Assessment of the Association of Vitamin D and the Risk of Tuberculosis among End-Stage Kidney Disease Population
Abstract
1. Introduction
2. Materials and Methods
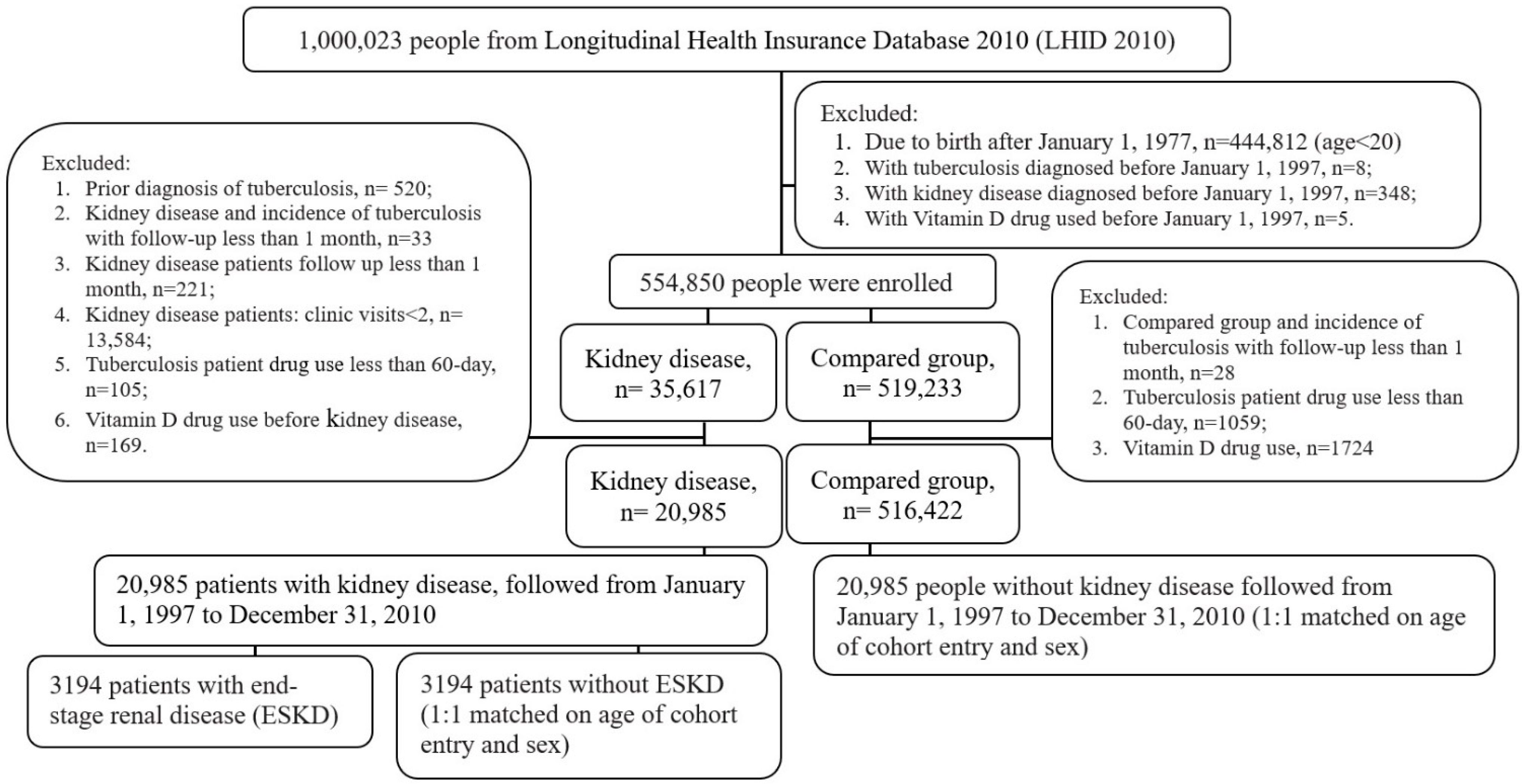
2.1. Source of Data and Study Population
2.2. Ascertainment of Kidney Diseases and Tuberculosis
2.3. Comorbidities
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, L.; Wan, X.; Huang, Z.; Zeng, F.; Wei, G.; Fang, D.; Deng, W.; Li, Y. Impact of vitamin D on chronic kidney diseases in nondialysis patients: A meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e61387. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.R.; Sigdel, M.R. Prevalence, clinical presentation, and outcome of tuberculosis in patients with chronic kidney disease at a tertiary care hospital in Nepal. Int. J. Nephrol. 2020, 2020, 7401541. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global prevalence of chronic kidney disease—A systematic review and meta-analysis. PLoS ONE 2016, 11, 158–765. [Google Scholar] [CrossRef]
- Yang, W.C.; Hwang, S.J.; Taiwan Society of Nephrology. Incidence, prevalence and mortality trends of dialysis end-stage renal disease in Taiwan from 1990 to 2001: The impact of national health insurance. Nephrol. Dial. Transplant. 2008, 23, 3977–3982. [Google Scholar] [CrossRef]
- Cahuayme-Zuniga, L.J.; Brust, K.B. Mycobacterial infections in patients with chronic kidney disease and kidney transplantation. Adv. Chronic Kidney Dis. 2019, 26, 35–40. [Google Scholar] [CrossRef]
- Talat, N.; Perry, S.; Parsonnet, J.; Dawood, G.; Hussain, R. Vitamin D deficiency and tuberculosis progression. Emerg. Infect. Dis. 2010, 16, 853–855. [Google Scholar] [CrossRef]
- Graidis, S.; Papavramidis, T.S.; Papaioannou, M. Vitamin D and acute kidney injury: A two-way causality relation and a predictive, prognostic, and therapeutic role of vitamin D. Front. Nutr. 2020, 7, 630951. [Google Scholar] [CrossRef]
- Christakos, S.; Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Mady, L.J. Vitamin D: Metabolism. Endocrinol. Metab. Clin. N. Am. 2010, 39, 243–253. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab. 2012, 97, 1153–1158. [Google Scholar] [CrossRef]
- Griffin, G.; Hewison, M.; Hopkin, J.; Kenny, R.A.; Quinton, R.; Rhodes, J.; Subramanian, S.; Thickett, D. Preventing vitamin D deficiency during the COVID-19 pandemic: UK definitions of vitamin D sufficiency and recommended supplement dose are set too low. Clin. Med. 2021, 21, e48–e51. [Google Scholar] [CrossRef]
- Investigators, J.D.; Shoji, T.; Inaba, M.; Fukagawa, M.; Ando, R.; Emoto, M.; Fujii, H.; Fujimori, A.; Fukui, M.; Hase, H.; et al. Effect of oral alfacalcidol on clinical outcomes in patients without secondary hyperparathyroidism receiving maintenance hemodialysis: The J-DAVID randomized clinical trial. JAMA 2018, 320, 2325–2334. [Google Scholar] [CrossRef]
- Hu, L.; Napoletano, A.; Provenzano, M.; Garofalo, C.; Bini, C.; Comai, G.; La Manna, G. Mineral bone disorders in kidney disease patients: The ever-current topic. Int. J. Mol. Sci. 2022, 23, 12223. [Google Scholar] [CrossRef] [PubMed]
- Iseki, K.; Kabata, D.; Shoji, T.; Inaba, M.; Emoto, M.; Mori, K.; Morioka, T.; Nakatani, S.; Shintani, A. Dialysate calcium, alfacalcidol, and clinical outcomes: A post-hoc analysis of the J-DAVID trial. PLoS ONE 2022, 17, e0273195. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.; Aspray, T.J.; Schoenmakers, I. Vitamin D supplementation for patients with chronic kidney disease: A systematic review and meta-analyses of trials investigating the response to supplementation and an overview of guidelines. Calcif. Tissue Int. 2021, 109, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Finch, J.L.; Tokumoto, M.; Nakamura, H.; Yao, W.; Shahnazari, M.; Lane, N.; Slatopolsky, E. Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2010, 298, F1315–F1322. [Google Scholar] [CrossRef]
- Banon, S.; Rosillo, M.; Gomez, A.; Perez-Elias, M.J.; Moreno, S.; Casado, J.L. Effect of a monthly dose of calcidiol in improving vitamin D deficiency and secondary hyperparathyroidism in HIV-infected patients. Endocrine 2015, 49, 528–537. [Google Scholar] [CrossRef]
- Martins, J.S.; Palhares, M.O.; Teixeira, O.C.; Gontijo Ramos, M. Vitamin D status and its association with parathyroid hormone concentration in Brazilians. J. Nutr. Metab. 2017, 2017, 9056470. [Google Scholar] [CrossRef]
- Bonkain, F.; De Clerck, D.; Dirix, V.; Singh, M.; Locht, C.; Mascart, F.; Corbiere, V. Early diagnosis of miliary tuberculosis in a hemodialysis patient by combining two interferon-gamma-release assays: A case report. BMC Nephrol. 2020, 21, 214. [Google Scholar] [CrossRef]
- Cardoso, M.P. Native vitamin D in pre-dialysis chronic kidney disease. Nefrología 2019, 39, 18–28. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chiu, Y.W.; Yang, H.R.; Chen, T.C.; Hsieh, M.H.; Wang, W.H.; Chen, Y.H. Association of vitamin D levels and risk of latent tuberculosis in the hemodialysis population. J. Microbiol. Immunol. Infect. 2021, 54, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Todoriko, L.D.; Toderika, Y.I.; Shevchenko, O.S.; Pidverbetska, O.V.; Pidverbetskyi, O.Y. The role of vitamin D deficiency in antituberculous protection. Infus. Chemother. 2021, 4, 38–44. [Google Scholar] [CrossRef]
- Shu, C.C.; Wei, Y.F.; Yeh, Y.C.; Lin, H.H.; Chen, C.Y.; Wang, P.H.; Cheng, S.L.; Wang, J.Y.; Yu, C.J. The impact on incident tuberculosis by kidney function impairment status: Analysis of severity relationship. Respir. Res. 2020, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Bardenheier, B.H.; Pavkov, M.E.; Winston, C.A.; Klosovsky, A.; Yen, C.; Benoit, S.; Gravenstein, S.; Posey, D.L.; Phares, C.R. Prevalence of Tuberculosis disease among adult US-bound refugees with chronic kidney disease. Immigr. Minor. Health 2019, 21, 1275–1281. [Google Scholar] [CrossRef]
- Baghaei, P.; Marjani, M.; Javanmard, P.; Tabarsi, P.; Masjedi, M.R. Diabetes mellitus and tuberculosis facts and controversies. J. Diabetes Metab. Disord. 2013, 12, 58. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, A.; Balasubramanian, V.; Gupta, N. Extensively drug-resistant tuberculosis in India: Current evidence on diagnosis & management. Indian J. Med. Res. 2017, 145, 271–293. [Google Scholar] [CrossRef]
- Segall, L.; Covic, A. Diagnosis of tuberculosis in dialysis patients: Current strategy. Clin. J. Am. Soc. Nephrol. 2010, 5, 1114–1122. [Google Scholar] [CrossRef][Green Version]
- Dobler, C.C.; McDonald, S.P.; Marks, G.B. Risk of tuberculosis in dialysis patients: A nationwide cohort study. PLoS ONE 2011, 6, e29563. [Google Scholar] [CrossRef]
- Ostermann, M.; Palchaudhuri, P.; Riding, A.; Begum, P.; Milburn, H.J. Incidence of tuberculosis is high in chronic kidney disease patients in South East England and drug resistance common. Ren. Fail. 2016, 38, 256–261. [Google Scholar] [CrossRef]
- Gao, L.; Tao, Y.; Zhang, L.; Jin, Q. Vitamin D receptor genetic polymorphisms and tuberculosis: Updated systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2010, 14, 15–23. [Google Scholar] [PubMed]
- Hu, Q.; Chen, Z.; Liang, G.; Mo, F.; Zhang, H.; Xu, S.; Wang, Y.; Kang, L.; Jin, T. Vitamin D receptor gene associations with pulmonary tuberculosis in a Tibetan Chinese population. BMC Infect. Dis. 2016, 16, 469. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zappulo, F.; Cappuccilli, M.; Cingolani, A.; Scrivo, A.; Chiocchini, A.L.C.; Nunzio, M.D.; Donadei, C.; Napoli, M.; Tondolo, F.; Cianciolo, G.; et al. Vitamin D and the Kidney: Two players, one console. Int. J. Mol. Sci. 2022, 23, 9135. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, N.A.; Kanis, J.A.; Beneton, M.N.; Brown, C.B.; Juttmann, J.R.; Jordans, J.G.; Josse, S.; Meyrier, A.; Lins, R.L.; Fairey, I.T. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ 1995, 310, 358–363. [Google Scholar] [CrossRef]
- Chang, D.P.S.; Guan, X.L. Metabolic versatility of mycobacterium tuberculosis during infection and dormancy. Metabolites 2021, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Kao Yang, Y.H.; Lai, E.C. Taiwan’s National health insurance research database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Schold, J.D.; Arrigain, S.; Jolly, S.E.; Jain, A.; Schreiber, M.J., Jr.; Simon, J.F.; Srinivas, T.R.; Nally, J.V., Jr. Low 25-hydroxyvitamin D levels and mortality in non-dialysis-dependent CKD. Am. J. Kidney Dis. 2011, 58, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Pagani, F.; Francucci, C.M.; Moro, L. Markers of bone turnover: Biochemical and clinical perspectives. J. Endocrinol. Investig. 2005, 28, 8–13. [Google Scholar]
| Kidney Disease | Compared Group | p Value | |
|---|---|---|---|
| N | 20,985 | 20,985 | |
| Dialysis, n (%) | |||
| Hemodialysis alone | 2780 (13.2) | - | - |
| Peritoneal dialysis alone | 80 (0.4) | - | - |
| Hemodialysis and peritoneal dialysis | 291 (1.4) | - | - |
| Kidney transplant recipients | 192 (0.9) | - | - |
| Tuberculosis *, n (%) | 290 (1.4) | 334 (1.6) | 0.0760 |
| Follow-up duration median (IQR), years | 3.7 (1.7–6.6) | 8.0 (4.9–11.2) | <0.0001 |
| Follow-up duration groups, n (%) | |||
| ≤3 years | 120 (41.4) | 34 (10.2) | |
| >3 to 6 years | 86 (29.7) | 79 (23.7) | |
| >6 to 9 years | 53 (18.3) | 70 (21.0) | |
| >9 years | 31 (10.7) | 151 (45.2) | <0.0001 |
| Age of cohort entry mean (SD), years | 51.8 (14.2) | 51.8 (14.2) | 0.9501 |
| Age group, n (%) | |||
| 20 to 30 | 1578 (7.5) | 1578 (7.5) | |
| >30 to 40 | 3177 (15.1) | 3177 (15.1) | |
| >40 to 50 | 4751 (22.6) | 4751 (22.6) | |
| >50 to 60 | 4525 (21.6) | 4525 (21.6) | |
| >60 to 70 | 4901 (23.4) | 4901 (23.4) | |
| >70 | 2053 (9.8) | 2053 (9.8) | 1.0000 |
| Sex, n (%) | |||
| Males | 11,242 (53.6) | 11,242 (53.6) | |
| Females | 9743 (46.4) | 9743 (46.4) | 1.0000 |
| Residential region, n (%) | |||
| Northern | 9597 (45.7) | 9748 (46.5) | |
| Central | 4678 (22.3) | 4899 (23.3) | |
| Southern | 6038 (28.8) | 5594 (26.7) | |
| Eastern and other region | 672 (3.2) | 744 (3.5) | <0.0001 |
| Comorbidities, n (%) | |||
| Alcohol abuse | 404 (1.9) | 214 (1.0) | <0.0001 |
| Lipid disorders | 10,780 (51.4) | 5993 (28.6) | <0.0001 |
| Obesity | 306 (1.5) | 149 (0.7) | <0.0001 |
| Hypertension | 15,274 (72.8) | 9986 (47.6) | <0.0001 |
| Myocardial infarction | 380 (1.8) | 176 (0.8) | <0.0001 |
| Congestive heart failure | 2709 (12.9) | 1009 (4.8) | <0.0001 |
| Peripheral vascular disease | 1418 (6.8) | 712 (3.4) | <0.0001 |
| Cerebrovascular disease | 3552 (16.9) | 2253 (10.7) | <0.0001 |
| Chronic pulmonary disease | 5330 (25.4) | 3859 (18.4) | <0.0001 |
| Rheumatologic disease | 1011 (4.8) | 501 (2.4) | <0.0001 |
| Liver disease | 5083 (24.2) | 2726 (13.0) | <0.0001 |
| Diabetes mellitus | 5286 (25.2) | 2768 (13.2) | <0.0001 |
| Any malignancy | 1720 (8.2) | 1061 (5.1) | <0.0001 |
| Vitamin D drugs (ATC code), n(%) | |||
| A11CC03 (alfacalcidol) | 297 (1.4) | - | - |
| A11CC04 (calcitriol) | 1138 (5.4) | - | - |
| A11CC03 or A11CC04 | 1306 (6.2) | - | - |
| Tuberculosis/ Total Subjects, % | Person–Years | Events Per 1000 Person–Years (95% CI) | IRR (95% CI) | p Value | Adjusted IRR (95% CI) | p Value | |
|---|---|---|---|---|---|---|---|
| Compared group | 334/20,985, 1.59 | 291,773.08 | 1.14 (1.14–1.15) | 1.00 | 1.00 | ||
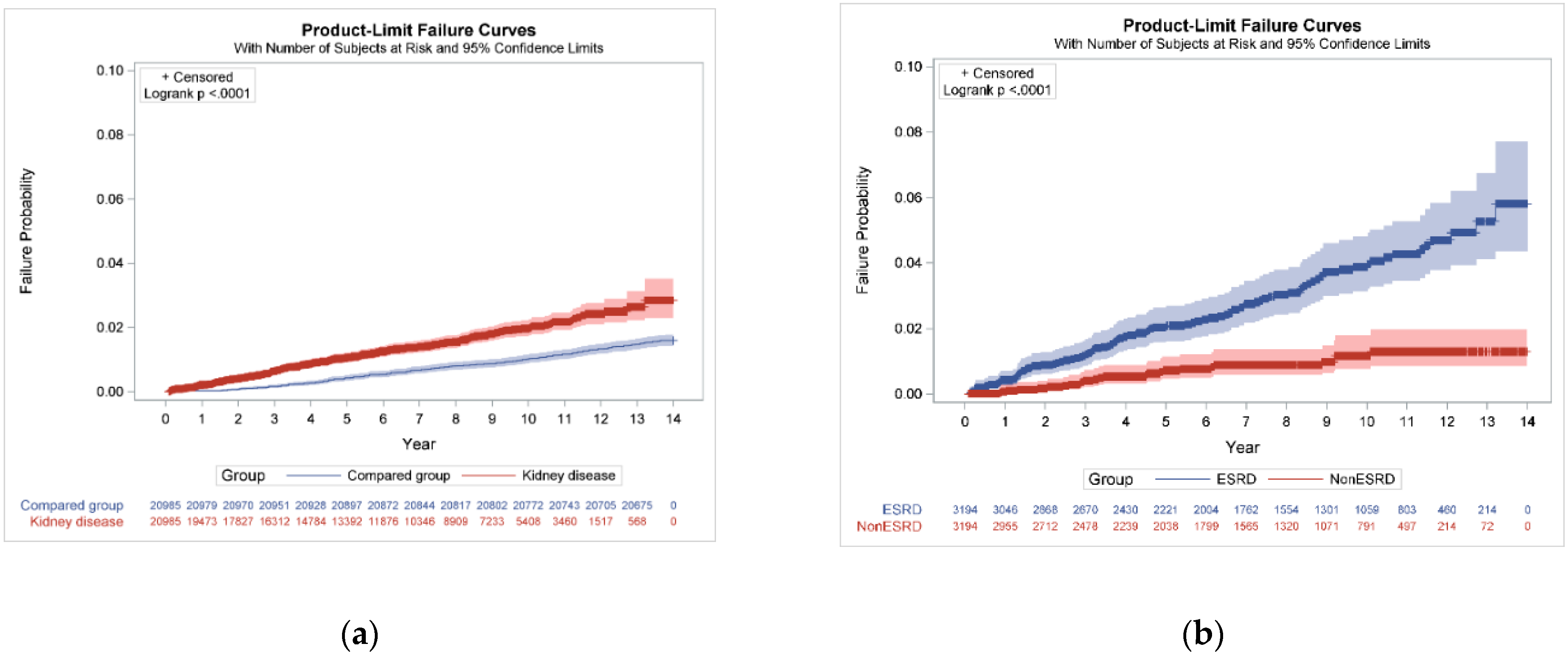
| Kidney disease | 290/20,985, 1.38 | 141,549.53 | 2.05 (2.04–2.06) | 1.79 (1.53–2.09) | <0.0001 | 1.57 (1.33–1.86) | <0.0001 |
| Follow-up duration * | |||||||
| ≤3 years | |||||||
| Compared group | 34/20,985, 0.16 | 62,920.18 | 0.54 (0.54–0.54) | 1.00 | 1.00 | ||
| Kidney disease | 120/20,985, 0.57 | 56,041.95 | 2.14 (2.12–2.16) | 3.96 (2.71–5.80) | <0.0001 | 3.79 (2.55–5.62) | <0.0001 |
| >3 to 6 years | |||||||
| Compared group | 79/20,951, 0.38 | 125,588.8 | 0.63 (0.63–0.63) | 1.00 | 1.00 | ||
| Kidney disease | 86/16,303, 0.53 | 91,174.27 | 0.94 (0.94–0.95) | 1.50 (1.10–2.04) | 0.0093 | 1.33 (0.96–1.84) | 0.0824 |
| ESKD | NonESKD | p Value | |
|---|---|---|---|
| N | 3194 | 3194 | |
| Dialysis, n (%) | |||
| Hemodialysis alone | 2657 (83.2) | - | - |
| Peritoneal dialysis alone | 74 (2.3) | - | - |
| Hemodialysis and peritoneal dialysis | 271 (8.5) | - | - |
| Kidney transplant recipients | 192 (6.0) | - | - |
| Tuberculosis, n (%) | 98 (3.1) | 25 (0.8) | <0.0001 |
| Follow-up duration median (IQR), years | 3.9 (1.7–7.4) | 3.3 (2.4–5.3) | 0.6085 |
| Follow-up duration groups, n (%) | |||
| ≤3 years | 36 (36.7) | 11 (44.0) | |
| >3 to 6 years | 26 (26.5) | 8 (32.0) | |
| >6 to 9 years | 24 (24.5) | 3 (12.0) | |
| >9 years | 12 (12.2) | 3 (12.0) | 0.5919 |
| Age of cohort entry mean (SD), years | 50.3 (13.1) | 50.4 (13.3) | 0.8923 |
| Age group, n (%) | |||
| 20 to 30 | 199 (6.2) | 199 (6.2) | |
| >30 to 40 | 519 (16.2) | 519 (16.2) | |
| >40 to 50 | 908 (28.4) | 908 (28.4) | |
| >50 to 60 | 726 (22.7) | 726 (22.7) | |
| >60 to 70 | 629 (19.7) | 629 (19.7) | |
| >70 | 213 (6.7) | 213 (6.7) | 1.0000 |
| Sex, n (%) | |||
| Males | 1575 (49.3) | 1575 (49.3) | |
| Females | 1619 (50.7) | 1619 (50.7) | 1.0000 |
| Residential region, n (%) | |||
| Northern | 1372 (43.0) | 1476(46.2) | |
| Central | 737 (23.1) | 752(23.5) | |
| Southern | 981 (30.7) | 893(28) | |
| Eastern and other | 104 (3.3) | 73(2.3) | 0.0037 |
| Comorbidities, n (%) | |||
| Alcohol abuse | 39 (1.2) | 64 (2.0) | 0.0130 |
| Lipid disorders | 1648 (51.6) | 1650 (51.7) | 0.9601 |
| Obesity | 23 (0.7) | 53 (1.7) | 0.0005 |
| Hypertension | 2851 (89.3) | 2172 (68.0) | <0.0001 |
| Myocardial infarction | 77 (2.4) | 49 (1.5) | 0.0118 |
| Congestive heart failure | 642 (20.1) | 341 (10.7) | <0.0001 |
| Peripheral vascular disease | 282 (8.8) | 174 (5.4) | <0.0001 |
| Cerebrovascular disease | 586 (18.3) | 481 (15.1) | 0.0004 |
| Chronic pulmonary disease | 683 (21.4) | 751 (23.5) | 0.0414 |
| Rheumatologic disease | 111 (3.5) | 169 (5.3) | 0.0004 |
| Liver disease | 668 (20.9) | 780 (24.4) | 0.0008 |
| Diabetes mellitus | 1048 (32.8) | 725 (22.7) | <0.0001 |
| Any malignancy | 314 (9.8) | 247 (7.7) | 0.0031 |
| Vitamin D drugs (ATC code), n(%) | |||
| A11CC03 (alfacalcidol) | 240 (7.5) | 8 (0.3) | <0.0001 |
| Patient visits median (IQR), frequency | 6.5 (3.0–16.0) | 4.0 (1.5–6.0) | 0.0587 |
| Total dose median (IQR) | 51.9 (17.3–171.6) | 29.0 (21.0–96.3) | 0.3592 |
| A11CC04 (calcitriol) | 996 (31.2) | 19 (0.6) | <0.0001 |
| Patient visits median (IQR), n | 9.0 (4.0–22.0) | 6.0 (2.0–16.0) | 0.1516 |
| Total dose median (IQR) | 54.1 (17.6–137.0) | 38.5 (14.0–117.5) | 0.5898 |
| A11CC03 or A11CC04 | 1113 (34.8) | 27 (0.8) | <0.0001 |
| Patient visits median (IQR) | 10.0 (4.0–23.0) | 5.0 (2.0–11.0) | 0.0061 |
| Total dose median (IQR) | 60.3 (18.8–152.1) | 31.5 (14.0–117.5) | 0.1975 |
| Tuberculosis /Total Subjects, % | Person–Years | Events Per 1000 Person–Years (95% CI) | IRR (95% CI) | p Value | Adjusted IRR (95% CI) | p Value | |
|---|---|---|---|---|---|---|---|
| ESKD | |||||||
| None | 25/3194, 0.78 | 21,330.40 | 1.17 (1.16–1.19) | 1.00 | 1.00 | ||
| Yes | 98/3194, 3.07 | 24,041.22 | 4.08 (4.03–4.13) | 3.48 (2.24–5.40) | <0.0001 | 3.67 (2.27–5.93) | <0.0001 |
| ESKD with vitamin D use | |||||||
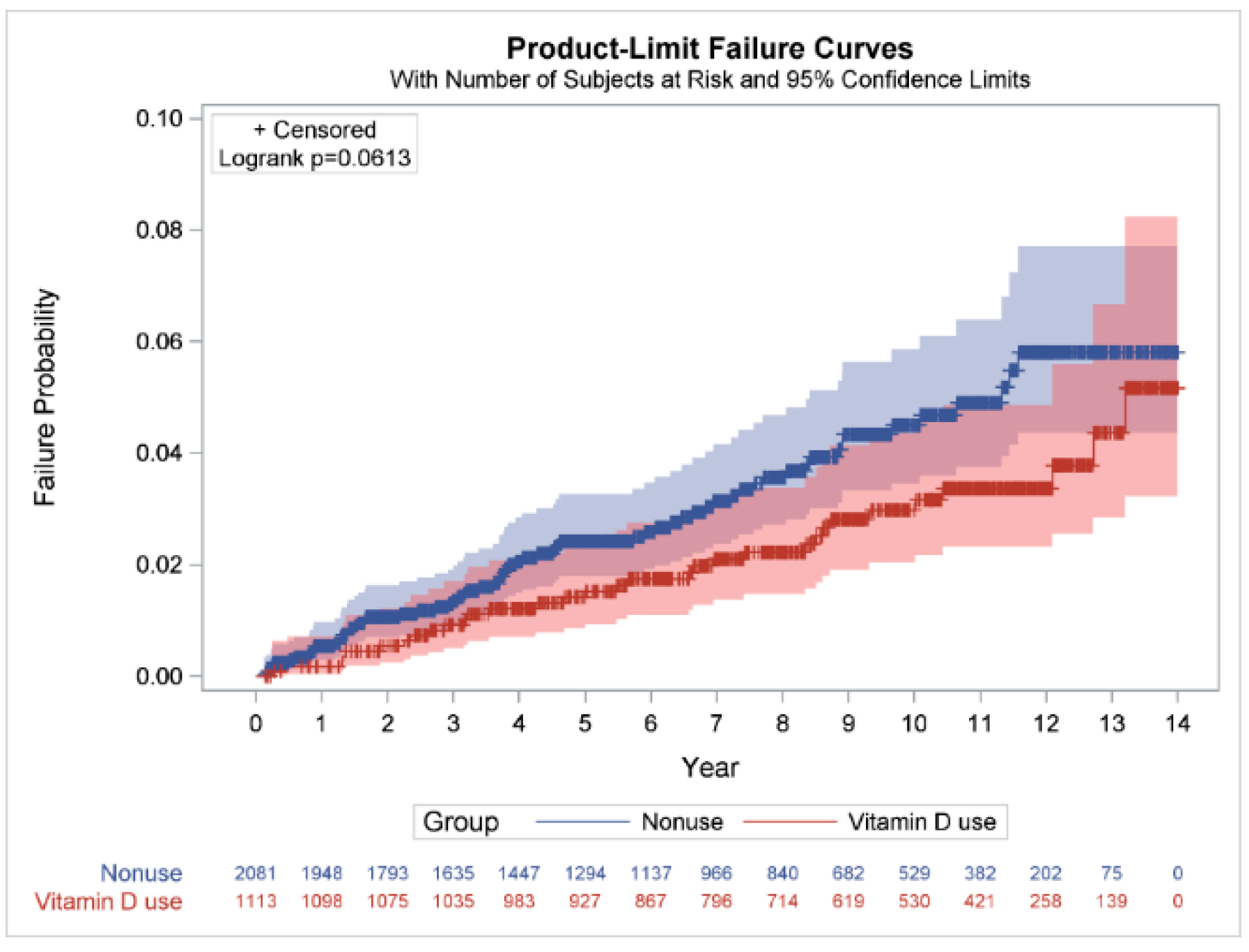
| Nonuse | 66/2081, 3.17 | 14,006.34 | 4.71 (4.63–4.79) | 1.00 | 1.00 | ||
| Use | 32/1113, 2.88 | 10,034.88 | 3.19 (3.13–3.25) | 0.68 (0.44–1.03) | 0.0699 | 0.77 (0.49–1.19) | 0.2351 |
| Vitamin D use | |||||||
| Nonuse | 66/2081, 3.17 | 14,006.34 | 4.71 (4.63–4.79) | 1.00 | |||
| 0–6750 | 6/279, 2.15 | 2334.04 | 2.57 (2.47–2.68) | 0.55 (0.24–1.26) | 0.1553 | 0.56 (0.24–1.30) | 0.1783 |
| 6751–21,690 | 8/275, 2.91 | 2304.91 | 3.47 (3.33–3.62) | 0.74 (0.35–1.53) | 0.4141 | 0.83 (0.39–1.74) | 0.6183 |
| 21,691–54,810 | 8/281, 2.85 | 2663.54 | 3.00 (2.89–3.12) | 0.64 (0.31–1.33) | 0.2290 | 0.75 (0.36–1.58) | 0.4520 |
| >54,810 | 10/278, 3.60 | 2732.38 | 3.66 (3.53–3.80) | 0.78 (0.40–1.51) | 0.4564 | 0.95 (0.48–1.89) | 0.8795 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlamini, S.T.; Htet, K.M.; Theint, E.C.C.; Li, W.-M.; Chang, H.-W.; Tu, H.-P. Assessment of the Association of Vitamin D and the Risk of Tuberculosis among End-Stage Kidney Disease Population. Life 2022, 12, 1881. https://doi.org/10.3390/life12111881
Dlamini ST, Htet KM, Theint ECC, Li W-M, Chang H-W, Tu H-P. Assessment of the Association of Vitamin D and the Risk of Tuberculosis among End-Stage Kidney Disease Population. Life. 2022; 12(11):1881. https://doi.org/10.3390/life12111881
Chicago/Turabian StyleDlamini, Sithembiso Tiyandza, Kyaw Moe Htet, Ei Chue Chue Theint, Wei-Ming Li, Hsin-Wen Chang, and Hung-Pin Tu. 2022. "Assessment of the Association of Vitamin D and the Risk of Tuberculosis among End-Stage Kidney Disease Population" Life 12, no. 11: 1881. https://doi.org/10.3390/life12111881
APA StyleDlamini, S. T., Htet, K. M., Theint, E. C. C., Li, W.-M., Chang, H.-W., & Tu, H.-P. (2022). Assessment of the Association of Vitamin D and the Risk of Tuberculosis among End-Stage Kidney Disease Population. Life, 12(11), 1881. https://doi.org/10.3390/life12111881






