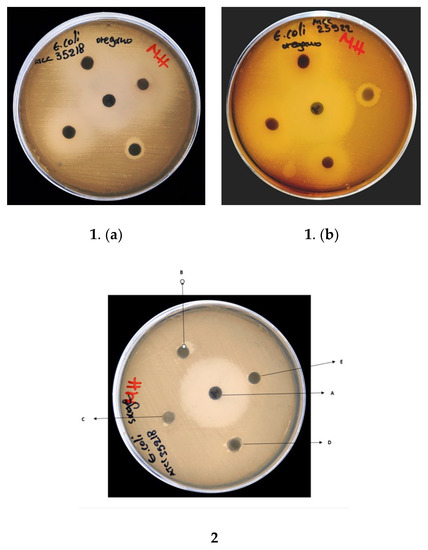
Figure 6.
Disk diffusion assay showing antibacterial activity of: (1) oregano EO against (a) E. coli ATCC 35,218 and (b) E. coli ATCC 25922; (2) sage EO against E. coli ATCC 35218. An enrofloxacin disc (A) (5 mg/mL) used as positive control; EOS were used at concentrations of (B) 100%, (C) 50%, (D) 20%, and (E) 5%.
3.6.2. Broth Microdilution Method
The EO MIC and MBC values (% v/v) against S. aureus ATCC 29213, E. coli ATCC 25922, E. coli ATCC 35218, and L. fermentum ATCC 9338 are presented in Table 5, along with the values of the aforementioned concentrations expressed as mg mL−1 (shown in Table 5). The results are displayed in Figure 7. While MIC is the lowest concentration of an antibacterial agent necessary to inhibit visible growth, MBC is the minimum concentration of an antibacterial agent that results in bacterial death. Thus, the closer the MIC to the MBC, the more bactericidal the compound [53].

Table 5.
MIC and MBC values (mg mL−1) of EOs against S. aureus ATCC 29213, E. coli ATCC 25922, E. coli ATCC 35218, and L. fermentum ATCC 9338.
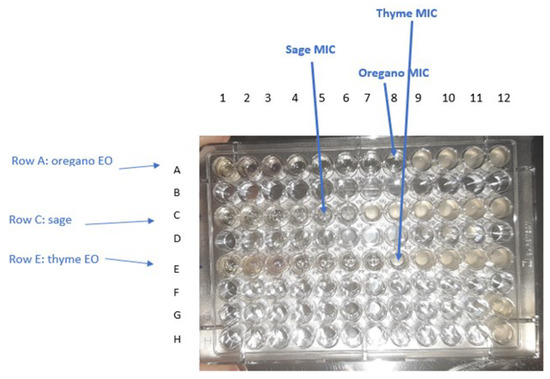
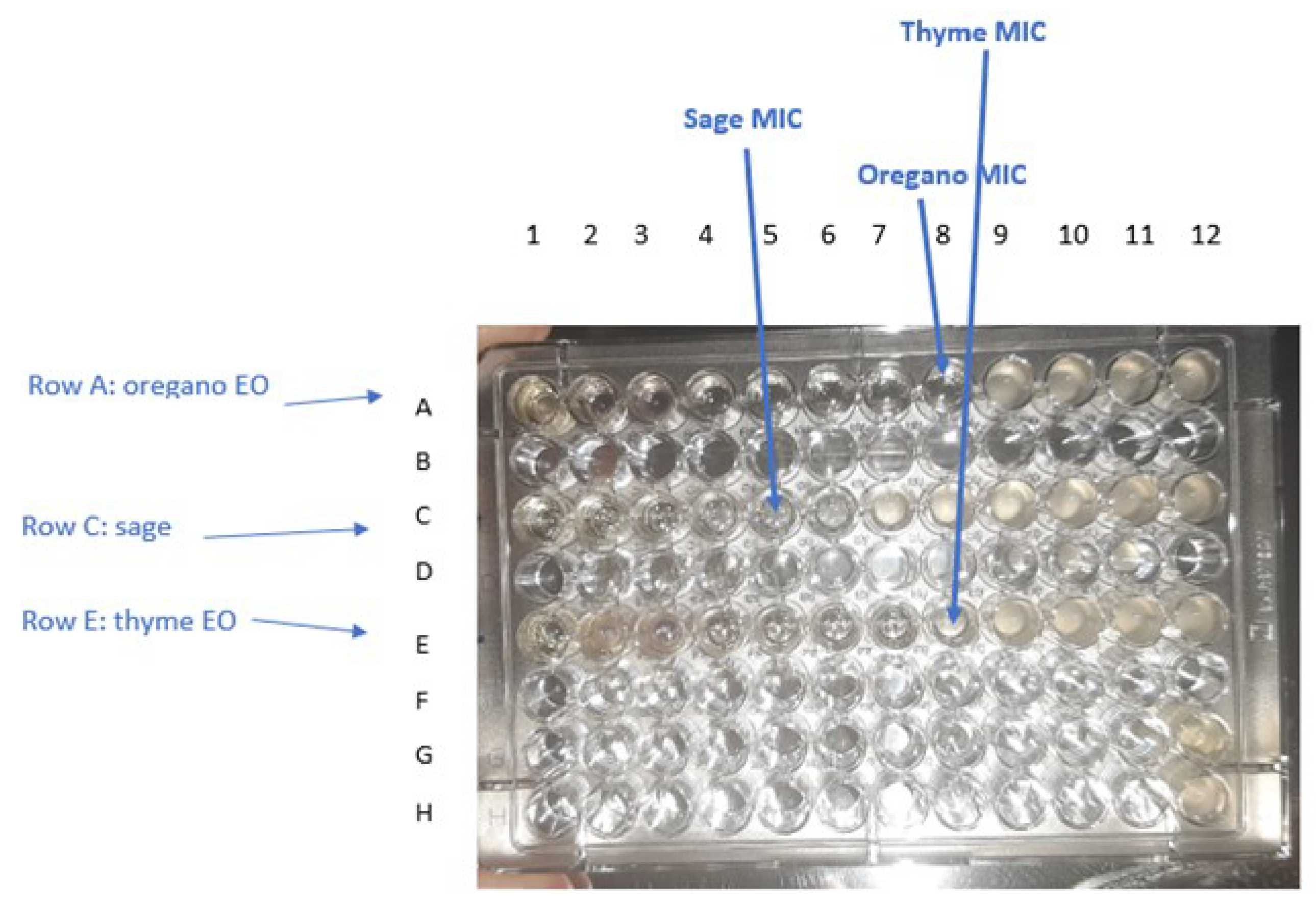
Figure 7.
Determination of MIC for oregano, sage, and thyme EOs against E. coli ATCC 25,922 after incubation period by observing turbidity. The range of each EO concentration in the wells is 50% to 0.024% (v/v). Well 8 of row A shows no turbidity, therefore the concentration of oregano ΕO in that column was taken as the MIC value (0.39% v/v). Well 5 of row C shows no turbidity, therefore the concentration of sage ΕO in that column was taken as the MIC value (3.125% v/v). Well 8 of raw E shows no turbidity, therefore the concentration of thyme ΕO in that column was taken as the MIC value (0.39% v/v). Well G12 and H12 are the negative and positive control, respectively.
For both values expressed in % v/v and mg mL−1, TEO had the best MIC and MBC values against S. aureus ATCC 29,213 compared to ORE, while SEO had no major effect. ORE presented the best results for E. coli ATCC 25922, E. coli ATCC 35,218, and L. fermentum ATCC 9338 strain. SEO had greater antimicrobial effects in E. coli ATCC 25,922 and E. coli ATCC 35,218 than S. aureus ATCC 29,213 or L. fermentum ATCC 9338 strains; however, its antimicrobial activity proved to be less than the other two EOs.
3.7. Antiparasitic Capacity
Anticoccidial Effect of EOs in Eimeria Tenella Using an In Vitro System
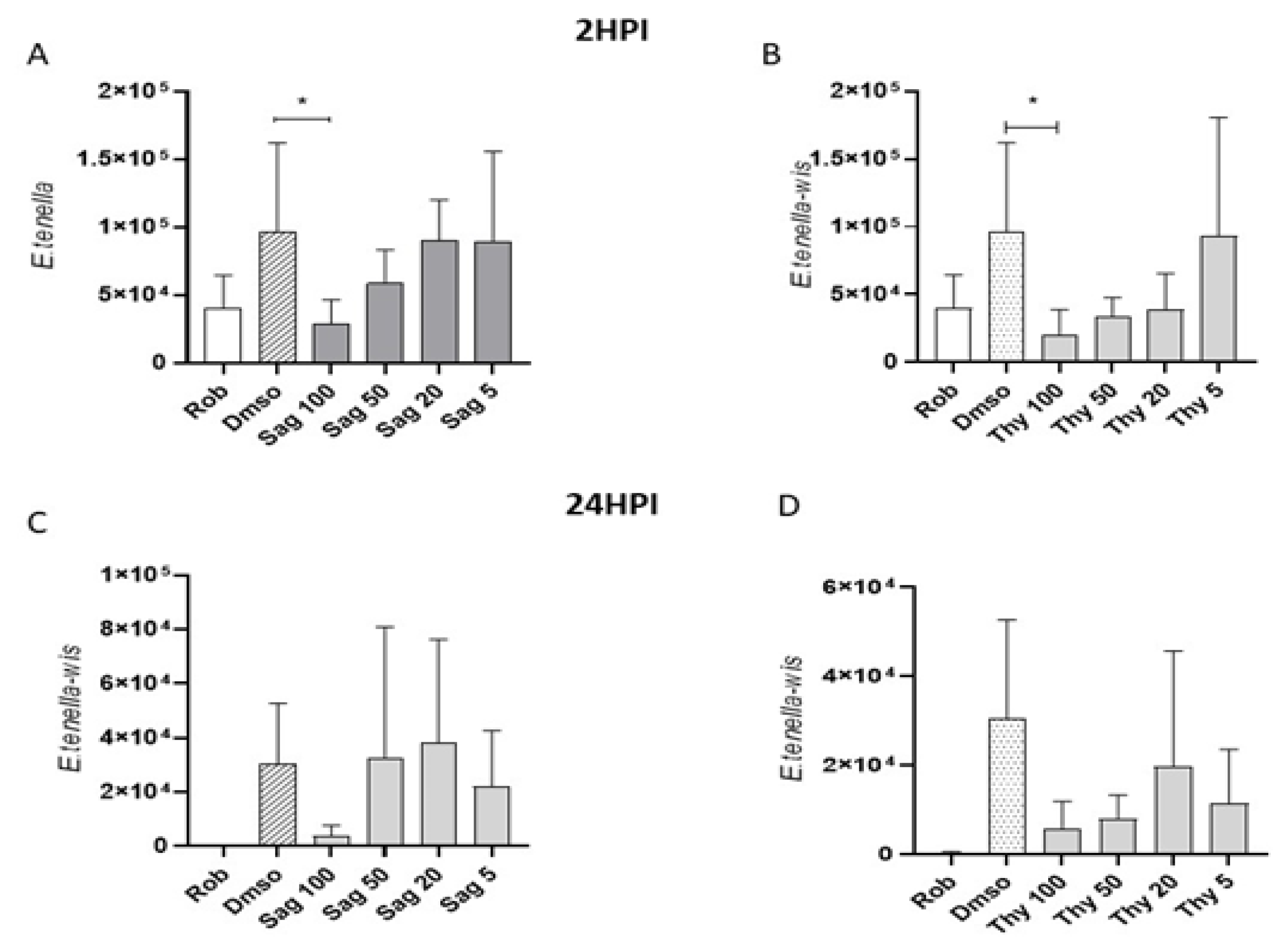
Effects of thyme and sage essential oils in reducing host cell invasion by E. tenella sporozoites were evaluated in an in vitro system using quantitative qPCR and were compared to previous effects reported for OEO [30]. The effects were tested at two different time points after invasion (2 and 24 hpi) at different concentrations (100, 50, 20, and 5 μg mL−1). The untreated control (DMSO) and inhibition control (robenidine) of invasion were included. Normal morphology was observed in MDBK cells exposed to concentrations of 100 μL mL−1 of oregano, thyme, or sage EOs. The invasion of E. tenella in the controls showed the expected effect (increase and decrease of infection for DMSO and robenidine, respectively). At 2 hpi, only the highest concentration of sage and thyme (100 μg mL−1) showed a significant reduction in comparison to the untreated control (DMSO vs. sage 100, thyme 100, Dunnett’s multiple comparisons test, p < 0.05) (Figure 8A,B). At 24 hpi, none of the tested concentrations for sage and thyme showed a significant reduction in relation to the untreated control (DMSO). However, there was a tendency towards the reduction of invaded sporozoites (Table 6, Figure 8C,D).
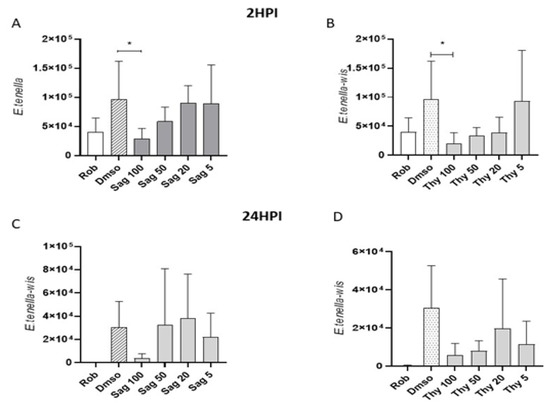
Figure 8.
Effects of sage and thyme essential oils in E. tenella host cell invasion. Graphs show the number of intracellular sporozoites for: 2 (A,B) and 24 (C,D) hpi after pretreatment with sage (Sag) and thyme (Thy) essential oil at different concentrations (100, 50, 20, and 5 μg mL−1) and for the untreated (DMSO; 10 μL mL−1) and inhibition (robenidine, Rob; 5 μg mL−1) controls. *Asterisks indicate significant differences (Dunnett’s multiple comparisons test, p < 0.05).

Table 6.
Sporozoite inhibition after treatment with oregano, sage, and thyme EOs at various concentrations.
4. Discussion
In the current study, oregano, thyme, and sage EOs were examined for their in vitro antioxidant, anti-inflammatory, antimicrobial, and antiparasitic efficacy. Based on the GC-MS results, the main bioactive compounds of the oregano EO were carvacrol and γ-terpinene, of thyme EO were thymol and p-cymene, and of sage EO were eucalyptol and camphor. Essential oils have already been used in veterinary medicine and may be classified as follows: oils attracting and repelling animals; insecticidal, pest-repellent, and antiparasitic oils; oils used in animal feed; and oils used in the disease treatment of animals [54].
Although their mechanisms of action have not been fully elucidated, Many EOs have been widely applied to treat infections due to their antimicrobial activity [55]. The results from the in vitro studies in this work showed that the essential oils inhibited bacterial growth and parasite invasion with varied effectiveness. The antioxidant effects were evaluated using three different tests providing comparisons for different antioxidant mechanisms. The significance of EOs in combating oxidative stress in cells and organisms, together with their antimicrobial, anti-inflammatory, and other functions, has been validated by many studies; however, only a few have conducted direct comparisons [30,56]. There are many methods measuring the antioxidant activity of OEO, TEO, and SEO components, usually based on the scavenging of free radical species, such as DPPH, ABTS, and FRAP, determining the absorbance of the reduced product using a photometric assay. Here, the three tested EOs were evaluated and proved to be potent LOX inhibitors as well. In this study, oregano EO showed the highest antioxidant activity, both as a reducing agent and a radical scavenger. In an earlier extensive study, it was reported that Mediterranean aromatic plants and spices, such as annatto, cumin, oregano, sweet and hot paprika, rosemary, and saffron, which are traditionally used for their aromatic properties in the preparation of Mediterranean food, exhibited strong antioxidant activity as scavengers of several reactive oxygen species. This study provides evidence in support of replacing synthetic antioxidants with natural spice extracts that could further enrich characteristic colors and flavors, encouraging the use of these spices in the design of new functional foods [56].
The anti-inflammatory effect was also tested by the lipoxygenase (LOX) inhibition assay. The practical value of in vitro screening and the value added by techniques such as the LOX inhibition assay was first recommended almost two decades ago [57], aiming to develop safer anti-inflammatory drugs and to supplement in vivo testing. The authors reviewed a large number of active plant extracts and compounds identified from a limited sampling of Africa’s medicinal flora, further emphasizing potential areas for the identification of the biological activity of plant extracts. It was highlighted that impurities, poor technique, or partial isolation of active components can hinder drug development. Routine testing for the toxicity, selectivity, and stability of compounds were presented as critical attributes of compounds considered for drug development, both for alternative human medicine and animal health care.
In the present study, thyme EO was observed to be the strongest inhibitor of soybean lipoxygenase, in accordance with the existing literature. Wei and Shibamoto [58] reported that the essential oil of Thymus vulgaris exerted a particularly powerful antioxidant and LOX-inhibitory action. Abdelli et al. [59], who studied the potential of Algerian TEO to mitigate carrageenan-induced paw edema in mice, also reported the inhibition of the inflammatory process, an action attributed mainly to thymol. On the other hand, carvacrol, another major constituent of TEO, was reported by Hotta et al. [60] to be an inhibitor of COX-2 via the activation of PPARα and γ. It is known that COX-2 is a key enzyme involved in prostaglandin biosynthesis and therefore in the inflammatory response, whose expression seems to be controlled by PPARα and γ in smooth muscle cells and macrophages, respectively. There is extended literature to support the suggestion that all three EOs have the potential to be employed as anti-inflammatory agents and alternatives to synthetic antioxidants in food preparations. For example, Leyva-López et al. [61] demonstrated that terpenes, such as thymol and carvacrol acetate, obtained from three Mexican oregano species, Lippia graveolens, Lippia palmeri, and Hedeoma patens, significantly reduced the levels of ROS and NO produced by macrophage cells stimulated with lipopolysaccharide (LPS). Furthermore, EOs of Origanum majorana (10 µg mL−1) reduced the production of tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 in LPS-activated THP-1 human macrophage cells [62]. Recently, Han and Parker [63] showed that EOs obtained from O. vulgare significantly inhibited levels of the inflammatory biomarkers monocyte chemoattractant protein-1 (MCP-1), vascular cell adhesion molecule-1 (VCAM-1), and intracellular cell-adhesion molecule-1 (ICAM-1) in activated primary human neonatal fibroblasts. These findings suggest that OEOs have anti-inflammatory properties. The individual components of EOs from oregano have also been studied to better understand their effect on inflammation. Thus, Lima et al. [64] demonstrated that carvacrol exerts anti-inflammatory activity in a mouse inflammation model. When carvacrol was administrated to mice (at 50 and 100 mg kg−1) presenting paw edema, the levels of IL-1β and prostaglandin E2 (PGE2) prostaglandins were diminished. The anti-inflammatory effect of carvacrol is due both to the reduction of pro-inflammatory mediators and the increase of anti-inflammatory cytokines (IL-10) [64]. Other EO components, such as p-cymene and β-caryophyllene [63,65,66,67], have also demonstrated anti-inflammatory properties. The studies mentioned above indicate that diverse oregano species might be used as anti-inflammatory agents and could be added to formulations for the prevention or treatment of inflammation-related diseases. Nevertheless, and since OEOs might exert toxic effect on cells, several in vivo and clinical studies are needed before the EOs can be used as an alternative to treat inflammation [68]. Salvia triloba antioxidant activity is due to high levels of phenolics, terpenoids, polyphenols, and flavonoids, as revealed by in vitro and in vivo studies [38,40,69].
Studies on the antimicrobial properties of essential oils against microorganisms with veterinary importance in vitro and in vivo are still limited. Rusenova and Parvanov [54] evaluated twelve essential oils for their inhibitory activity against some microorganisms of veterinary interest using disk diffusion and the most active were selected for further study using the agar dilution method. Disk diffusion showed variation in the antimicrobial activity of selected essential oils. According to the agar dilution method, the most potent essential oils were cinnamon, oregano, lemongrass, and thyme. MICs were tested at concentrations ranging from 2.0 to 0.008% (v/v). These inhibitory effects are interesting in relation to treatment of bacterial and yeast infections in animals [55]. Moreover, the inhibitory effects of sage and oregano essential oils against E. coli were noted when these EOs were applied in meat preparations such as minced beef [69].
Finally, in this study, the in vitro anticoccidial activity of oregano, thyme, and sage essential oils was evaluated, based on their effect on the inhibition of coccidial (E. tenella) invasion in MDBK cells along with their cytotoxic effects. Previous results showed that oregano possesses very strong anticoccidial activity in vitro, evidenced by the inhibition of sporozoite invasion at the higher concentrations tested, potentially caused by a toxic effect that left few parasites fit to invade cells [30]. These results were similar to those previously reported for sporozoites pretreated with oregano essential oil [30], where only the highest concentration (100 μg mL−1) showed a significant reduction in the number of sporozoites to the untreated control at 2 hpi, despite a clear reduction tendency between groups. Following identical protocols, sage and thyme present a potent inhibitory effect on sporozoite invasion. Both had a noticeable reduction at higher concentrations, with thyme showing more consistent effects at lower concentrations. Oregano essential oil exhibited an effect comparable with robenidine, a well-known anticoccidial drug. The same high essential oil concentration did not show any deleterious effects on the host cells based upon a microscopic assessment of the cell morphology within the monolayer. Cytotoxic effects on the host cells could have affected parasite invasion and proliferation [70]. Sporozoites have been shown to begin endogenous development into schizonts from 28 hpi [71]. Further studies to evaluate the effects of the tested essential oils in this part of the eimerian lifecycle, and the extent to which the pretreatment of free sporozoites has an effect would be of great interest. Although the mode(s) of action or mechanisms involved have not been elucidated, a reasonable explanation for this anticoccidial activity is the hydrophobic character and low molecular weight of the main phenolic compounds present in these essential oils that might allow them to disintegrate the outer cell membranes [72]. This may cause an increase in cytoplasmic membrane permeability and lead to cell death caused by the leakage of ions, energy loss, and the diffusion of cell contents [14]. Furthermore, the high lipid solubility of oregano and other essential oils is likely to permit rapid diffusion through parasite and host cell membranes. Other possible mechanisms include interference with the calcium-mediated signaling that is a necessary mechanism for invasion by E. tenella sporozoites [72]. The hydrophobic character of these compounds may suggest interaction with membrane components and permeability [73].
It is believed that the hydrophobic compounds in EOs may penetrate bacterial and parasitic cells causing cell deformities and organelle dysfunction [74]. If the carvacrol concentration increases, then more molecules interact with the phospholipid bilayer, upsetting the membrane fluidity [75,76]. Accordingly, carvacrol, thymol, and the major bioactive ingredients of the EOs tested here may also exert a toxic effect on the upper layer of mature enterocytes of the intestinal mucosa. Recent scientific research has shown that many plants used as food or in traditional medicine are potentially toxic, mutagenic, and carcinogenic [77,78,79,80,81]. For this reason, in this study, their in vitro cytotoxic effects on MDBK cells were assessed and were detected to be very low. Moreover, other studies have demonstrated that MDBK cells are able to produce cytokines when stimulated by exposure to viruses, suggesting that an immune response could also be involved in the anticoccidial effect of these metabolite compounds of the plants in question [28,30,82]. Traditionally used medicinal plants are assumed to be safe based on their long usage in the treatment of various ailments, according to knowledge accumulated over centuries.
The results of this study are in agreement with several studies conducted on natural plant essential oils to indicate their in vitro antioxidant and antimicrobial properties, justifying their potential use in industrial applications, as, for example, Melaleuca alternifiolia [83]. Renewed interest in traditional pharmacopeias means that researchers are concerned not only with determining the scientific rationale for the plant’s usage, but also the discovery of novel compounds of pharmaceutical value. Instead of relying on trial and error, as in random screening procedures, traditional knowledge helped scientists to target plants that may be medicinally useful. An estimated 122 drugs from 94 plant species have already been discovered through ethnobotanical leads. Through the last two decades, 33% of the 1394 small molecules that were approved as new drugs were either natural products or natural derivatives, and another 35% were created around a natural pharmacophore acknowledging their significance. Various assays can be used to test for biological activity, firstly in vitro and later, for promising natural products, in vivo. Crude or fractionated extracts and sometimes individual compounds have been screened for antibacterial, anti-inflammatory, antioxidant, anthelmintic, anti-amoebic, antischistosomal, and/or antimalarial activity, as well as psychotropic and neurotropic properties.
5. Conclusions
This study confirms the potent antioxidant, anti-inflammatory, and antimicrobial (both antibacterial and anticoccidial) activity of selected essential oils derived from oregano, thyme, and sage. These oils also exhibited very low cytotoxic activity. Oregano and thyme oils, according to the agar dilution method, were the most effective. Thyme presented the highest anti-inflammatory effect, and the antioxidant activity was in the order of oregano > thyme > sage. Together, these essential oils can comprise an effective combination that could be further tested in vivo by challenging broilers with coccidia, E. coli, and other pathogens and may potentiate the efficacy of chemotherapeutics/prophylactics with economic relevance to broiler rearing. Bioactive compounds derived from natural resources such as oregano, thyme, and sage plants merit great interest due to their pharmacological and medicinal properties, low adverse effects, and economic value. Although it is difficult to extrapolate the doses employed in vitro to in vivo, further work needs to be undertaken to determine the appropriate doses of essential oils showing both antimicrobial activity and very low detrimental effect on animal cells.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life12111783/s1, Figure S1. GC-MS chromatogram of oregano essential oil (OEO); Figure S2. GC-MS chromatogram of thyme essential oil (TEO); Figure S3. GC-MS chromatogram of sage essential oil (SEO); Table S1. Total phenolic content (TPC) of EOs; Table S2. Antioxidant activity of EOs: Interaction with DPPH and ABTS radicals; Table S3. Antioxidant activity of EOs: FRAP values; Table S4. Inhibition of soybean LOX by EOs.
Author Contributions
Conceptualization, I.G. and I.S.; methodology, E.S., V.M.-H., K.A.-M., D.L., T.P., K.F., and A.T.; software, E.B.; resources, K.G., D.L., and T.P.; writing—original draft preparation, I.G., I.S., K.G., D.P.B., and E.B.; writing—review and editing, all authors; funding acquisition, I.S. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research project is co-financed by the European Regional Development Fund (ERDF) under the Operational Program “Epirus 2014–2020”, NSRF 2014–2020. Project Code: HΠ1AB-0028192. Acronym: Innochicken.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Oregano, thyme, and sage plant materials were kindly donated by Fotis Stavratis, “Aromata Epirus”, Palaiohori, Filiates Thesprotia, Epirus, Greece.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kosakowska, O.; Węglarz, Z.; Pióro-Jabrucka, E.; Przybył, J.L.; Kraśniewska, K.; Gniewosz, M.; Bączek, K. Antioxidant and Antibacterial Activity of Essential Oils and Hydroethanolic Extracts of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare). Molecules 2021, 26, 988. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadou, K.; Trikka, F.A.; Tsoktouridis, G.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Maloupa, E.; Makris, A.M. Μicropropagation and cultivation of Salvia sclarea for essential oil and sclareol production in northern Greece. In Vitro Cell. Dev. Biol. Plant 2020, 56, 51–59. [Google Scholar] [CrossRef]
- Socaci, S.A.; Fărcaş, A.C.; Tofană, M. Functional ingredients derived from aromatic plants. In Feed Additives; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 133–146. [Google Scholar]
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.M. Potential of essential oils for poultry and pigs. Anim. Nutr. 2018, 4, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Bonos, E.; Filliousis, G.; Stylianaki, I.; Kumar, P.; Lazari, D.; Christaki, E.; Florou-Paneri, P. Effect of a Polyherbal or an Arsenic-Containing Feed Additive on Growth Performance of Broiler Chickens, Intestinal Microbiota, Intestinal Morphology, and Lipid Oxidation of Breast and Thigh Meat. J. Appl. Poult. Res. 2019, 28, 164–175. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Phcog. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Jin, S.; Zong, Y.; Gao, Q.; Zhu, Z.; Wang, Y.; Qin, P.; Liang, C.; Wang, D.; Qiu, J.-L.; Zhang, F.; et al. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science 2019, 364, 292–295. [Google Scholar] [CrossRef]
- Giannenas, I.; Bonos, E.; Skoufos, I.; Tzora, A.; Stylianaki, I.; Lazari, D.; Tsinas, A.; Christaki, E.; Florou-Paneri, P. Effect of herbal feed additives on performance parameters, intestinal microbiota, intestinal morphology and meat lipid oxidation of broiler chickens. Br. Poult. Sci. 2018, 59, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.A.; Ortega-Ramirez, L.A.; Leyva, J.M.; Siddiqui, M.W.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Oregano Essential Oil as an Antimicrobial and Antioxidant Additive in Food Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Skoufogianni, E.; Solomou, D.; Alexandra, N.; Danalatos, G. Ecology, Cultivation and Utilization of the Aromatic Greek Oregano (Origanum vulgare L.): A Review. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 545–552. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Skoufos, I.; Tzora, A.; Giannenas, I.; Tontis, D.; Bartzanas, T.; Kittas, C.; Panagakis, P. Effects of oregano essential oil and attapulgite on growth performance, intestinal microbiota and morphometry in broilers. S. Afr. J. Anim. Sci. 2016, 46, 77–88. [Google Scholar] [CrossRef]
- Socaci, S.A.; Rugină, D.O.; Diaconeasa, Z.M.; Pop, O.L.; Fărcaș, A.C.; Păucean, A.; Tofană, M.; Pintea, A. Antioxidant compounds recovered from food wastes. In Functional Food-Improve Health Through Adequate Food; Chavarri Hueda, M., Ed.; In Tech: Rijeka, Croatia, 2017. [Google Scholar]
- Ultee, A.; Smid, E.J. Influence of carvacrol on growth and toxin production by Bacillus cereus. Internat. J. Food Microbiol. 2001, 64, 373–378. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.A.; Tjeerdsma-Van Bokhoven, J.L.M.; Zweijtzer, C.; Burt, S.A.; Haagsman, H.P. Structural Requirements for the Antimicrobial Activity of Carvacrol. J. Agric. Food Chem. 2006, 54, 1874–1879. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil-Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Hashemipour, H.; Kermanshahi, H.; Golian, A.; Veldkamp, T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013, 92, 2059–2069. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Elnesr, S.S. A review on the beneficial effect of thymol on health and production of fish. Rev. Aquacult. 2021, 13, 632–641. [Google Scholar] [CrossRef]
- Avila-Ramos, F.; Pro-Martínez, A.; Sosa-Montes, E.; Cuca-García, J.M.; Becerril-Pérez, C.M.; Figueroa-Velasco, J.L.; Narciso-Gaytán, C. Effects of dietary oregano essential oil and vitamin E on the lipid oxidation stability of cooked chicken breast meat. Poult. Sci. 2012, 91, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and Antioxidant Properties of Rosemary and Sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) Essential Oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef]
- Selmi, S.; Sadok, S. The effect of natural antioxidant (Thymus vulgaris Linnaeus) on flesh quality of tuna (Thunnus thynnus (Linnaeus)) during chilled storage. Pan-Am. J. Aquat. Sci. 2008, 3, 36–45. [Google Scholar]
- Gumus, C.E.; Decker, E.A.; McClements, D.J. Impact of legume protein type and location on lipid oxidation in fish oil-in-water emulsions: Lentil, pea, and faba bean proteins. Food Res. Int. 2017, 100, 175–185. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Sanches-Silva, A.; Ramos de Melo, N. Essential Oils for Food Application: Natural Substances with Established Biological Activities. Food Bioprocess Technol. 2018, 11, 43–71. [Google Scholar] [CrossRef]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) essential oil provides anti-inflammatory activity and facilitates wound healing in a human keratinocytes cell model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.; Juan, S.; Andrade, E. Effect of oregano essential oil and carvacrol on Cryptosporidium parvum infectivity in HCT-8 cells. Parasitol. Int. 2018, 67, 170–175. [Google Scholar] [CrossRef]
- Tasdemir, D.; Kaiser, M.; Demirci, B.; Demirci, F.; Baser, K.H.C. Antiprotozoal Activity of Turkish Origanum onites Essential Oil and Its Components. Molecules 2019, 24, 4421. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Tersteeg-Zijderveld, M.H.G.; Jongerius-Gortemaker, B.G.M.; Vervelde, L.; Vernooij, J.C. In vitro inhibition of Eimeria tenella invasion of epithelial cells by phytochemicals. Vet. Parasitol. 2013, 191, 374–378. [Google Scholar] [CrossRef][Green Version]
- Giannenas, I.; Florou-Paneri, P.; Papazahariadou, M.; Christaki, E.; Botsoglou, N.A.; Spais, A.B. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch. Anim. Nutr. 2003, 57, 99–106. [Google Scholar] [CrossRef]
- Aljabeili, H.S.; Barakat, H.; Abdel-Rahman, H.A. Chemical Composition, Antibacterial and Antioxidant Activities of Thyme Essential Oil (Thymus vulgaris). Food Nutr. Sci. 2018, 9, 433–446. [Google Scholar] [CrossRef]
- Sidiropoulou, E.; Skoufos, I.; Marugán-Hernández, V.; Giannenas, I.; Bonos, E.; Aguiar-Martins, K.; Lazari, D.; Blake, D.P.; Tzora, A. In vitro Anticoccidial Study of Oregano and Garlic Essential Oils and Effects on Growth Performance, Fecal Oocyst Output, and Intestinal Microbiota in vivo. Front. Vet. Sci. 2020, 7, 420. [Google Scholar] [CrossRef]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.T.; Horhat, F.G. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life 2014, 7, 56–60. [Google Scholar]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Ezzat Abd El-Hack, M.; Alagawany, M.; Ragab Farag, M.; Tiwari, R.; Karthik, K.; Dhama, K.; Zorriehzahra, J.; Adel, M. Beneficial impacts of thymol essential oil on health and production of animals, fish and poultry: A review. J. Essent. Oil Res. 2016, 28, 365–382. [Google Scholar] [CrossRef]
- Ghabraie, M.; Dang, V.K.; Tata, L.; Salmieri, S.; Lacroix, M. Antimicrobial effect of essential oils in combinations against five bacteria and their effect on sensorial quality of ground meat. LWT 2016, 66, 332–339. [Google Scholar] [CrossRef]
- Severino, R.; Vu, K.D.; Donsì, F.; Salmieri, S.; Ferrari, G.; Lacroix, M. Antibacterial and physical effects of modified chitosan based-coating containing nanoemulsion of mandarin essential oil and three non-thermal treatments against Listeria innocua in green beans. Int. J. Food Microbiol. 2014, 191, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Mikus, J.; Steverding, D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue®. Parasitol. Int. 2000, 48, 265–269. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Risaliti, L.; Kehagia, A.; Daoultzi, E.; Lazari, D.; Bergonzi, M.C.; Vergkizi-Nikolakaki, S.; Hadjipavlou-Litina, D.; Bilia, A.R. Liposomes loaded with Salvia triloba and Rosmarinus officinalis essential oils: In vitro assessment of antioxidant, antiinflammatory and anti-bacterial activities. J. Drug Deliv. Sci. Technol. 2019, 51, 493–498. [Google Scholar] [CrossRef]
- Poulios, E.; Giaginis, C.; Vasios, G.K. Current advances on the extraction and identification of bioactive components of sage (Salvia spp.). Curr. Pharm. Biotechnol. 2019, 20, 845–857. [Google Scholar] [CrossRef]
- Poulios, E.; Giaginis, C.; Vasios, G.K. Current State of the Art on the Antioxidant Activity of Sage (Salvia spp.) and Its Bioactive Components. Planta Med. 2020, 86, 224–238. [Google Scholar] [CrossRef]
- Cutillas, A.B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Salvia officinalis L. Essential Oils from Spain: Determination of Composition, Antioxidant Capacity, Anti-enzymatic, and Antimicrobial Bioactivities. Chem. Biodivers. 2017, 14, e1700102. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Eldeeb, H.M.; Khan, R.A.; Al-Omar, M.S.; Mohammed, S.A.A.; Sajid, M.S.M.; Aly, M.S.A.; Ahmad, A.M.; Abdellatif, A.A.H.; Eid, S.Y.; et al. Sage, Salvia officinalis L., Constituents, Hepatoprotective Activity, and Cytotoxicity Evaluations of the Essential Oils Obtained from Fresh and Differently Timed Dried Herbs: A Comparative Analysis. Molecules 2021, 26, 5757. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456, p. 8. [Google Scholar]
- Massada, Y. Analysis of Essential Oil by Gas Chromatography and Spectrometry; John Willey & Sons: New York, NY, USA, 1976; p. 334. [Google Scholar]
- Kiritsakis, K.; Kontominas, M.G.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Moustakas, A.; Kiritsakis, A. Composition and Antioxidant Activity of Olive Leaf Extracts from Greek Olive Cultivars. J. Am. Oil Chem. Soc. 2010, 87, 369–376. [Google Scholar] [CrossRef]
- Zhen, J.; Villani, T.S.; Guo, Y.; Qi, Y.; Chin, K.; Pan, M.H.; Ho, C.T.; Simon, J.E.; Wu, Q. Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem. 2016, 190, 673–680. [Google Scholar] [CrossRef]
- Benzie, I.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: New York, NY, USA, 2017; Volume 11, pp. 77–106. [Google Scholar] [CrossRef]
- Ondua, M.; Njoya, E.M.; Abdalla, M.A.; McGaw, L.J. Anti-inflammatory and antioxidant properties of leaf extracts of eleven South African medicinal plants used traditionally to treat inflammation. J. Ethnopharmacol. 2019, 234, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Fernández, I.; Pegg, E.; Macdonald, S.; Tomley, F.M.; Blake, D.P.; Marugán-Hernández, V. Laboratory growth and genetic manipulation of Eimeria tenella. Curr. Prot. Microbiol. 2019, 53, e81. [Google Scholar] [CrossRef]
- Thabet, A.; Alnassan, A.A.; Daugschies, A.; Bangoura, B. Combination of cell culture and qPCR to assess the efficacy of different anticoccidials on Eimeria tenella sporozoites. Parasitol. Res. 2015, 114, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Marugán-Hernández, V.; Cockle, C.; Macdonald, S.; Pegg, E.; Crouch, C.; Blake, D.P.; Tomley, F.M. Viral proteins expressed in the protozoan parasite Eimeria tenella are detected by the chicken immune system. Parasit. Vectors 2016, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.D. Essentials of Medical Pharmacology, 7th ed.; Jaypee Bros Medical Publishers (Ltd.): New Delhi, India, 2013. [Google Scholar]
- Rusenova, N.; Parvanov, P. Antimicrobial activities of twelve essential oils against microorganism of veterinary importance. Trakia J. Sci. 2009, 7, 37–43. [Google Scholar]
- Hitokoto, H.; Morozumi, S.; Wauke, T.; Sakai, S.; Kurata, H. Inhibitory effects of spices on growth and toxin production of toxigenic fungi. Appl. Envir. Microbiol. 1980, 39, 818–822. [Google Scholar] [CrossRef]
- Martínez-Tomé, M.; Jiménez-Monreal, A.; Ruggieri, S.; Frega, N.; Strabbioli, R.; Murcia, M. Antioxidant Properties of Mediterranean Spices Compared with Common Food Additives. J. Food Prot. 2001, 64, 1412–1419. [Google Scholar] [CrossRef]
- McGaw, L.J.; Jäger, A.K.; van Staden, J. Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J. Ethnopharmacol. 2000, 72, 247–263. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J. Agric. Food Chem. 2010, 58, 7218–7225. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, W.; Bahri, F.; Romane, A.; Höferl, M.; Wanner, J.; Schmidt, E.; Jirovetz, L. Chemical composition and anti-inflammatory activity of Algerian Thymus vulgaris essential oil. Nat. Prod. Com. 2017, 12, 611–614. [Google Scholar] [CrossRef]
- Hotta, M.; Nakata, R.; Katsukawa, M.; Hori, K.; Takahashi, S.; Inoue, H. Carvacrol, a component of thyme oil, activates PPARα and γ and suppresses COX-2 expression. J. Lipid Res. 2010, 51, 132–139. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Arranz, L.; Sánchez-Aguilera, A.; Martín-Pérez, D.; Isern, J.; Langa, X.; Tzankov, A.; Lundberg, P.; Muntión, S.; Tzeng, Y.S.; Lai, D.M.; et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 2014, 512, 78–81. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef]
- Lima, M.D.S.; Quintans-Júnior, L.J.; de Santana, W.A.; Martins Kaneto, C.; Pereira Soares, M.B.; Villarreal, C.F. Anti-inflammatory effects of carvacrol: Evidence for a key role of interleukin-10. Eur. J. Pharmacol. 2013, 699, 112–117. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Anti-inflammatory Activity of Cinnamon (Cinnamomum zeylanicum) Bark Essential Oil in a Human Skin Disease Model. Phytother. Res. 2017, 31, 1034–1038. [Google Scholar] [CrossRef]
- Alberti, T.B.; Barbosa, W.L.R.; Vieira, J.L.F.; Raposo, N.R.B.; Dutra, R. (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef]
- Berdowska, I.; Zieliński, B.; Fecka, I.; Kulbacka, J.; Saczko, J.; Gamian, A. Cytotoxic impact of phenolics from Lamiaceae species on human breast cancer cells. Food Chem. 2013, 141, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Benoist, C.; Bluestone, J.; Campbell, D.J.; Ghosh, S.; Hori, S.; Jiang, S.; Kuchroo, V.K.; Mathis, D.; Roncarolo, G.M.; et al. Regulatory T cells: Recommendations to simplify the nomenclature. Nat. Immunol. 2013, 14, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichia coli O157:H7 in minced beef during refrigerated storage. Meat Sci. 2008, 80, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, R.E.; Müller, U.; Shahiduzzaman, M.; Dyachenko, V.; Desouky, A.Y.; Alber, G.; Daugschies, A. Effects of curcumin (diferuloylmethane) on Eimeria tenella sporozoites in vitro. Parasitol. Res. 2011, 108, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Marugán-Hernández, V.; Jeremiah, G.; Aguiar-Martins, K.; Burrell, A.; Vaughan, S.; Xia, D.; Randle, N.; Tomley, F. The growth of Eimeria tenella: Characterization and application of quantitative methods to assess sporozoite invasion and endogenous development in cell culture. Front. Cell. Infect. Microbiol. 2020, 10, 568. [Google Scholar] [CrossRef]
- Jitviriyanon, S.; Phanthong, P.; Lomarat, P.; Bunyapraphatsara, N.; Porntrakulpipat, S.; Paraksa, N. In vitro study of anti-coccidial activity of essential oils from indigenous plants against Eimeria tenella. Vet. Parasitol. 2016, 228, 96–102. [Google Scholar] [CrossRef]
- Sikkema, J.; De Bont, J.A.M.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Bhatia, S.; Behl, T.; Kaushik, D.; Ahmed, M.M.; Anwer, K. Antibacterial Mechanism of Action of Essential Oils. In Role of Essential Oils in the Management of COVID-19; CRC Press: Boca Raton, FL, USA, 2022; pp. 227–237. [Google Scholar]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Schimmer, O.; Häfele, F.; Krüger, A. The mutagenic potencies of plant extracts containing quercetin in Salmonella typhimurium TA98 and TA100. Mutat. Res. Genet. Toxicol. 1988, 206, 201–208. [Google Scholar] [CrossRef]
- Schimmer, O.; Krüger, A.; Paulini, H.; Haefele, F. An evaluation of 55 commercial plant extracts in the Ames mutagenicity test. Pharmazie 1994, 49, 448–451. [Google Scholar] [PubMed]
- Higashimoto, M.; Purintrapiban, J.; Kataoka, K.; Kinouchi, T.; Vinitketkumnuen, U.; Akimoto, S.; Matsumoto, H.; Ohnishi, Y. Mutagenicity and antimutagenicity of extracts of three spices and a medicinal plant in Thailand. Mut. Res. Let. 1993, 303, 135–142. [Google Scholar] [CrossRef]
- De Sã Ferrira, I.C.F.; Ferrão Vargas, V.M. Mutagenicity of medicinal plant extracts in Salmonella/microsome assay. Phytother. Res. 1999, 13, 397–400. [Google Scholar] [CrossRef]
- Kassie, F.; Parzefall, W.; Musk, S.; Johnson, I.; Lamprecht, G.; Sontag, G.; Knasmüller, S. Genotoxic effects of crude juices from Brassica vegetables and juices and extracts from phytopharmaceutical preparations and spices of cruciferous plants origin in bacterial and mammalian cells. Chem.-Biol. Interact. 1996, 102, 1–16. [Google Scholar] [CrossRef]
- Hessenberger, S.; Schatzmayer, G.; Teichmann, K. In vitro inhibition of Eimeria tenella sporozoite invasion into host cells by probiotics. Vet. Parasitol. 2016, 229, 93–98. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Guo, L.; Jiang, H.; Ji, Q. In Vitro Evaluation of Antioxidant and Antimicrobial Activities of Melaleuca alternifolia Essential Oil. Biomed Res. Int. 2018, 2018, 2396109. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).