Immunology of Oral Squamous Cell Carcinoma—A Comprehensive Insight with Recent Concepts
Abstract
:1. Introduction
2. Materials and Methods
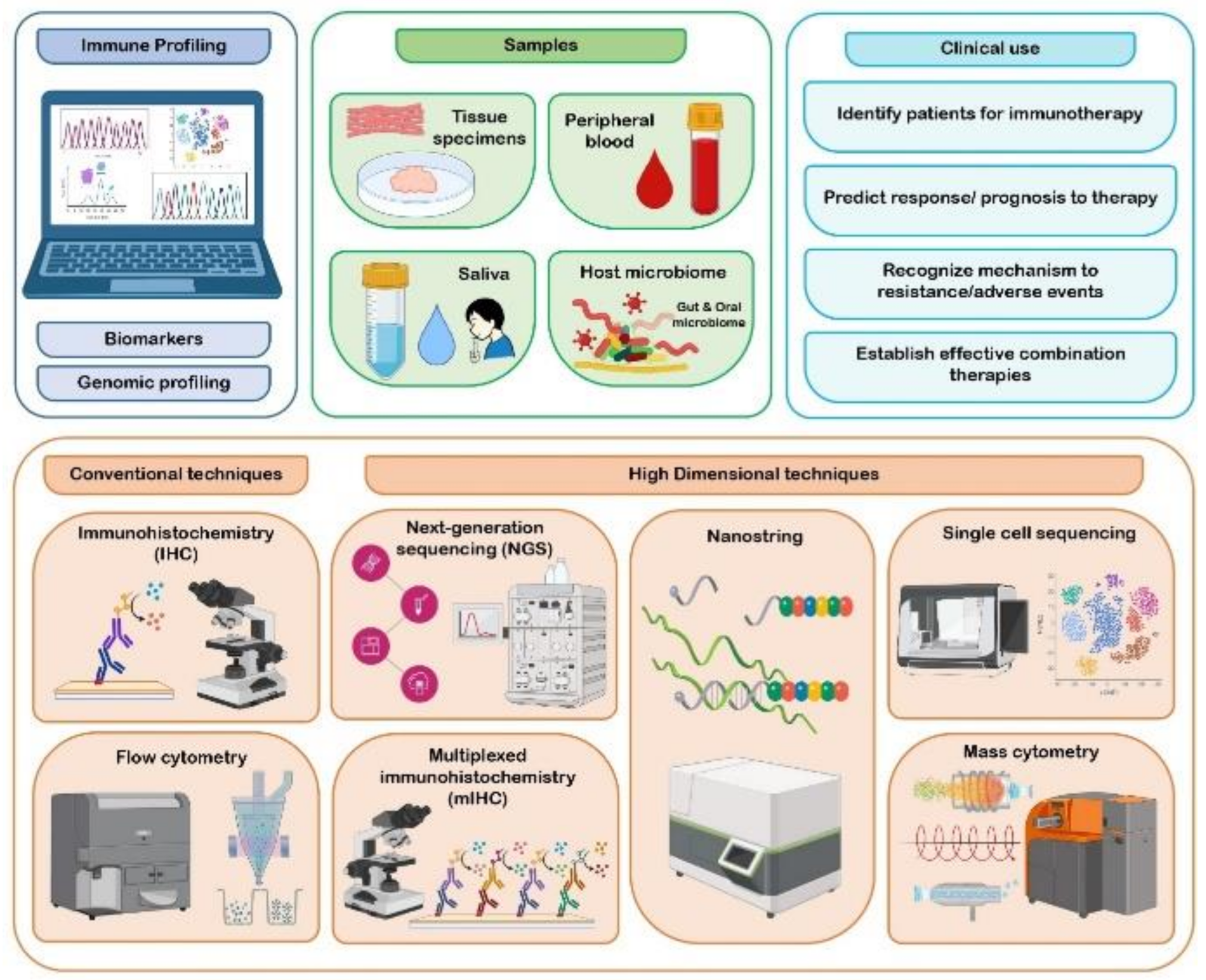
3. Immune Profiling of Oral Cancer
3.1. Cross Talk between Immune System and Tumor Cells within TME
3.2. Key Biomarkers of TME
3.2.1. Immune and Non-Immune Cell Markers
3.2.2. Inflammatory Biomarkers
3.3. Immune Gene Expression Profiling
3.4. Methods Adopted in Immune Profiling
3.5. Clinical Use of Immune Profiles
4. Immunoediting in Oral Cancer
4.1. Mechanism of Cancer Immunoediting
4.2. Implication of Cancer Immunoediting in Oral Cancer Immunotherapy
5. Immunotherapy in Oral Cancer
5.1. Immune Checkpoint Inhibitors (ICIs)
5.2. Targeted Monoclonal Antibodies
5.3. Oral Cancer Stem Cells and Escape from the Host Immune Surveillance
5.4. Cancer Vaccines
5.5. Other Therapies and Advances
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hadler-Olsen, E.; Wirsing, A.M. Tissue-infiltrating immune cells as prognostic markers in oral squamous cell carcinoma: A systematic review and meta-analysis. Br. J. Cancer 2019, 120, 714–727. [Google Scholar] [CrossRef] [Green Version]
- Freeman, P.; Mielgo, A. Cancer-associated fibroblast mediated inhibition of CD8+ cytotoxic T cell accumulation in Tumours: Mechanisms and therapeutic opportunities. Cancers 2020, 12, 2687. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, D.C.; Shevde, L.A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazemi, M.; Rainero, E. Cross-talk between the tumor microenvironment, extracellular matrix, and cell metabolism in cancer. Front. Oncol. 2020, 10, 239. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Li, D.; Cang, H.; Guo, B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med. 2019, 8, 4709–4721. [Google Scholar] [CrossRef]
- Finotello, F.; Eduati, F. Multi-omics profiling of the tumor microenvironment: Paving the way to precision immuno-oncology. Front. Oncol. 2018, 8, 430. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, J.S.; Teng, M.W.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Wagner, M.; Koyasu, S. Cancer immunoediting by innate lymphoid cells. Trends Immunol. 2019, 40, 415–430. [Google Scholar] [CrossRef]
- Ostroumov, D.; Fekete-Drimusz, N.; Saborowski, M.; Kühnel, F.; Woller, N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell. Mol. Life Sci. 2018, 75, 689–713. [Google Scholar] [CrossRef]
- Billan, S.; Kaidar-Person, O.; Gil, Z. Treatment after progression in the era of immunotherapy. Lancet Oncol. 2020, 21, e463–e476. [Google Scholar] [CrossRef]
- Christofi, T.; Baritaki, S.; Falzone, L.; Libra, M.; Zaravinos, A. Current perspectives in cancer immunotherapy. Cancers 2019, 11, 1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollaers, K.; Hinton-Bayre, A.; Friedland, P.L.; Farah, C.S. AJCC 8th Edition oral cavity squamous cell carcinoma staging–Is it an improvement on the AJCC 7th Edition? Oral Oncol. 2018, 82, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lyons, Y.A.; Wu, S.Y.; Overwijk, W.W.; Baggerly, K.A.; Sood, A.K. Immune cell profiling in cancer: Molecular approaches to cell-specific identification. NPJ Precis. Oncol. 2017, 1, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santosh, A.B.; Jones, T.; Harvey, J. A review on oral cancer biomarkers: Understanding the past and learning from the present. J. Cancer Res. Ther. 2016, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; Kong, X.; Ge, Y.; Yin, P.; Liu, Z.; Chen, J.; Gao, F.; Fang, S. Immune-related gene signature for predicting the prognosis of head and neck squamous cell carcinoma. Cancer Cell Int. 2020, 20, 22. [Google Scholar] [CrossRef] [Green Version]
- Pilla, L.; Maccalli, C. Immune profiling of cancer patients treated with immunotherapy: Advances and challenges. Biomedicines 2018, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Terry, R.L.; Meyran, D.; Ziegler, D.S.; Haber, M.; Ekert, P.G.; Trapani, J.A.; Neeson, P.J. Immune profiling of pediatric solid tumors. J. Clin. Investig. 2020, 130, 3391–3402. [Google Scholar] [CrossRef]
- Alves, A.; Diel, L.; Ramos, G.; Pinto, A.; Bernardi, L.; Yates, J., 3rd; Lamers, M. Tumor microenvironment and Oral Squamous Cell Carcinoma: A crosstalk between the inflammatory state and tumor cell migration. Oral Oncol. 2021, 112, 105038. [Google Scholar] [CrossRef]
- de Sousa Lopes, M.L.; Liu, Y.; Liu, K.Y.; da Silveira, É.J.; Poh, C.F. Tumor-associated immune aggregates in oral cancer: Their cellular composition and potential prognostic significance. Med. Hypotheses 2017, 108, 17–23. [Google Scholar] [CrossRef]
- Hu, Y.; He, M.Y.; Zhu, L.F.; Yang, C.C.; Zhou, M.L.; Wang, Q.; Zhang, W.; Zheng, Y.Y.; Wang, D.M.; Xu, Z.Q.; et al. Tumor-associated macrophages correlate with the clinicopathological features and poor outcomes via inducing epithelial to mesenchymal transition in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2016, 35, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Miao, C.; Liu, W.; Qiao, X.; Yang, W.; Li, L.; Li, C. TGF-β1/TβRII/Smad3 signaling pathway promotes VEGF expression in oral squamous cell carcinoma tumor-associated macrophages. Biochem. Biophys. Res. Commun. 2018, 497, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Moriyama, M.; Furukawa, S.; Rafiul, H.A.; Maruse, Y.; Jinno, T.; Tanaka, A.; Ohta, M.; Ishiguro, N.; Yamauchi, M.; et al. CD163+ CD204+ tumor-associated macrophages contribute to T cell regulation via interleukin-10 and PD-L1 production in oral squamous cell carcinoma. Sci. Rep. 2017, 7, 1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, A.R.; Moriyama, M.; Kubota, K.; Ishiguro, N.; Sakamoto, M.; Chinju, A.; Mochizuki, K.; Sakamoto, T.; Kaneko, N.; Munemura, R.; et al. CD206+ tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci. Rep. 2019, 9, 14611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Liu, F.Y.; Kirkwood, K.L. The p38/MKP-1 signaling axis in oral cancer: Impact of tumor-associated macrophages. Oral Oncol. 2020, 103, 104591. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Lee, Y.S.; Radford, K.J. The role of dendritic cells in cancer. Int. Rev. Cell Mol. Biol. 2019, 348, 123–178. [Google Scholar]
- Han, N.; Li, X.; Wang, Y.; Wang, L.; Zhang, C.; Zhang, Z.; Ruan, M.; Zhang, C. Increased tumor-infiltrating plasmacytoid dendritic cells promote cancer cell proliferation and invasion via TNF-α/NF-κB/CXCR-4 pathway in oral squamous cell carcinoma. J. Cancer 2021, 12, 3045. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Yin, K.; Li, T.; Bao, Y.; Chen, Z. Variation and significance of secretory immunoglobulin A, interleukin 6 and dendritic cells in oral cancer. Oncol. Lett. 2017, 13, 2297–2303. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Li, H.; Mao, L.; Yang, Q.C.; Fu, L.Q.; Wu, C.C.; Liu, B.; Sun, Z.J. CD103+ T and dendritic cells indicate a favorable prognosis in oral cancer. J. Dent. Res. 2019, 98, 1480–1487. [Google Scholar] [CrossRef]
- Jewett, A.; Kos, J.; Kaur, K.; Safaei, T.; Sutanto, C.; Chen, W.; Wong, P.; Namagerdi, A.K.; Fang, C.; Fong, Y.; et al. Natural killer cells: Diverse functions in tumor immunity and defects in pre-neoplastic and neoplastic stages of tumorigenesis. Mol. Ther.-Oncolytics 2020, 16, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Melsen, J.E.; Lugthart, G.; Lankester, A.C.; Schilham, M.W. Human circulating and tissue-resident CD56bright natural killer cell populations. Front. Immunol. 2016, 7, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, K.; Safaie, T.; Ko, M.W.; Wang, Y.; Jewett, A. ADCC against MICA/B is Mediated against Differentiated Oral and Pancreatic and Not Stem-Like/Poorly Differentiated Tumors by the NK Cells; Loss in Cancer Patients due to Down-Modulation of CD16 Receptor. Cancers 2021, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, D.; Li, J.; Zhang, D.; Chen, Q. Regulatory T cells in oral squamous cell carcinoma. J. Oral Pathol. Med. 2016, 45, 635–639. [Google Scholar] [CrossRef]
- Park, J.Y.; Chung, H.; DiPalma, D.T.; Tai, X.; Park, J.H. Immune quiescence in the oral mucosa is maintained by a uniquely large population of highly activated Foxp3+ regulatory T cells. Mucosal Immunol. 2018, 11, 1092–1102. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.J.; Kao, K.C.; Chiu, Y.L.; Jung, C.J.; Liu, C.J.; Cheng, S.J.; Chang, Y.L.; Ko, J.Y.; Chia, J.S. Enrichment of human CCR6+ regulatory T cells with superior suppressive activity in oral cancer. J. Immunol. 2017, 199, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, S.; Sharma, S.C.; NDas, S. Dynamics of regulatory T cells (Tregs) in patients with oral squamous cell carcinoma. J. Surg. Oncol. 2017, 116, 1103–1113. [Google Scholar] [CrossRef]
- Piersiala, K.; da Silva, P.F.; Hjalmarsson, E.; Kolev, A.; Kågedal, Å.; Starkhammar, M.; Elliot, A.; Marklund, L.; Margolin, G.; Munck-Wikland, E.; et al. CD4+ and CD8+ T cells in sentinel nodes exhibit distinct pattern of PD-1, CD69, and HLA-DR expression compared to tumor tissue in oral squamous cell carcinoma. Cancer Sci. 2021, 112, 1048. [Google Scholar] [CrossRef]
- Wirsing, A.M.; Ervik, I.K.; Seppola, M.; Uhlin-Hansen, L.; Steigen, S.E.; Hadler-Olsen, E. Presence of high-endothelial venules correlates with a favorable immune microenvironment in oral squamous cell carcinoma. Mod. Pathol. 2018, 31, 910–922. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.L.; Parkinson, E.K.; Yap, L.F.; Paterson, I.C. Autophagy is deregulated in cancer-associated fibroblasts from oral cancer and is stimulated during the induction of fibroblast senescence by TGF-β1. Sci. Rep. 2021, 11, 584. [Google Scholar] [CrossRef]
- Spenlé, C.; Loustau, T.; Murdamoothoo, D.; Erne, W.; Beghelli-de la Forest Divonne, S.; Veber, R.; Petti, L.; Bourdely, P.; Mörgelin, M.; Brauchle, E.M.; et al. Tenascin-C orchestrates an immune suppressive tumor microenvironment in oral squamous cell carcinoma. Cancer Immunol. Res. 2020, 8, 1122–1138. [Google Scholar] [CrossRef] [PubMed]
- Domnich, M.; Riedesel, J.; Pylaeva, E.; Kürten, C.H.; Buer, J.; Lang, S.; Jablonska, J. Oral Neutrophils: Underestimated Players in Oral Cancer. Front. Immunol. 2020, 11, 2529. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Liu, S.; Yang, L.; Liu, Z.; Hu, Y.; Yao, Z.; Tang, Z.; Fang, L.; Quan, H. Repertoire of peripheral T cells in patients with oral squamous cell carcinoma. Oral Dis. 2020, 26, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Fan, H.Y.; Tang, Y.L.; Wang, S.S.; Cao, M.X.; Wang, H.F.; Dai, L.L.; Wang, K.; Yu, X.H.; Wu, J.B.; et al. Myeloid derived suppressor cells contribute to the malignant progression of oral squamous cell carcinoma. PLoS ONE 2020, 15, e0229089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, A.M.; Diel, L.F.; Lamers, M.L. Macrophages and prognosis of oral squamous cell carcinoma: A systematic review. J. Oral Pathol. Med. 2018, 47, 460–467. [Google Scholar] [CrossRef]
- Chakraborty, P.; Karmakar, T.; Arora, N.; Mukherjee, G. Immune and genomic signatures in oral (head and neck) cancer. Heliyon 2018, 4, e00880. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.; Büttner-Herold, M.; Hyckel, P.; Moebius, P.; Distel, L.; Ries, J.; Amann, K.; Neukam, F.W.; Wehrhan, F. Small oral squamous cell carcinomas with nodal lymphogenic metastasis show increased infiltration of M2 polarized macrophages–an immunohistochemical analysis. J. Cranio-Maxillofac. Surgery 2014, 42, 1087–1094. [Google Scholar] [CrossRef]
- Jardim, J.F.; Gondak, R.; Galvis, M.M.; Pinto, C.A.; Kowalski, L.P. A decreased peritumoral CD 1a+ cell number predicts a worse prognosis in oral squamous cell carcinoma. Histopathology 2018, 72, 905–913. [Google Scholar] [CrossRef]
- Taghavi, N.; Bagheri, S.; Akbarzadeh, A. Prognostic implication of CD 57, CD 16, and TGF-β expression in oral squamous cell carcinoma. J. Oral Pathol. Med. 2016, 45, 58–62. [Google Scholar] [CrossRef]
- Mukherjee, G.; Bag, S.; Chakraborty, P.; Dey, D.; Roy, S.; Jain, P.; Roy, P.; Soong, R.; Majumder, P.P.; Dutt, S. Density of CD3+ and CD8+ cells in gingivo-buccal oral squamous cell carcinoma is associated with lymph node metastases and survival. PLoS ONE 2020, 15, e0242058. [Google Scholar] [CrossRef]
- Lao, X.M.; Liang, Y.J.; Su, Y.X.; Zhang, S.E.; Zhou, X.I.; Liao, G.Q. Distribution and significance of interstitial fibrosis and stroma-infiltrating B cells in tongue squamous cell carcinoma. Oncol. Lett. 2016, 11, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Li, R.; Wang, Y.; Li, S.; Hong, Z.; Han, Z. Landscape of myeloid-derived suppressor cell in tumor immunotherapy. Biomark. Res. 2021, 9, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Teófilo, C.R.; Junior, A.E.; Batista, A.C.; Jamacaru, F.V.; Sousa, F.B.; Mota, M.R.; e Silva, M.F.; de Barros Silva, P.G.; Alves, A.P. Mast Cells and Blood Vessels Profile in Oral Carcinogenesis: An Immunohistochemistry Study. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 1097. [Google Scholar] [CrossRef]
- Patel, J.B.; Shah, F.D.; Joshi, G.M.; Patel, P.S. Clinical significance of inflammatory mediators in the pathogenesis of oral cancer. J. Cancer Res. Ther. 2016, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Dantas, T.S.; de Barros Silva, P.G.; Verde, M.E.; Júnior, A.D.; Cunha, M.D.; Mota, M.R.; Alves, A.P.; de Carvalho Leitão, R.F.; Sousa, F.B. Role of inflammatory markers in prognosis of oral squamous cell carcinoma. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 3635. [Google Scholar] [CrossRef] [Green Version]
- Mitran, M.I.; Mitran, C.I.; Sarbu, M.I.; Matei, C.; Nicolae, I.; Caruntu, A.; Tocut, S.M.; Popa, M.I.; Caruntu, C.; Georgescu, S.R. Mediators of inflammation–a potential source of biomarkers in oral squamous cell carcinoma. J. Immunol. Res. 2018, 2018, 1061780. [Google Scholar]
- Hasegawa, T.; Iga, T.; Takeda, D.; Amano, R.; Saito, I.; Kakei, Y.; Kusumoto, J.; Kimoto, A.; Sakakibara, A.; Akashi, M. Neutrophil-lymphocyte ratio associated with poor prognosis in oral cancer: A retrospective study. BMC Cancer 2020, 20, 568. [Google Scholar] [CrossRef]
- Zhang, B.; Du, W.; Gan, K.; Fang, Q.; Zhang, X. Significance of the neutrophil-to-lymphocyte ratio in young patients with oral squamous cell carcinoma. Cancer Manag. Res. 2019, 11, 7597. [Google Scholar] [CrossRef] [Green Version]
- Tai, S.F.; Chien, H.T.; Young, C.K.; Tsao, C.K.; de Pablo, A.; Fan, K.H.; Liao, C.T.; Wang, H.M.; Kang, C.J.; Chang, J.T.; et al. Roles of preoperative C-reactive protein are more relevant in buccal cancer than other subsites. World J Surg. Oncol. 2017, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dan, H.; Liu, S.; Liu, J.; Liu, D.; Yin, F.; Wei, Z.; Wang, J.; Zhou, Y.; Jiang, L.; Ji, N.; et al. RACK1 promotes cancer progression by increasing the M2/M1 macrophage ratio via the NF-κB pathway in oral squamous cell carcinoma. Mol. Oncol. 2020, 14, 795–807. [Google Scholar] [CrossRef] [Green Version]
- Chiamulera, M.M.; Zancan, C.B.; Remor, A.P.; Cordeiro, M.F.; Gleber-Netto, F.O.; Baptistella, A.R. Salivary cytokines as biomarkers of oral cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 205. [Google Scholar] [CrossRef]
- Ferrari, E.; Pezzi, M.E.; Cassi, D.; Pertinhez, T.A.; Spisni, A.; Meleti, M. Salivary Cytokines as Biomarkers for Oral Squamous Cell Carcinoma: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6795. [Google Scholar] [CrossRef]
- Thomas, N.; Krishnapillai, R.; Bindhu, P.R.; Thomas, P. Immunohistochemical expression of cyclooxygenase-2 in oral squamous cell carcinoma. Indian J. Dent. Res. 2019, 30, 102. [Google Scholar] [PubMed]
- Miguel, A.F.; Mello, F.W.; Melo, G.; Rivero, E.R. Association between immunohistochemical expression of matrix metalloproteinases and metastasis in oral squamous cell carcinoma: Systematic review and meta-analysis. Head Neck 2020, 42, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.C.; Lin, S.Y.; Chien, K.Y.; Chen, S.F.; Wu, C.C.; Chang, Y.T.; Chi, L.M.; Chu, L.J.; Chiang, W.F.; Chien, C.Y.; et al. An immuno-MALDI mass spectrometry assay for the oral cancer biomarker, matrix metalloproteinase-1, in dried saliva spot samples. Anal. Chim. Acta 2020, 1100, 118–130. [Google Scholar] [CrossRef]
- Canning, M.; Guo, G.; Yu, M.; Myint, C.; Groves, M.W.; Byrd, J.K.; Cui, Y. Heterogeneity of the head and neck squamous cell carcinoma immune landscape and its impact on immunotherapy. Front. Cell Dev. Biol. 2019, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yan, Z.; Lian, S.; Wei, L.; Zhou, C.; Feng, D.; Zhang, Y.; Yang, J.; Li, M.; Chen, Y. Prognostic value of novel immune-related genomic biomarkers identified in head and neck squamous cell carcinoma. J. Immunother. Cancer 2020, 8, e000444. [Google Scholar] [CrossRef]
- Basu, B.; Chakraborty, J.; Chandra, A.; Katarkar, A.; Baldevbhai, J.R.; Chowdhury, D.D.; Ray, J.G.; Chaudhuri, K.; Chatterjee, R. Genome-wide DNA methylation profile identified a unique set of differentially methylated immune genes in oral squamous cell carcinoma patients in India. Clin. Epigenetics 2017, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Guo, Q.; Zhang, X.; Dong, X.; Liu, W.; Zhang, A.; Li, Y.; Yan, J.; Jia, G.; Zheng, Z.; et al. Oral cancer-associated tertiary lymphoid structures: Gene expression profile and prognostic value. Clin. Exp. Immunol. 2020, 199, 172–181. [Google Scholar] [CrossRef]
- Meehan, K.; Leslie, C.; Lucas, M.; Jacques, A.; Mirzai, B.; Lim, J.; Bulsara, M.; Khan, Y.; Wong, N.C.; Solomon, B.; et al. Characterization of the immune profile of oral tongue squamous cell carcinomas with advancing disease. Cancer Med. 2020, 9, 4791–4807. [Google Scholar] [CrossRef]
- Chatzopoulos, K.; Sotiriou, S.; Collins, A.R.; Kartsidis, P.; Schmitt, A.C.; Chen, X.; Khazaie, K.; Hinni, M.L.; Ramsower, C.A.; Zarka, M.A.; et al. Transcriptomic and Immunophenotypic Characterization of Tumor Immune Microenvironment in Squamous Cell Carcinoma of the Oral Tongue. Head Neck Pathol. 2021, 15, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.N.; Li, G.S.; Zhou, X.G.; Chen, X.Y.; Yao, Y.X.; Zhang, X.G.; Liang, Y.; Li, M.X.; Chen, G.; Huang, Z.G.; et al. Identification of an immune score-based gene panel with prognostic power for oral squamous cell carcinoma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e922854-1. [Google Scholar] [CrossRef] [PubMed]
- Chuah, S.; Chew, V. High-dimensional immune-profiling in cancer: Implications for immunotherapy. J. Immunother. Cancer 2020, 8, e000363. [Google Scholar] [CrossRef] [PubMed]
- Kamps, R.; Brandão, R.D.; Bosch, B.J.; Paulussen, A.D.; Xanthoulea, S.; Blok, M.J.; Romano, A. Next-generation sequencing in oncology: Genetic diagnosis, risk prediction and cancer classification. Int. J. Mol. Sci. 2017, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Cameron Goertzen, H.M.; Laliberte, C.; Meirson, T.; Eymael, D.; Gil-Henn, H.; Magalhaes, M. Oral inflammation promotes oral squamous cell carcinoma invasion. Oncotarget 2018, 9, 29047. [Google Scholar] [CrossRef] [Green Version]
- Suvà, M.L.; Tirosh, I. Single-cell RNA sequencing in cancer: Lessons learned and emerging challenges. Mol. Cell 2019, 75, 7–12. [Google Scholar] [CrossRef]
- Goytain, A.; Ng, T. NanoString nCounter technology: High-throughput RNA validation. In InChimeric RNA; Humana: New York, NY, USA, 2020; pp. 125–139. [Google Scholar]
- Tan, W.C.; Nerurkar, S.N.; Cai, H.Y.; Ng, H.H.; Wu, D.; Wee, Y.T.; Lim, J.C.; Yeong, J.; Lim, T.K. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. 2020, 40, 135–153. [Google Scholar] [CrossRef] [Green Version]
- Qiao, B.; Huang, J.; Mei, Z.; Lam, A.K.; Zhao, J.; Ying, L. Analysis of immune microenvironment by multiplex immunohistochemistry staining in different oral diseases and oral squamous cell carcinoma. Front. Oncol. 2020, 10, 2509. [Google Scholar] [CrossRef]
- Almangush, A.; Leivo, I.; Mäkitie, A.A. Biomarkers for Immunotherapy of Oral Squamous Cell Carcinoma: Current Status and Challenges. Front. Oncol. 2021, 11, 388. [Google Scholar] [CrossRef]
- Rebelatto, M.C.; Midha, A.; Mistry, A.; Sabalos, C.; Schechter, N.; Li, X.; Jin, X.; Steele, K.E.; Robbins, P.B.; Blake-Haskins, J.A.; et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn. Pathol. 2016, 11, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Goltz, D.; Gevensleben, H.; Dietrich, J.; Schroeck, F.; de Vos, L.; Droege, F.; Kristiansen, G.; Schroeck, A.; Landsberg, J.; Bootz, F.; et al. PDCD1 (PD-1) promoter methylation predicts outcome in head and neck squamous cell carcinoma patients. Oncotarget 2017, 8, 41011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorta-Estremera, S.; Hegde, V.L.; Slay, R.B.; Sun, R.; Yanamandra, A.V.; Nicholas, C.; Nookala, S.; Sierra, G.; Curran, M.A.; Sastry, K.J. Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV+ oral cancer. J. Immunother. Cancer 2019, 7, 252. [Google Scholar] [CrossRef] [PubMed]
- Foy, J.P.; Bertolus, C.; Michallet, M.C.; Deneuve, S.; Incitti, R.; Bendriss-Vermare, N.; Albaret, M.A.; Ortiz-Cuaran, S.; Thomas, E.; Colombe, A.; et al. The immune microenvironment of HPV-negative oral squamous cell carcinoma from never-smokers and never-drinkers patients suggests higher clinical benefit of IDO1 and PD1/PD-L1 blockade. Ann. Oncol. 2017, 28, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Shayan, G.; Kansy, B.A.; Gibson, S.P.; Srivastava, R.M.; Bryan, J.K.; Bauman, J.E.; Ohr, J.; Kim, S.; Duvvuri, U.; Clump, D.A.; et al. Phase Ib study of immune biomarker modulation with neoadjuvant cetuximab and TLR8 stimulation in head and neck cancer to overcome suppressive myeloid signals. Clin. Cancer Res. 2018, 24, 62–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shayan, G.; Srivastava, R.; Li, J.; Schmitt, N.; Kane, L.P.; Ferris, R.L. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology 2017, 6, e1261779. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Ponzetta, A.; Inforzato, A.; Jaillon, S. Innate immunity, inflammation and tumour progression: Double-edged swords. J. Intern. Med. 2019, 285, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Derakhshani, A.; Vahidian, F.; Alihasanzadeh, M.; Mokhtarzadeh, A.; Nezhad, P.L.; Baradaran, B. Mast cells: A double-edged sword in cancer. Immunol. Lett. 2019, 209, 28–35. [Google Scholar] [CrossRef]
- Aragon-Sanabria, V.; Kim, G.B.; Dong, C. From cancer immunoediting to new strategies in cancer immunotherapy: The roles of immune cells and mechanics in oncology. In Biomechanics in Oncology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 113–138. [Google Scholar]
- Silva, E.V.; Nascente, E.D.; Miguel, M.P.; Alves, C.E.; Moura, V.M. Elucidating tumor immunosurveillance and immunoediting: A comprehensive review. Ciência Anim. Bras. 2021, 22. [Google Scholar] [CrossRef]
- Perri, F.; Ionna, F.; Longo, F.; Scarpati, G.D.; De Angelis, C.; Ottaiano, A.; Botti, G.; Caponigro, F. Immune response against head and neck cancer: Biological mechanisms and implication on therapy. Transl. Oncol. 2020, 13, 262–274. [Google Scholar] [CrossRef]
- Tavakoli, F.; Sartakhti, J.S.; Manshaei, M.H.; Basanta, D. Cancer Immunoediting: A Game Theoretical Approach. In Silico Biol. 2020; Preprint. [Google Scholar] [CrossRef]
- Eckert, A.W.; Wickenhauser, C.; Salins, P.C.; Kappler, M.; Bukur, J.; Seliger, B. Clinical relevance of the tumor microenvironment and immune escape of oral squamous cell carcinoma. J. Transl. Med. 2016, 14, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stasikowska-Kanicka, O.; Wągrowska-Danilewicz, M.; Danilewicz, M. CD8+ and CD163+ infiltrating cells and PD-L1 immunoexpression in oral leukoplakia and oral carcinoma. Apmis 2018, 126, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Sim, F.; Leidner, R.; Bell, R.B. Immunotherapy for head and neck cancer. Oral Maxillofac. Surg. Clin. 2019, 31, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Sánchez, F.J.; Lequerica-Fernández, P.; Suárez-Canto, J.; Rodrigo, J.P.; Rodriguez-Santamarta, T.; Domínguez-Iglesias, F.; García-Pedrero, J.M.; de Vicente, J.C. Macrophages in oral carcinomas: Relationship with cancer stem cell markers and PD-L1 expression. Cancers 2020, 12, 1764. [Google Scholar] [CrossRef]
- Varadé, J.; Magadán, S.; González-Fernández, Á. Human immunology and immunotherapy: Main achievements and challenges. Cell Mol. Immunol. 2021, 18, 805–828. [Google Scholar] [CrossRef]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases—Elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Abbott, M.; Ustoyev, Y. Cancer and the immune system: The history and background of immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef]
- Zolkind, P.; Uppaluri, R. Checkpoint immunotherapy in head and neck cancers. Cancer Metastasis Rev. 2017, 36, 475–489. [Google Scholar] [CrossRef]
- Cramera, J.D.; Barbara Burtness, B.; Ferris, R.L. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. 2019, 99, 104460. [Google Scholar] [CrossRef]
- Mei, Z.; Huang, J.; Qiao, B.; Lam, A.K. Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma. Int. J Oral Sci. 2020, 12, 16. [Google Scholar] [CrossRef]
- Lee, M.Y.; Allen, C.T. Mechanisms of resistance to T cell-based immunotherapy in head and neck cancer. Head Neck 2020, 42, 2722–2733. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.P.; Bhaskaran, M.K.; George, A.L.; Thirutheri, A.; Somasundaran, M.; Pavithran, A. Immunotherapy in oral cancer. J. Pharm. Bioallied Sci. 2019, 11 (Suppl. 2), S107. [Google Scholar] [CrossRef]
- Johnson, D.B.; Sullivan, R.J.; Menzies, A.M. Immune checkpoint inhibitors in challenging populations. Cancer 2017, 123, 1904–1911. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Wang, H.; Mustafa, A.; Liu, S.; Liu, J.; Lv, D.; Yang, H.; Zou, J. Immune checkpoint inhibitor toxicity in head and neck cancer: From identification to management. Front. Pharmacol. 2019, 10, 1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Felice, F.; Musio, D.; Tombolini, V. Immune Check-Point Inhibitors and Standard Chemoradiotherapy in Definitive Head and Neck Cancer Treatment. J. Pers. Med. 2021, 11, 393. [Google Scholar] [CrossRef]
- Qin, Y.; Zheng, X.; Gao, W.; Wang, B.; Wu, Y. Tumor microenvironment and immune-related therapies of head and neck squamous cell carcinoma. Mol. Ther.-Oncolytics 2021, 20, 342–351. [Google Scholar] [CrossRef]
- Kim, H.; Kwon, M.; Kim, B.; Jung, H.A.; Sun, J.M.; Lee, S.H.; Park, K.; Ahn, M.J. Clinical outcomes of immune checkpoint inhibitors for patients with recurrent or metastatic head and neck cancer: Real-world data in Korea. BMC Cancer 2020, 20, 727. [Google Scholar] [CrossRef]
- Sacco, A.G.; Chen, R.; Worden, F.P.; Wong, D.J.; Adkins, D.; Swiecicki, P.; Chai-Ho, W.; Oppelt, P.; Ghosh, D.; Bykowski, J.; et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: An open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021, 22, 883–892. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.W.; Park, Y.S. The Application of Next-Generation Sequencing to Define Factors Related to Oral Cancer and Discover Novel Biomarkers. Life 2020, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J.; Cai, X.; Yao, Z.; Huang, J. Progress in targeted therapeutic drugs for oral squamous cell carcinoma. Surg. Oncol. 2019, 31, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Jameson, M.J. The EGFR inhibitor gefitinib enhanced the response of human oral squamous cell carcinoma to cisplatin in vitro. Drugs R D 2017, 17, 545–555. [Google Scholar] [CrossRef] [Green Version]
- Macha, M.A.; Rachagani, S.; Qazi, A.K.; Jahan, R.; Gupta, S.; Patel, A.; Seshacharyulu, P.; Lin, C.; Li, S.; Wang, S.; et al. Afatinib radiosensitizes head and neck squamous cell carcinoma cells by targeting cancer stem cells. Oncotarget 2017, 8, 20961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Yoshimura, H.; Matsuda, S.; Ryoke, T.; Kiyoshima, T.; Kobayashi, M.; Sano, K. Effects of peritumoral bevacizumab injection against oral squamous cell carcinoma in a nude mouse xenograft model: A preliminary study. Oncol. Lett. 2018, 15, 8627–8634. [Google Scholar] [CrossRef] [PubMed]
- Ganjibakhsh, M.; Monshizadeh, R.; Nasimian, A.; Aminishakib, P.; Farzaneh, P.; Tavakoli Shiraji, S.; Gharajei, A.; Rahrotaban, S.; Baghaei, F.; Gohari, N.S. Anti-angiogenic efficacy of aflibercept and bevacizumab in primary oral squamous cell carcinoma cells. J. Oral Pathol. Med. 2018, 47, 575–582. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Shiota, G. Immune evasion by cancer stem cells. Regen Ther. 2021, 17, 20–33. [Google Scholar] [CrossRef]
- Shibata, H.; Zhou, L.; Xu, N.; Egloff, A.M.; Uppaluri, R. Personalized cancer vaccination in head and neck cancer. Cancer Sci. 2021, 112, 978. [Google Scholar] [CrossRef]
- Zolkind, P.; Przybylski, D.; Marjanovic, N.; Nguyen, L.; Lin, T.; Johanns, T.; Alexandrov, A.; Zhou, L.; Allen, C.T.; Miceli, A.P.; et al. Cancer immunogenomic approach to neoantigen discovery in a checkpoint blockade responsive murine model of oral cavity squamous cell carcinoma. Oncotarget 2018, 9, 4109. [Google Scholar] [CrossRef] [Green Version]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Kumai, T.; Lee, S.; Cho, H.I.; Sultan, H.; Kobayashi, H.; Harabuchi, Y.; Celis, E. Optimization of peptide vaccines to induce robust antitumor CD4 T-cell responses. Cancer Immunol. Res. 2017, 5, 72–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norberg, S.M.; Hinrichs, C.S. Advances in Adoptive Cell Therapy for Head and Neck Cancer. Otolaryngol. Clin. N. Am. 2021, 54, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, J.; Bari, S.; Kirtane, K.; Chung, C.H. Recent Advances and Future Directions in Clinical Management of Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 338. [Google Scholar] [CrossRef] [PubMed]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the treatment of cancer. J. Interferon Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R.L.; Saba, N.F.; Gitlitz, B.J.; Haddad, R.; Sukari, A.; Neupane, P.; Morris, J.C.; Misiukiewicz, K.; Bauman, J.E.; Fenton, M.; et al. Effect of adding motolimod to standard combination chemotherapy and cetuximab treatment of patients with squamous cell carcinoma of the head and neck: The Active8 randomized clinical trial. JAMA Oncol. 2018, 4, 1583–1588. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.J.; Ferris, R.L.; Schmitt, N.C. Costimulatory and coinhibitory immune checkpoint receptors in head and neck cancer: Unleashing immune responses through therapeutic combinations. Cancers Head Neck 2016, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Philips, R.; Han, C.; Swendseid, B.; Curry, J.; Argiris, A.; Luginbuhl, A.; Johnson, J. Preoperative Immunotherapy in the Multidisciplinary Management of Oral Cavity Cancer. Front. Oncol. 2021, 11, 682075. [Google Scholar] [CrossRef]
- Augustine, D.; Sekar, B.; Murali, S.; Ramesh, M.; Madhavan, R.N.; Patil, S.G.; Rao, R.S. Expression of inducible nitric oxide synthase in carcinomas and sarcomas affecting the oral cavity. South Asian J. Cancer 2015, 4, 78–82. [Google Scholar] [CrossRef]
- Prasad, K.; Rao, R.; Augustine, D.; Sowmya, S.V.; Haragannavar, V.; Sagar, P.; Sreedhar, P. Pathway based prognostic gene expression profile of buccal and gingivo-buccal oral squamous cell carcinoma in smokeless tobacco chewers. Head Neck 2019, 41, 388–397. [Google Scholar] [CrossRef]
- Singh, P.; Augustine, D.; Rao, R.S.; Patil, S.; Sowmya, S.V.; Haragannavar, V.C.; Nambiar, S. Interleukin-1beta and Caspase-3 expression serve as independent prognostic markers for metastasis and survival in oral squamous cell carcinoma. Cancer Biomark. 2019, 26, 109–122. [Google Scholar] [CrossRef]
- Raju, K.L.; Haragannavar, V.C.; Patil, S.; Rao, R.S.; Nagaraj, T.; Augustine, D.; Venkatesiah, S.S.; Nambiar, S. Expression of hTERT in Oral Submucous Fibrosis and Oral Squamous Cell Carcinoma-an Immunohistochemical Analysis. Pathol. Oncol. Res. 2020, 26, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.; Raju, K.L.; Augustine, D.; Patil, S. Prognostic Significance of ALDH1, Bmi1, and OCT4 Expression in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Cancer Control 2020, 27, 1073274820904959. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Haragannavar, V.C.; Rao, R.S.; Prasad, K.; Sowmya, S.V.; Augustine, D.; Patil, S. P-Cadherin and WNT5A expression in assessment of lymph node metastasis in oral squamous cell carcinoma. Clin. Oral. Investig. 2022, 26, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Sudarshini, N.; Banavar, S.R.; Nambiar, S.K.; Augustine, D.; Haragannavar, V.C.; Sowmya, S.; Rao, R.S. Immunohistochemical Stain-Phosphohistone H3: Most Specific Mitotic Marker. J. Clin. Diagn. Res. 2018, 12, 2018. [Google Scholar] [CrossRef]



| No. | Cell Type | Biomarkers | References |
|---|---|---|---|
| 1 | Macrophages | CD68 pan marker | Alves et al.; (2018) [47] |
| a. | M1 TAM | CD11c, CD80 and HLA–DR | Alves et al.; (2018) [47] |
| b. | M2 TAM | CD163, CD11b, CD206, MRC1 | Alves et al.; (2018) [47] |
| 2 | DC | CD1a-immature, CD83-mature | Jardim et al.; (2018) [48] |
| 3 | NK | CD57 | Taghavi et al.; (2016) [49] |
| 4 | MDSC | CD33 and CD11b | Pang et al.; (2020) [52] |
| 5 | T cells | CD3 pan marker | Olsen et al.; (2020) [1] |
| a. | Tregs | CD4+ CD25+ FOXP3+ and CD4+ CD25+ CD127low | Liu et al.; (2016) [34] |
| b. | Cytotoxic T cells | CD8 pan marker | Olsen et al.; (2020) [1] |
| 6 | B cells | CD19 and CD20 pan marker | Olsen et al.; (2020) [1] |
| 7 | CAF | α-SMA | Lao et al.; (2016) [51] |
| 8 | Endothelial cells | CD31, CD34 | Teofilo et al.; (2020) [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesiah, S.S.; Augustine, D.; Mishra, D.; Gujjar, N.; Haragannavar, V.C.; Awan, K.H.; Patil, S. Immunology of Oral Squamous Cell Carcinoma—A Comprehensive Insight with Recent Concepts. Life 2022, 12, 1807. https://doi.org/10.3390/life12111807
Venkatesiah SS, Augustine D, Mishra D, Gujjar N, Haragannavar VC, Awan KH, Patil S. Immunology of Oral Squamous Cell Carcinoma—A Comprehensive Insight with Recent Concepts. Life. 2022; 12(11):1807. https://doi.org/10.3390/life12111807
Chicago/Turabian StyleVenkatesiah, Sowmya Samudrala, Dominic Augustine, Deepika Mishra, Neethi Gujjar, Vanishri C. Haragannavar, Kamran Habib Awan, and Shankargouda Patil. 2022. "Immunology of Oral Squamous Cell Carcinoma—A Comprehensive Insight with Recent Concepts" Life 12, no. 11: 1807. https://doi.org/10.3390/life12111807
APA StyleVenkatesiah, S. S., Augustine, D., Mishra, D., Gujjar, N., Haragannavar, V. C., Awan, K. H., & Patil, S. (2022). Immunology of Oral Squamous Cell Carcinoma—A Comprehensive Insight with Recent Concepts. Life, 12(11), 1807. https://doi.org/10.3390/life12111807







