Chitosan: An Autocidal Molecule of Plant Pathogenic Fungus
Abstract
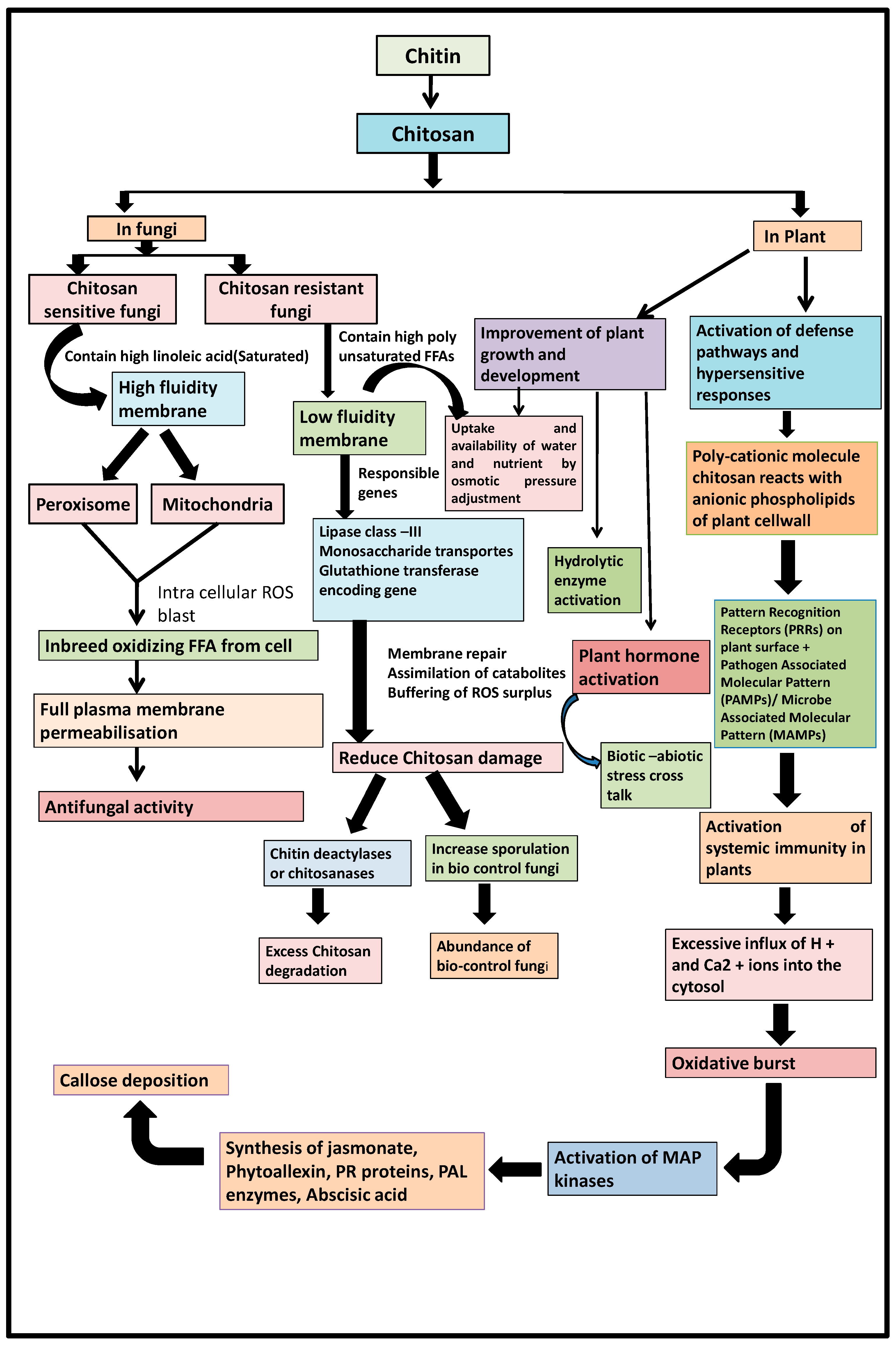
:1. Introduction
2. Structure and Formation of Chitin
Deacetylation and Hydrolysis of Chitin/Chitosan
- The chitinolytic process requires direct hydrolysis of the beta-1,4 glycosidic bonds between the GlcNAc units by chitinases. Chitinases are produced by higher plants, which use the enzymes to defend themselves against pathogenic attacks by degrading chitin in the cell walls of fungi and bacteria [27]. Plant chitinases have molecular weights ranging from 25 to 40 kD and can be acidic or basic. Endochitinases and exochitinases are the two types of chitinases [28]. Chitinase genes from biocontrol fungi such as Trichoderma have significantly higher antifungal activity than comparable plant genes. These fungal genes encode for chitinolytic enzymes, which have higher antifungal activity similar to chemical fungicides [29].
3. Successful Use of Chitin/Chitosan against Plant Pathogen with Special Reference of Pathogenic Fungus
4. Application of Chitosan
4.1. Seed Treatment
4.2. Chitosan Used for Soil Amendment
4.3. Chitosan Used as Foliar Spray
4.4. Chitosan Used as Post Harvest Fruit Treatment
4.5. Effect of Chitosan in Plant Disease Control
- Indirect treatment of disease by activating defenses and improving plant health
- 2.
- Direct method of pathogen control.
5. Conclusions
6. Future Aspect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghule, M.R.; Ramteke, P.K.; Ramteke, S.D.; Kodre, P.S.; Langote, A.; Gaikwad, A.V.; Holkar, S.K.; Jambhekar, H. Impact of chitosan seed treatment of fenugreek for management of root rot disease caused by Fusarium solani under in vitro and in vivo conditions. 3 Biotech 2021, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, C.; Zhang, H.; Wang, Q. Benzoylurea Chitin Synthesis Inhibitors. J. Agric. Food Chem. 2015, 63, 6847–6865. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Prasad, R.; Chandrika, K.; Godbole, V. A novel chitosan biopolymer based Trichoderma delivery system: Storage stability, persistence and bio efficacy against seed and soil borne diseases of oilseed crops. Microbiol. Res. 2020, 237, 126487. [Google Scholar] [CrossRef] [PubMed]
- Amine, R.; Tarek, C.; Hassane, E.; Noureddine, E.H.; Khadija, O. Chemical Proprieties of Biopolymers (Chitin/Chitosan) and their Synergic Effects with Endophytic Bacillus Species: Unlimited Applications in Agriculture. Molecules 2021, 26, 1117. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Hassan, O.; Chang, T. Chitosan for Eco-friendly Control of Plant Disease. Asian J. Plant Pathol. 2017, 11, 53–70. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-K.; Shen, C.-R.; Liu, C.-L. N-Acetylglucosamine: Production and Applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef] [Green Version]
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef]
- Wang, S.L.; Chang, W.T. Purification and characterization of two bifunctional chitinases/lysozymes extracellularly produced by Pseudomonas aeruginosa K-187 in a shrimp and crab shell powder medium. Appl. Environ. Microbiol. 1997, 63, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Mourya, V.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, Properties, and Some New Applications of Chitin and Its Derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef]
- Kramer, K.J.; Koga, D. Insect chitin: Physical state, synthesis, degradation and metabolic regulation. Insect Biochem. 1986, 16, 851–877. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Chen, L.; Xiang, X.; Yang, R.; Yu, S.; Wu, X. Proteomic analysis of peritrophic membrane (PM) from the midgut of fifth-instar larvae, Bombyx mori. Mol. Biol. Rep. 2012, 39, 3427–3434. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Èntomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Salama, D.M.; El-Aziz, M.A.; Rizk, F.A.; Elwahed, M.A. Applications of nanotechnology on vegetable crops. Chemosphere 2021, 266, 129026. [Google Scholar] [CrossRef]
- Suresh, P.V.; Kumar, P.K.A. Enhanced degradation of α-chitin materials prepared from shrimp processing byproduct and production of N-acetyl-d-glucosamine by thermoactive chitinases from soil mesophilic fungi. Biogeochemistry 2012, 23, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of Chitosan for the Generation of Functional Derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Chatterjee, S.; Adhya, M.; Guha, A.; Chatterjee, B. Chitosan from Mucor rouxii: Production and physico-chemical characterization. Process Biochem. 2005, 40, 395–400. [Google Scholar] [CrossRef]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Arnold, N.D.; Brück, W.M.; Garbe, D.; Brück, T.B. Enzymatic Modification of Native Chitin and Conversion to Specialty Chemical Products. Mar. Drugs 2020, 18, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Sharma, K.; Gaur, R.; Gupta, V. Role of Chitinase in Plant Defense. Asian J. Biochem. 2011, 6, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Rathore, A.S.; Gupta, R.D. Chitinases from Bacteria to Human: Properties, Applications, and Future Perspectives. Enzym. Res. 2015, 2015, 791907. [Google Scholar] [CrossRef] [Green Version]
- Loc, N.H.; Huy, N.D.; Quang, H.T.; Lan, T.T.; Ha, T.T.T. Characterisation and antifungal activity of extracellular chitinase from a biocontrol fungus, Trichoderma asperellum PQ34. Mycology 2019, 11, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Roy, J.C.; Salaun, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of chitin: Solvents, solution behaviors and their related mechanisms. In Solubility of Polysaccharides; Xu, Z., Ed.; Intech Open: London, UK, 2017. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Zhang, C.; Guo, Z.; Wu, X.; An, H. Co-Application of Allicin and Chitosan Increases Resistance of Rosa roxburghii against Powdery Mildew and Enhances Its Yield and Quality. Antibiotics 2021, 10, 1449. [Google Scholar] [CrossRef]
- Ferrante, P.; Scortichini, M. Molecular and phenotypic features of Pseudomonas syringae pv. actinidiae isolated during recent epidemics of bacterial canker on yellow kiwifruit (Actinidia chinensis) in central Italy. Plant Pathol. 2010, 59, 954–962. [Google Scholar] [CrossRef]
- Huda, M.S. Antifungal and Antioxidant Activities of methanol extract of Chitin, Chitosan and Shrimp shell waste. Int. J. Pharm. Phytopharm. Res. 2018, 8, 25–30. [Google Scholar] [CrossRef]
- Zhang, J.; Luan, F.; Li, Q.; Gu, G.; Dong, F.; Guo, Z. Synthesis of Novel Chitin Derivatives Bearing Amino Groups and Evaluation of Their Antifungal Activity. Mar. Drugs 2018, 16, 380. [Google Scholar] [CrossRef] [Green Version]
- Shakeel, Q.; Lyu, A.; Zhang, J.; Wu, M.; Li, G.; Hsiang, T.; Yang, L. Biocontrol of Aspergillus flavus on Peanut Kernels Using Streptomyces yanglinensis 3-10. Front. Microbiol. 2018, 9, 1049. [Google Scholar] [CrossRef] [Green Version]
- Xing, K.; Shen, X.; Zhu, X.; Ju, X.; Miao, X.; Tian, J.; Feng, Z.; Peng, X.; Jiang, J.; Qin, S. Synthesis and in vitro antifungal efficacy of oleoyl-chitosan nanoparticles against plant pathogenic fungi. Int. J. Biol. Macromol. 2016, 82, 830–836. [Google Scholar] [CrossRef]
- Zachetti, V.G.L.; Cendoya, E.; Nichea, M.J.; Chulze, S.N.; Ramirez, M.L. Preliminary Study on the Use of Chitosan as an Eco-Friendly Alternative to Control Fusarium Growth and Mycotoxin Production on Maize and Wheat. Pathogens 2019, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Akter, J.; Jannat, R.; Hossain, M.; Ahmed, J.U.; Rubayet, T. Chitosan for Plant Growth Promotion and Disease Suppression against Anthracnose in Chilli. Int. J. Environ. Agric. Biotechnol. 2018, 3, 806–817. [Google Scholar] [CrossRef] [Green Version]
- Riad, S.R.; El-Mohamedy, M.M.; Abdel-Kader, F.; Abd-El-Kareem, M.M.; El-Mougy, N.S. Inhibitory effect of antagonistic bio-agents and chitosan on the growth of tomato root rot pathogens In vitro. J. Agric. Technol. 2013, 9, 1521–1533. [Google Scholar]
- El-Mohamedy, S.R.R.; Shafeek, M.R.; Abd El-Samad, E.E.-D.H.; Salama, D.M.; Rizk, F.A. Field application of plant resistance inducers (PRIs) to control important root rot diseases and improvement growth and yield of green bean (Phaseolus vulgaris L.). Aust. J. Crop Sci. 2017, 11, 496–505. [Google Scholar] [CrossRef]
- Li, R.; He, J.; Xie, H.; Wang, W.; Bose, S.K.; Sun, Y.; Hu, J.; Yin, H. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2019, 126, 91–100. [Google Scholar] [CrossRef]
- Xue, H.; Yang, Z. Potato Dry Rot Caused by Fusarium spp. and Mycotoxins Accumulation and Management; Intech Open: London, UK, 2021. [Google Scholar] [CrossRef]
- Zohara, F.; Surovy, M.Z.; Khatun, A.; Prince, F.R.K.; Akanda, A.M.; Rahman, M.; Islam, T. Chitosan biostimulant controls infection of cucumber by Phytophthora capsici through suppression of asexual reproduction of the pathogen. Acta Agrobot. 2019, 72, 1763. [Google Scholar] [CrossRef]
- Corsi, B.; Riccioni, L.; Forni, C. In vitro cultures of Actinidia deliciosa (A. Chev) C.F. Liang & A.R. Ferguson: A tool to study the SAR induction of chitosan treatment. Org. Agric. 2015, 5, 189–198. [Google Scholar] [CrossRef]
- Murphy, J.G.; Rafferty, S.M.; Cassells, A.C. Stimulation of wild strawberry (Fragaria vesca) arbuscular mycorrhizas by addition of shellfish waste to the growth substrate: Interaction between mycorrhization, substrate amendment and susceptibility to red core (Phytophthora fragariae). Appl. Soil Ecol. 2000, 15, 153–158. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; Van Der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.A.; Farrag, S.A.A.; Abu-Elamayem, M.M.; Ahmed, N.S. Extraction, characterization, and nematicidal activity of chitin and chitosan derived from shrimp shell wastes. Biol. Fertil. Soils 2012, 48, 463–468. [Google Scholar] [CrossRef]
- Rahman, H.; Shovan, L.R.; Hjeljord, L.G.; Aam, B.B.; Eijsink, V.G.H.; Sørlie, M.; Tronsmo, A. Inhibition of Fungal Plant Pathogens by Synergistic Action of Chito-Oligosaccharides and Commercially Available Fungicides. PLoS ONE 2014, 9, e93192. [Google Scholar] [CrossRef]
- Dodgson, J.; Dodgson, W. Comparison of Effects of Chitin and Chitosan for Control of Colletotrichum sp. on Cucumbers. J. Pure Appl. Microbiol. 2017, 11, 87–93. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Panda, K.; Acharya, K. Chitosan-induced immunity in Camellia sinensis (L.) O. Kuntze against blister blight disease is mediated by nitric-oxide. Plant Physiol. Biochem. 2017, 115, 298–307. [Google Scholar] [CrossRef]
- Anusuya, S.; Sathiyabama, M. Effect of Chitosan on Rhizome Rot Disease of Turmeric Caused by Pythium aphanidermatum. ISRN Biotechnol. 2014, 2014, 305349. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food Sci. Nutr. 2017, 57, 579–601. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Lennox, C.L.; Meitz-Hopkins, J.C.; Caleb, O.J.; Sigge, G.O.; Opara, U.L. Investigating the effects of crab shell chitosan on fungal mycelial growth and postharvest quality attributes of pomegranate whole fruit and arils. Sci. Hortic. 2017, 220, 78–89. [Google Scholar] [CrossRef]
- Wang, L.; Wu, H.; Qin, G.; Meng, X. Chitosan disrupts Penicillium expansum and controls postharvest blue mold of jujube fruit. Food Control 2014, 41, 56–62. [Google Scholar] [CrossRef]
- Zheng, F.; Zheng, W.; Li, L.; Pan, S.; Liu, M.; Zhang, W.; Liu, H.; Zhu, C. Chitosan Controls Postharvest Decay and Elicits Defense Response in Kiwifruit. Food Bioprocess Technol. 2017, 10, 1937–1945. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, L.; Yan, H.; Kennedy, J.F.; Meng, X. Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohydr. Polym. 2013, 94, 272–277. [Google Scholar] [CrossRef]
- Plainsirichai, M.; Leelaphatthanapanich, S.; Wongsachai, N. Effect of chitosan on the quality of rose apples (Syzygium agueum Alston) cv. tabtimchan stored at an ambient temperature. APCBEE Procedia 2014, 8, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Siddaiah, C.N.; Prasanth, K.V.H.; Satyanarayana, N.R.; Mudili, V.; Gupta, V.K.; Kalagatur, N.K.; Satyavati, T.; Dai, X.-F.; Chen, J.-Y.; Mocan, A.; et al. Chitosan nanoparticles having higher degree of acetylation induce resistance against pearl millet downy mildew through nitric oxide generation. Sci. Rep. 2018, 8, 2485. [Google Scholar] [CrossRef] [Green Version]
- Zeng, K.; Deng, Y.; Ming, J.; Deng, L. Induction of disease resistance and ROS metabolism in navel oranges by chitosan. Sci. Hortic. 2010, 126, 223–228. [Google Scholar] [CrossRef]
- Meng, X.; Yang, L.; Kennedy, J.F.; Tian, S. Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohydr. Polym. 2010, 81, 70–75. [Google Scholar] [CrossRef]
- Li, S.-J.; Zhu, T.-H. Biochemical response and induced resistance against anthracnose (Colletotrichum camelliae) of camellia (Camellia pitardii) by chitosan oligosaccharide application. For. Pathol. 2013, 43, 67–76. [Google Scholar] [CrossRef]
- Li, B.; Liu, B.; Shan, C.; Ibrahim, M.; Lou, Y.; Wang, Y.; Xie, G.; Li, H.-Y.; Sun, G. Antibacterial activity of two chitosan solutions and their effect on rice bacterial leaf blight and leaf streak. Pest Manag. Sci. 2013, 69, 312–320. [Google Scholar] [CrossRef]
- Zeng, D.; Luo, X.; Tu, R. Application of Bioactive Coatings Based on Chitosan for Soybean Seed Protection. Int. J. Carbohydr. Chem. 2012, 2012, 104565. [Google Scholar] [CrossRef] [Green Version]
- Hadwiger, L.A. Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rincona, J.; Vega-Perezb, J.; Guerra-Sanchezb, M.G.; Hernandez-Lauzardoa, A.N.; Pena-Diazc, A.; Vallea, M.G.V.-D. Effect of chitosan on growth and plasma membrane properties of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Pestic. Biochem. Physiol. 2010, 97, 275–278. [Google Scholar] [CrossRef]
- Ghaouth, A.; Arul, J.; Ponnampalam, R.; Boulet, M. Chitosan Coating Effect on Storability and Quality of Fresh Strawberries. J. Food Sci. 1991, 56, 1618–1620. [Google Scholar] [CrossRef]
- Verleet, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Colom-Valiente, M.F.; Martinez-Peinado, P.; Martinez-Lopez, J.E.; Puelles, E.; Sempere-Ortells, J.M.; Lopez-Llorca, L.V. Carbon and nitrogen limitation increase chitosan antifungal activity in Neurospora crassa and fungal human pathogens. Fungal Biol. 2015, 119, 154–169. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Moya, F.; Kowbel, D.; Nueda, M.J.; Palma-Guerrero, J.; Glass, N.L.; Lopez-Llorca, L.V. Neurospora crassa transcriptomics reveals oxidative stress and plasma membrane homeostasis biology genes as key targets in response to chitosan. Mol. BioSyst. 2016, 12, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Jaime, M.D.; Lopez-Llorca, L.V.; Conesa, A.; Lee, A.Y.; Proctor, M.; Heisler, L.E.; Gebbia, M.; Giaever, G.; Westwood, J.T.; Nislow, C. Identification of yeast genes that confer resistance to chitosan oligosaccharide (COS) using chemogenomics. BMC Genom. 2012, 13, 267. [Google Scholar] [CrossRef] [Green Version]
- Palma-Guerrero, J.; Huang, I.-C.; Jansson, H.-B.; Salinas, J.; Lopez-Llorca, L.V.; Read, N. Chitosan permeabilizes the plasma membrane and kills cells of Neurospora crassa in an energy dependent manner. Fungal Genet. Biol. 2009, 46, 585–594. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef] [Green Version]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Singh, T.; Chittenden, C. Synergistic Ability of Chitosan and Trichoderma harzianum to Control the Growth and Discolouration of Common Sapstain Fungi of Pinus radiata. Forests 2021, 12, 542. [Google Scholar] [CrossRef]



| Sl.No | Chitin Derivative | Target Pathogen | Remarks | References |
|---|---|---|---|---|
| 1 | Chitosan supplemented with 0.05% boron and 0.05% | Pseudomonas syringae pv. actinidiae | Inhibition of the growth of bacteriumin in vitro condition | [32] |
| 2 | Chitin (Shrimp shell) | Aspergillus fumigatus, Aspergillus flavus and Aspergillus niger | In in vitro condition, highest inhibition (19.5 mm) in case of Aspergillus fumigatus | [33] |
| 3 | Chitin methanol extract | Aspergillus fumigatus, Aspergillus flavus and Aspergillus niger | Highest inhibition (16.5 mm) in case of A. fumigatus | [33] |
| 4 | Chitosan | Aspergillus fumigates, Aspergillus flavus and Aspergillus niger | Highest inhibition (14 mm) in case of A. fumigatus | [33] |
| 5 | Chitosan | Alternaria solani | Complete inhibition in in vitro condition at 5.0 g/lit | [1] |
| 6 | Chitin (CT), 6-amino-chitin (NCT) and 3,6-diamino-chitin (DNCT) | F. oxysporum f. sp. cucumerium, B. cinerea, C. lagenarium, P. asparagi, F. oxysporum f. niveum, and G. zeae | In in vitro condition, DNCT showed Highest inhibition zone (11.4–20.4 mm) > NCT > CT | [34] |
| 7 | Chitosan | Aspergillus flavus, Rhizoctonia solani and Alterneria alterneta | Growth inhibition was highest in case of Aspergillus flavus (10.66 mm.) | [35] |
| 8 | Chitosan-polyacrylic acid nanoparticles | Aspergillus flavus, Fusarium oxysporum, Fusarium solani, Aspergillus terreus, Alternaria tenuis, Aspergillus niger and Sclerotium rolfsii | Inhibition percentage was highest in case of Aspergillus flavus (60%), | [36] |
| 9 | Chitosan | F. proliferatum and F. verticillioides | Reduce deoxynivalenol (DON) and fumonisin (FBs) production on irradiated maize and wheat grains and growth rates of both the pathogens decreased. | [37] |
| 10 | Chitosan | Colletotrichum capsici | 7.67% post-emergence seedling mortality where Seeds were treated with 1% chitosan. | [38] |
| 11 | Chitosan | Fusarium oxysporum radicisycopersici, F. oxysporum lycopersici, F. solani, Rhizoctonia solani, Sclerotium rolfsii, Macrophomina phaseolinae, Pythium sp. and Phytophthora sp. | In 5 g/lit concentration, 100% inhibition can occur against every tested pathogen | [39] |
| 12 | Chitosan | Fusarium oxysporum f. sp. radicislycopersici | 16.60% and 42.8% reduction of disease severity in application of chitosan 1 g/lit and T. harzianum + Chitosan 1.0 g/lit | [40] |
| Sl. No | Crop Name | Respective Dose | Efficiency on Target Pathogen | Reference |
|---|---|---|---|---|
| 1 | Fenugreek | 2.0 g/lit | In pot and field studies, seeds treated with chitosan greatly reduced root rot disease severity of Fusarium solani | [1] |
| 2 | Potato | 4.0 g/lit of acetic acid-distilled water solution | Reduced dry rot severity observed in case of F. oxysporum (60.0%)and F. sambucinum (48.2%) by chitosan treatment. | [42] |
| 3 | Chilli | 1% | 100% mycelium growth inhibition and the lowest (7.67%) post-emergence seedling mortality was observed against Colletotrichum capsici | [38] |
| 5 | Cucumber | 500 ppm | 500 ppm chitosan seed treatment showed 100% disease resistance against damping off caused by Phytophthora capsici | [43] |
| Sl.No | Crop | Effective Dose | Pathogen | Activity | Reference |
|---|---|---|---|---|---|
| 1 | Tomato | 0.5 g/lit | Rhizoctonia solani | 58.8% disease reduction in Pre-emergence damping off after 10 days | [40] |
| 2 | Cucumber | 0.05–0.1% | Colletotrichum sp. | Disease control and reduction of lesion than the untreated one | [49] |
| 3 | Tea | 0.01% | Exobasidium vexans | 67.73% less disease incidence than control | [50] |
| 4 | Turmeric | 0.1% | Pythium aphanidermatum | Reduced disease severity and increased chitinase activity. | [51] |
| 5 | Grape | 0.8% | Plasmopara vitccola | 81% disease reduction | [52] |
| Sl.No | Crop | Effective Dose | Pathogen | Activity | Reference |
|---|---|---|---|---|---|
| 1 | Pomegranate | 0.1–10 g/lit of chitosan | Botrytis sp., Penicillium sp. and Pilidiella granati | Reduced rot incidence by 18–66% | [53] |
| 2 | Jujube fruit. | 20 mg/ml | Penicillium expansum | More than 80% inhibition of incidence one day after treatment. | [54] |
| 3 | Kiwi fruit | 5 gm/lit | Gray mold (B. cinerea) and blue mold (P. expansum) | Disease Incidence was 46% (gray mold) and 65% (blue mold) comparing to untreated control. | [55] |
| 4 | Peach | Chitosan and oligochitosan 5 g/lit | Brown rot (Monilinia fruiticola) | Disease incidence drastically reduced and in both the cases only 20% DI occurred. | [56] |
| 5 | Rose Apple | 2% | Penecillium expansum | Disease incidence was only 14% which was 24% less than control | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debnath, D.; Samal, I.; Mohapatra, C.; Routray, S.; Kesawat, M.S.; Labanya, R. Chitosan: An Autocidal Molecule of Plant Pathogenic Fungus. Life 2022, 12, 1908. https://doi.org/10.3390/life12111908
Debnath D, Samal I, Mohapatra C, Routray S, Kesawat MS, Labanya R. Chitosan: An Autocidal Molecule of Plant Pathogenic Fungus. Life. 2022; 12(11):1908. https://doi.org/10.3390/life12111908
Chicago/Turabian StyleDebnath, Debanjana, Ipsita Samal, Chinmayee Mohapatra, Snehasish Routray, Mahipal Singh Kesawat, and Rini Labanya. 2022. "Chitosan: An Autocidal Molecule of Plant Pathogenic Fungus" Life 12, no. 11: 1908. https://doi.org/10.3390/life12111908
APA StyleDebnath, D., Samal, I., Mohapatra, C., Routray, S., Kesawat, M. S., & Labanya, R. (2022). Chitosan: An Autocidal Molecule of Plant Pathogenic Fungus. Life, 12(11), 1908. https://doi.org/10.3390/life12111908








