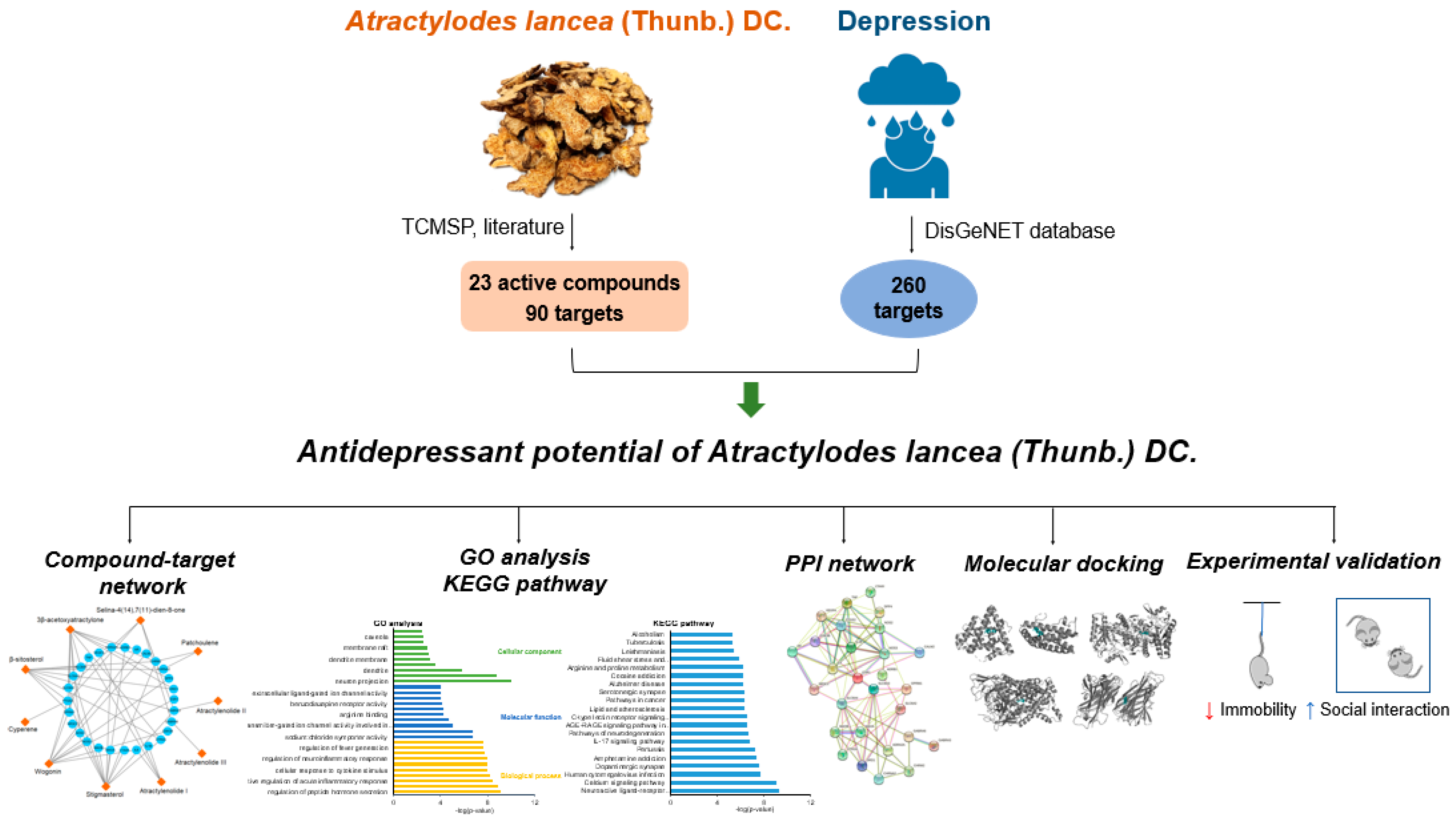
Network Pharmacology and Experimental Validation to Investigate the Antidepressant Potential of Atractylodes lancea (Thunb.) DC.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening for Active Components of Atractylodes lancea (Thunb.) DC. (AL)
2.2. Screening for Antidepressant Targets of AL
2.3. Compound-Target Network Construction
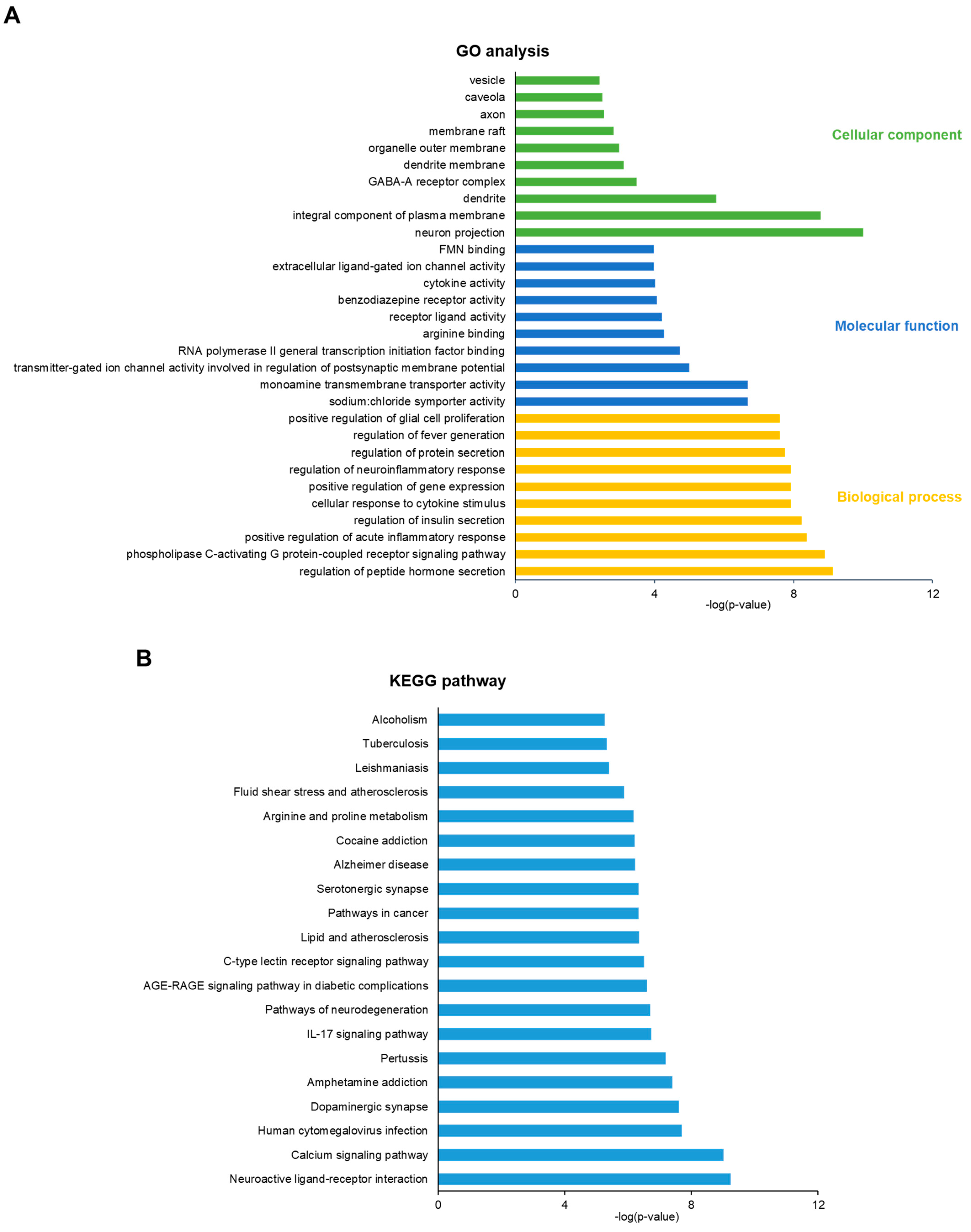
2.4. Gene Ontology (GO) and Kyoto Encyclopedia Genes and Genomes (KEGG) Analysis
2.5. Protein–Protein Interaction (PPI) Network Construction
2.6. Molecular Docking Analysis
2.7. Preparation of AL Extract
2.8. Animal Experiment
2.9. Tail Suspension Test (TST)
2.10. Social Interaction Test
2.11. Open Field Test (OFT)
2.12. Statistical Analysis
3. Results
3.1. Screening for Active Components of AL
3.2. Potential Antidepressant Targets of AL and Network Analysis
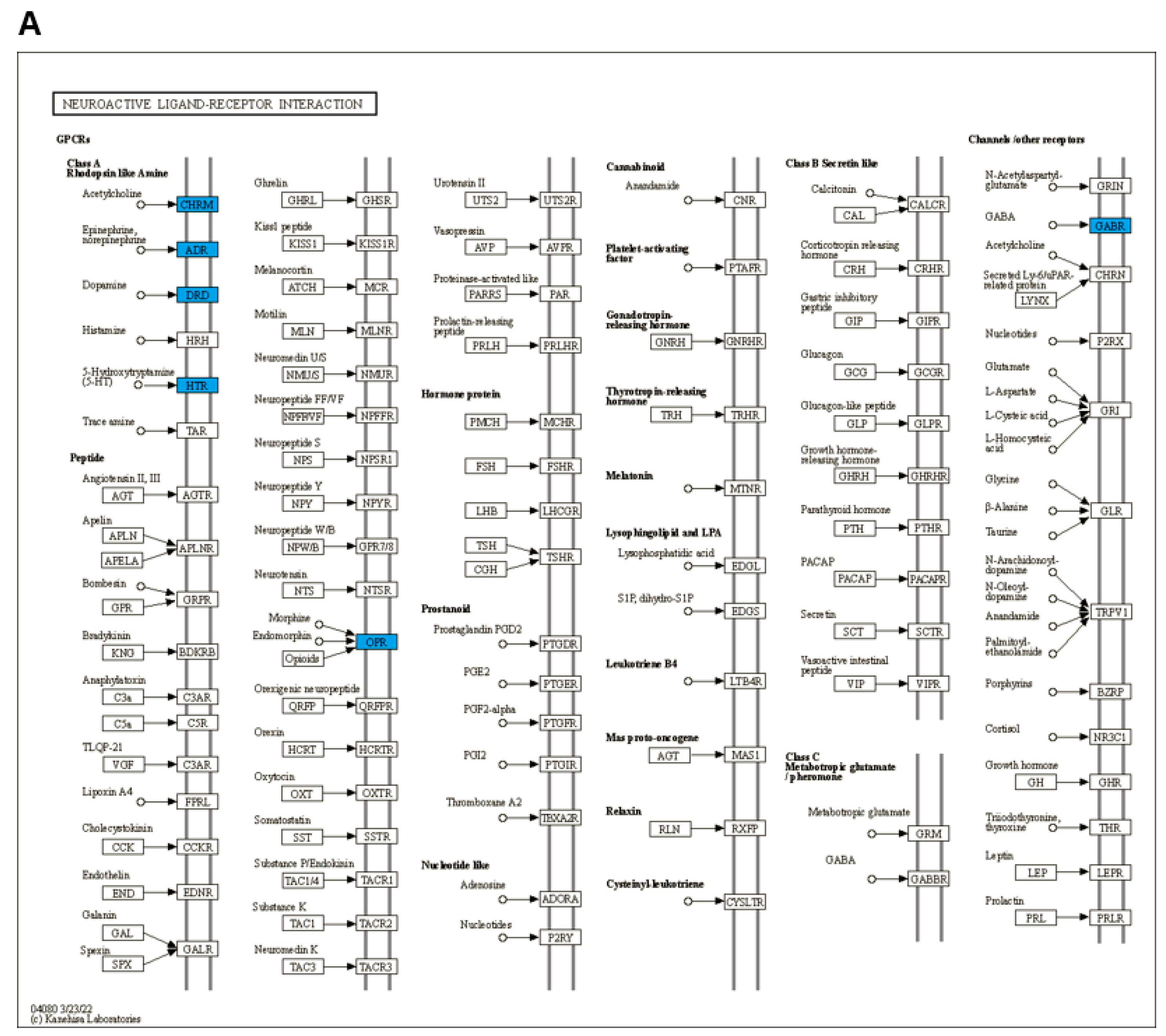
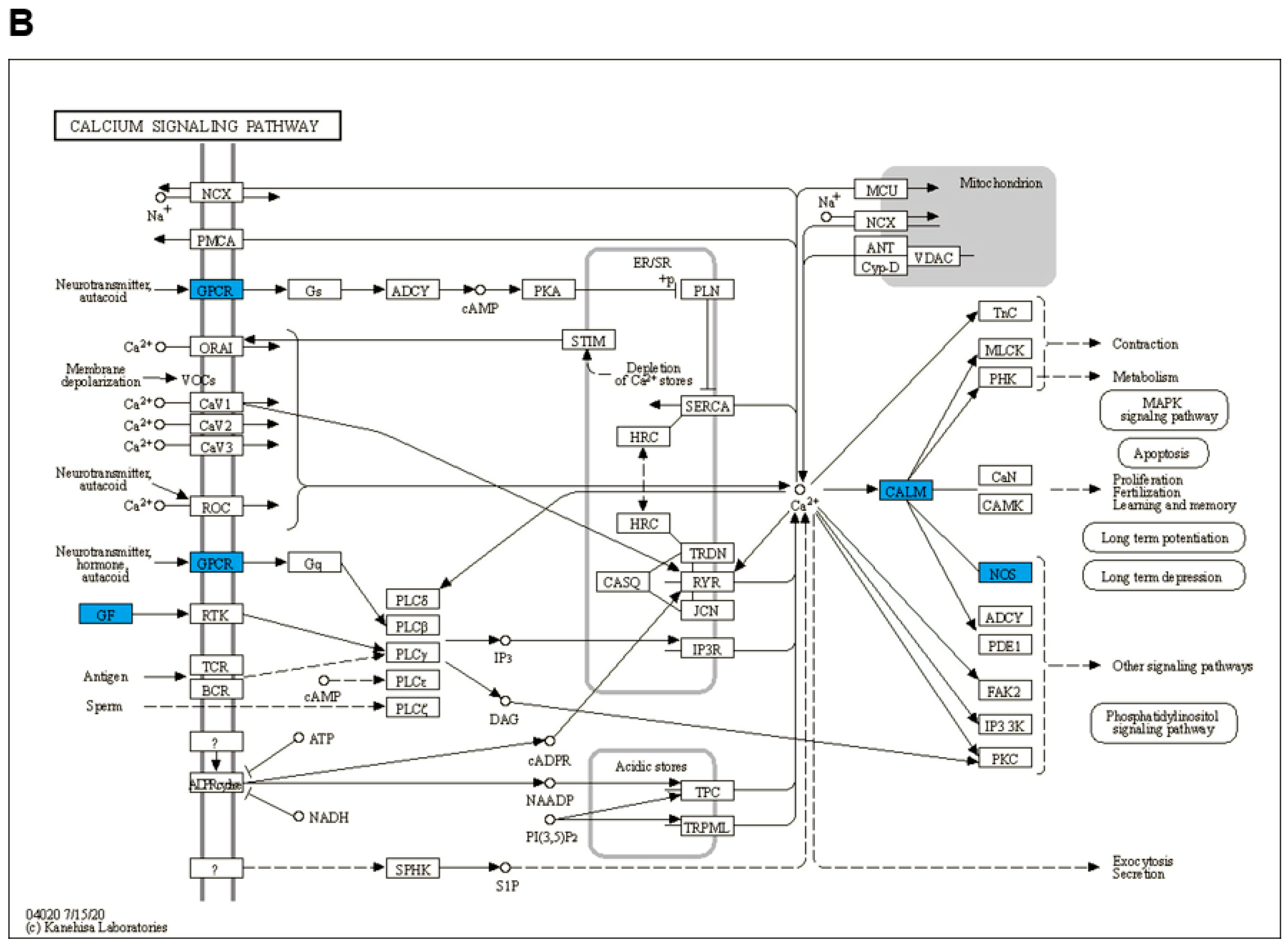
3.3. GO and KEGG Enrichment Analysis
3.4. PPI Network Construction
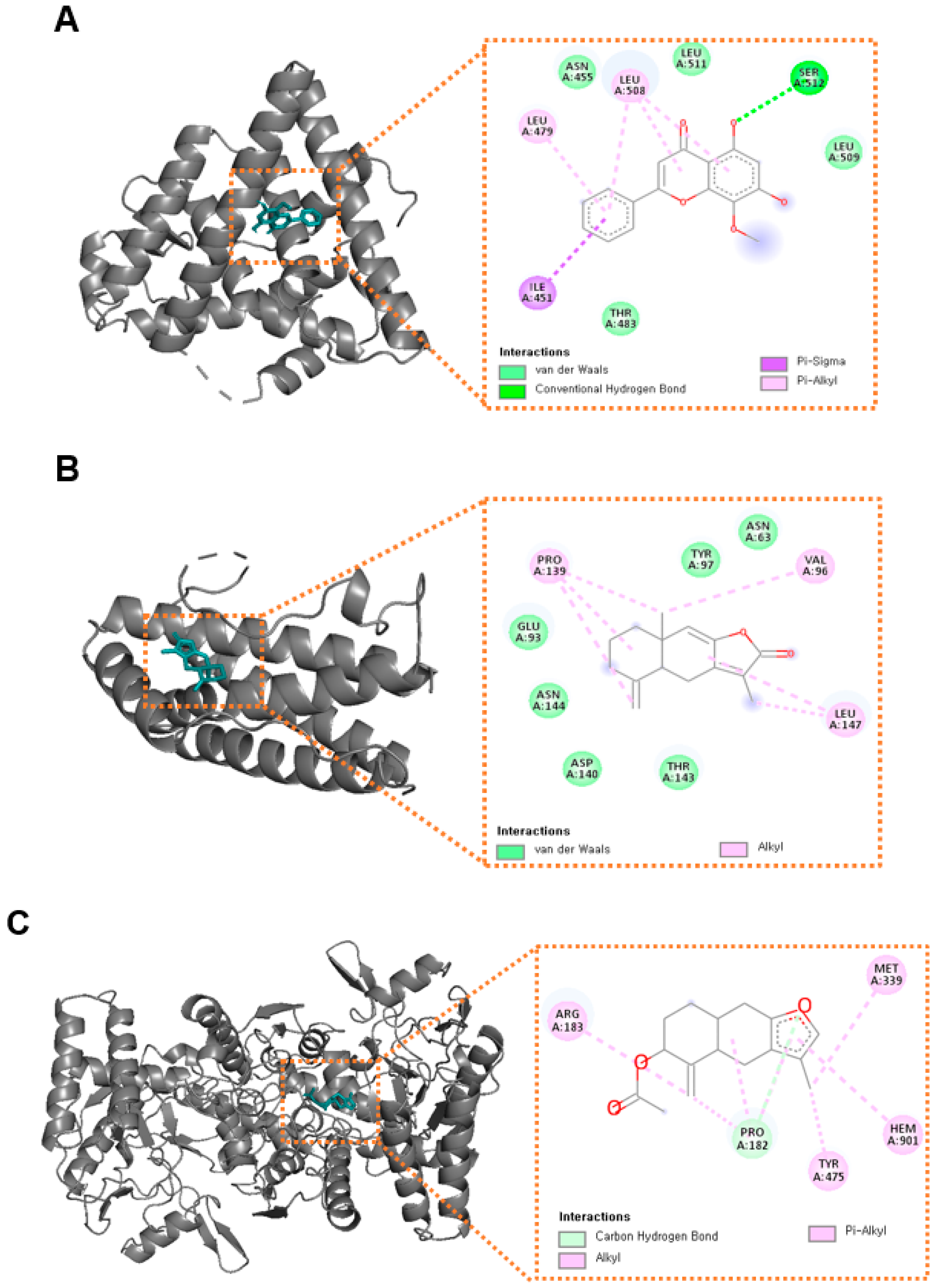
3.5. Molecular Docking Analysis
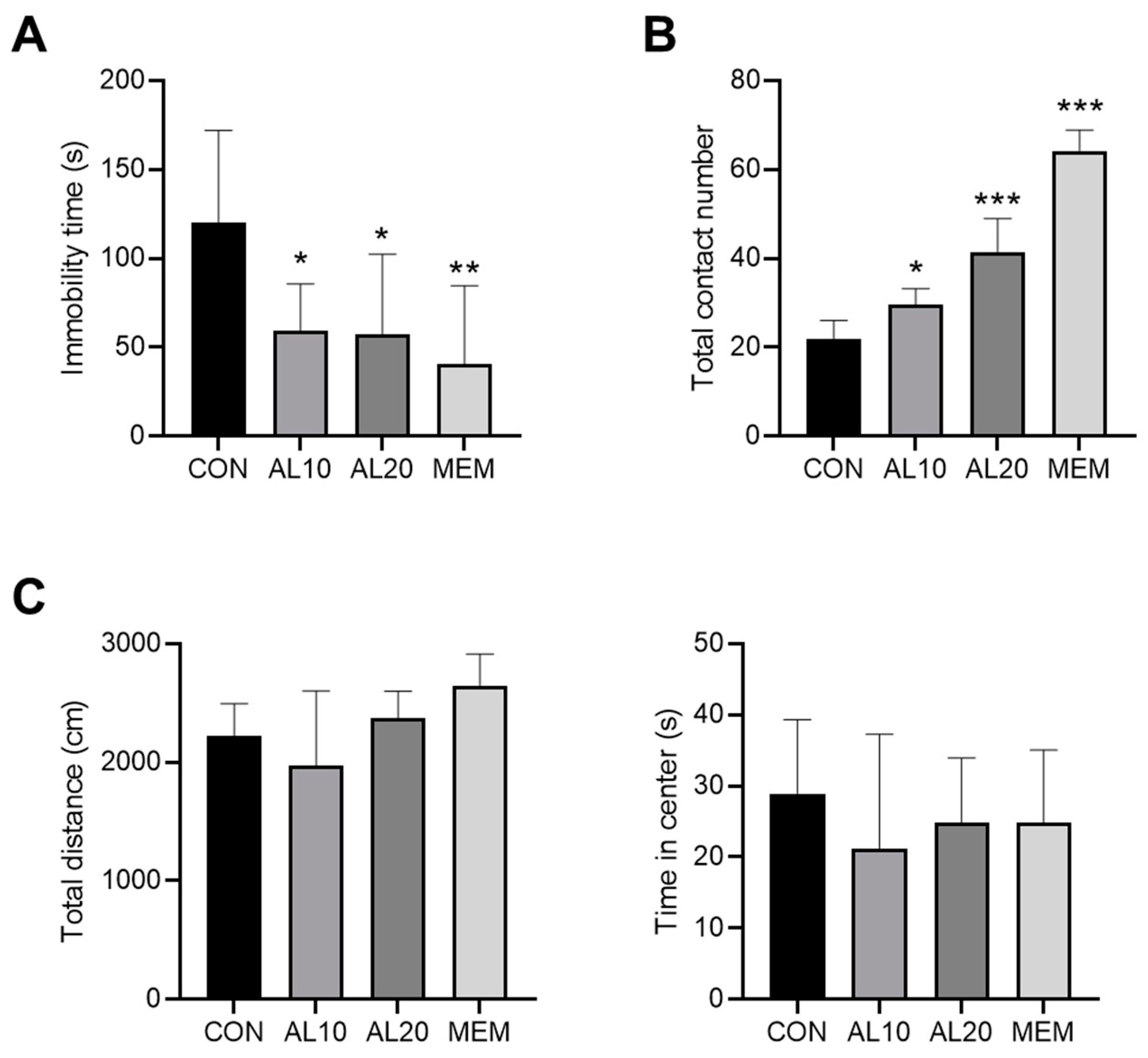
3.6. Validation of the Antidepressant Effect of AL in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Kang, H.J.; Kim, S.Y.; Bae, K.Y.; Kim, S.W.; Shin, I.S.; Yoon, J.S.; Kim, J.M. Comorbidity of depression with physical disorders: Research and clinical implications. Chonnam Med. J. 2015, 51, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voinov, B.; Richie, W.D.; Bailey, R.K. Depression and chronic diseases: It is time for a synergistic mental health and primary care approach. Prim. Care Companion CNS Disord. 2013, 15, 26226. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.S.; Goodwin, R.D.; Stinson, F.S.; Grant, B.F. Epidemiology of major depressive disorder: Results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch. Gen. Psychiatry 2005, 62, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Thaipisuttikul, P.; Ittasakul, P.; Waleeprakhon, P.; Wisajun, P.; Jullagate, S. Psychiatric comorbidities in patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2014, 10, 2097–2103. [Google Scholar]
- Steffen, A.; Nübel, J.; Jacobi, F.; Bätzing, J.; Holstiege, J. Mental and somatic comorbidity of depression: A comprehensive cross-sectional analysis of 202 diagnosis groups using German nationwide ambulatory claims data. BMC Psychiatry 2020, 20, 142. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults With Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Focus 2018, 16, 420–429. [Google Scholar] [CrossRef]
- Bet, P.M.; Hugtenburg, J.G.; Penninx, B.W.J.H.; Hoogendijk, W.J.G. Side effects of antidepressants during long-term use in a naturalistic setting. Eur. Neuropsychopharmacol. 2013, 23, 1443–1451. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shi, Y.H.; Xu, Z.; Fu, H.; Zeng, H.; Zheng, G.Q. Efficacy and safety of Chinese herbal medicine for depression: A systematic review and meta-analysis of randomized controlled trials. J. Psychiatr. Res. 2019, 117, 74–91. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Qu, H.; Gao, X.; Cheng, Y. Strategies and Techniques for Multi-Component Drug Design from Medicinal Herbs and Traditional Chinese Medicine. Curr. Top. Med. Chem. 2012, 12, 1356–1362. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Q.; Shen, M.; Lin, H.; Feng, J.; Peng, L.; Huang, M.; Zhan, X.; Chen, Z.; Ma, T. Identification of the Ingredients and Mechanisms of Curcumae Radix for Depression Based on Network Pharmacology and Molecular Docking. Nat. Prod. Commun. 2021, 16, 1934578X211016643. [Google Scholar] [CrossRef]
- Koonrungsesomboon, N.; Na-Bangchang, K.; Karbwang, J. Therapeutic potential and pharmacological activities of Atractylodes lancea (Thunb.) DC. Asian Pac. J. Trop. Med. 2014, 7, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Hao, W.; Wu, J.; Yuan, N.; Gong, L.; Huang, J.; Ma, Q.; Zhu, H.; Gan, H.; Da, X.; Deng, L.; et al. Xiaoyaosan Improves Antibiotic-Induced Depressive-Like and Anxiety-Like Behavior in Mice Through Modulating the Gut Microbiota and Regulating the NLRP3 Inflammasome in the Colon. Front. Pharmacol. 2021, 12, 619103. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.H.; Cheng, X.M.; Shen, J.S.; Wang, Z.T. Antidepressant effect of Yueju-Wan ethanol extract and its fractions in mice models of despair. J. Ethnopharmacol. 2008, 117, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhu, X.; Xi, Y.; Li, Q.; Shen, Z.; Yang, Y. Anti-depressant-like effect of atractylenolide I in a mouse model of depression induced by chronic unpredictable mild stress. Exp. Ther. Med. 2018, 15, 1574–1579. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Liu, X.; Liu, J.; Cai, E.; Zhao, Y.; Li, H.; Zhang, L.; Li, P.; Gao, Y. The effect of beta-sitosterol and its derivatives on depression by the modification of 5-HT, DA and GABA-ergic systems in mice. RSC Adv. 2018, 8, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminformatics 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [Green Version]
- Syafrizayanti; Betzen, C.; Hoheisel, J.D.; Kastelic, D. Methods for analyzing and quantifying protein–protein interaction. Expert Rev. Proteomics 2014, 11, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Timalsina, P.; Charles, K.; Mondal, A.M. STRING PPI score to characterize protein subnetwork biomarkers for human diseases and pathways. In Proceedings of the 2014 IEEE International Conference on Bioinformatics and Bioengineering, Boca Raton, FL, USA, 10–12 November 2014. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Di Costanzo, L.; Christie, C.; Dalenberg, K.; Duarte, J.M.; Dutta, S. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019, 47, D464–D474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Sharma, A.; Gupta, U. Molecular Docking studies on the Anti-fungal activity of Allium sativum (Garlic) against Mucormycosis (black fungus) by BIOVIA discovery studio visualizer 21.1. 0.0. Ann. Antivir. Antiretrovir. 2021, 5, 028–032. [Google Scholar]
- Almeida, R.C.; Souza, D.G.; Soletti, R.C.; López, M.G.; Rodrigues, A.L.S.; Gabilan, N.H. Involvement of PKA, MAPK/ERK and CaMKII, but not PKC in the acute antidepressant-like effect of memantine in mice. Neurosci. Lett. 2006, 395, 93–97. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef] [Green Version]
- Linck, V.M.; da Silva, A.L.; Figueiro, M.; Caramao, E.B.; Moreno, P.R.; Elisabetsky, E. Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef]
- Liu, Y.; Grimm, M.; Dai, W.-t.; Hou, M.-c.; Xiao, Z.-X.; Cao, Y. CB-Dock: A web server for cavity detection-guided protein–ligand blind docking. Acta Pharmacol. Sin. 2020, 41, 138–144. [Google Scholar] [CrossRef]
- Bosc, M. Assessment of social functioning in depression. Compr. Psychiatry 2000, 41, 63–69. [Google Scholar] [CrossRef]
- Norman, G.J.; Karelina, K.; Morris, J.S.; Zhang, N.; Cochran, M.; DeVries, A.C. Social interaction prevents the development of depressive-like behavior post nerve injury in mice: A potential role for oxytocin. Psychosom. Med. 2010, 72, 519–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022, 42, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bi, L.; Xia, K.; Gao, K.; Chen, J.; Guo, S.; Wang, T.; Ma, X.; Wang, W.; Zhao, H.; et al. Biological basis of “depression with liver-qi stagnation and spleen deficiency syndrome”: A digital gene expression profiling study. J. Tradit. Chin. Med. Sci. 2015, 2, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Szmelskyj, I.; Aquilina, L.; Szmelskyj, A.O. Chapter 5—Investigations from a TCM perspective. In Acupuncture for IVF and Assisted Reproduction; Szmelskyj, I., Aquilina, L., Szmelskyj, A.O., Eds.; Churchill Livingstone: London, UK, 2015; pp. 97–142. [Google Scholar]
- Ghosh, S.; Kumar, A.; Sachan, N.; Chandra, P. Evaluation of the Antidepressant-like Effect of Total Sterols Fraction and Stigmasterol Isolated from Leaves of Aegle marmelos and Possible Mechanism(s) of Action Involved. Curr. Drug Discov. Technol. 2022, 19, e290721195144. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, S.; Wu, F.; Zheng, Q.; Zhang, F.; Luo, Y.; Jian, X. Atractylenolide III reduces depressive- and anxiogenic-like behaviors in rat depression models. Neurosci. Lett. 2021, 759, 136050. [Google Scholar] [CrossRef]
- Morales-Medina, J.C.; Witchey, S.K.; Caldwell, H.K. The Role of Vasopressin in Anxiety and Depression. In Melatonin, Neuroprotective Agents and Antidepressant Therapy; López-Muñoz, F., Srinivasan, V., de Berardis, D., Álamo, C., Kato, T.A., Eds.; Springer: New Delhi, India, 2016; pp. 667–685. [Google Scholar]
- Choi, K.W.; Na, E.J.; Fava, M.; Mischoulon, D.; Cho, H.; Jeon, H.J. Increased adrenocorticotropic hormone (ACTH) levels predict severity of depression after six months of follow-up in outpatients with major depressive disorder. Psychiatry Res. 2018, 270, 246–252. [Google Scholar] [CrossRef]
- Slattery, D.A.; Neumann, I.D. Oxytocin and Major Depressive Disorder: Experimental and Clinical Evidence for Links to Aetiology and Possible Treatment. Pharmaceuticals 2010, 3, 702–724. [Google Scholar] [CrossRef] [Green Version]
- Senese, N.B.; Rasenick, M.M.; Traynor, J.R. The Role of G-proteins and G-protein Regulating Proteins in Depressive Disorders. Front. Pharmacol. 2018, 9, 1289. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Giuliani, F. The Role of Inflammation in Depression and Fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Melikian, H.E.; Rye, D.B.; Levey, A.I.; Blakely, R.D. Identification and characterization of antidepressant-sensitive serotonin transporter proteins using site-specific antibodies. J. Neurosci. 1995, 15, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Bowman, M.A.; Daws, L.C. Targeting Serotonin Transporters in the Treatment of Juvenile and Adolescent Depression. Front. Neurosci. 2019, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Imbrici, P.; Camerino, D.C.; Tricarico, D. Major channels involved in neuropsychiatric disorders and therapeutic perspectives. Front. Genet. 2013, 4, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adkins, D.E.; Khachane, A.N.; McClay, J.L.; Aberg, K.; Bukszar, J.; Sullivan, P.F.; van den Oord, E.J.C.G. SNP-based analysis of neuroactive ligand-receptor interaction pathways implicates PGE2 as a novel mediator of antipsychotic treatment response: Data from the CATIE study. Schizophr. Res. 2012, 135, 200–201. [Google Scholar] [CrossRef] [Green Version]
- Paul, I.A. Antidepressant activity and calcium signaling cascades. Hum. Psychopharmacol. 2001, 16, 71–80. [Google Scholar] [CrossRef]
- Catena-Dell’Osso, M.; Bellantuono, C.; Consoli, G.; Baroni, S.; Rotella, F.; Marazziti, D. Inflammatory and neurodegenerative pathways in depression: A new avenue for antidepressant development? Curr. Med. Chem. 2011, 18, 245–255. [Google Scholar] [CrossRef]
- Waisman, A.; Hauptmann, J.; Regen, T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015, 129, 625–637. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Fan, N.; Luo, Y.; Ou, Y.; He, H. Altered serum levels of TNF-alpha, IL-6, and IL-18 in depressive disorder patients. Hum. Psychopharmacol. 2017, 32, e2588. [Google Scholar] [CrossRef]
- Garcia-Garcia, M.L.; Tovilla-Zarate, C.A.; Villar-Soto, M.; Juarez-Rojop, I.E.; Gonzalez-Castro, T.B.; Genis-Mendoza, A.D.; Ramos-Mendez, M.A.; Lopez-Narvaez, M.L.; Saucedo-Osti, A.S.; Ruiz-Quinones, J.A.; et al. Fluoxetine modulates the pro-inflammatory process of IL-6, IL-1beta and TNF-alpha levels in individuals with depression: A systematic review and meta-analysis. Psychiatry Res. 2022, 307, 114317. [Google Scholar] [CrossRef]
- Dhir, A.; Kulkarni, S.K. Nitric oxide and major depression. Nitric Oxide 2011, 24, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Pezawas, L.; Meyer-Lindenberg, A.; Goldman, A.L.; Verchninski, B.A.; Chen, G.; Kolachana, B.S.; Egan, M.F.; Mattay, V.S.; Hariri, A.R.; Weinberger, D.R. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Mol. Psychiatry 2008, 13, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Pinsonneault, J.K.; Sullivan, D.; Sadee, W.; Soares, C.N.; Hampson, E.; Steiner, M. Association study of the estrogen receptor gene ESR1 with postpartum depression—A pilot study. Arch. Womens Ment. Health 2013, 16, 499–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shringarpure, M.; Gharat, S.; Momin, M.; Omri, A. Management of epileptic disorders using nanotechnology-based strategies for nose-to-brain drug delivery. Expert Opin. Drug Deliv. 2021, 18, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Jun, X.; Fu, P.; Lei, Y.; Cheng, P. Pharmacological effects of medicinal components of Atractylodes lancea (Thunb.) DC. Chin. Med. 2018, 13, 59. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Mai, J.Y.; Hou, T.L.; Ping, J.; Chen, J.J. Antiviral activities of atractylon from Atractylodis Rhizoma. Mol. Med. Rep. 2016, 14, 3704–3710. [Google Scholar] [CrossRef]



| Mol ID | Molecule Name | MW | OB (%) | Caco-2 | BBB | DL |
|---|---|---|---|---|---|---|
| MOL000086 | (24S)-5β-stigmastan-3β-ol | 416.81 | 25.32 | 1.41 | 1.18 | 0.75 |
| MOL000188 | 3β-acetoxyatractylone | 274.39 | 40.57 | 1.22 | 1.04 | 0.22 |
| MOL000167 | 3β-hydroxyatractylone | 232.35 | 21.17 | 1.18 | 0.97 | 0.15 |
| MOL000189 | Acetyl atractylodinol | 240.27 | 25.47 | 1.42 | 0.69 | 0.13 |
| MOL000043 | Atractylenolide I | 230.33 | 37.37 | 1.3 | 1.29 | 0.15 |
| MOL000044 | Atractylenolide II | 232.35 | 47.5 | 1.3 | 1.37 | 0.15 |
| MOL000178 | Atractylenolide III | 248.35 | 31.66 | 0.75 | 0.64 | 0.17 |
| MOL000164 | Atractylone | 216.35 | 33.91 | 1.74 | 1.83 | 0.13 |
| MOL000187 | Butenolide B | 234.32 | 61 | 0.65 | 0.45 | 0.15 |
| MOL000175 | Cyperene | 204.39 | 51.1 | 1.81 | 2.13 | 0.11 |
| MOL000092 | Daucosterin_qt | 414.79 | 36.91 | 1.42 | 1.15 | 0.76 |
| MOL000094 | Daucosterol_qt | 414.79 | 36.91 | 1.3 | 0.87 | 0.76 |
| MOL000194 | Patchoulene | 204.39 | 51.71 | 1.8 | 2.21 | 0.11 |
| MOL000060 | Selina-4(14),7(11)-dien-8-one | 218.37 | 32.31 | 1.42 | 1.57 | 0.1 |
| MOL000184 | Stigmastenone | 412.77 | 39.25 | 1.42 | 1.22 | 0.76 |
| MOL000449 | Stigmasterol | 412.77 | 43.83 | 1.44 | 1 | 0.76 |
| MOL000186 | Stigmasterol-3-O-β-D-glucopyranoside_qt | 412.77 | 43.83 | 1.31 | 0.9 | 0.76 |
| MOL000173 | Wogonin | 284.28 | 30.68 | 0.79 | 0.04 | 0.23 |
| MOL000085 | β-daucosterol_qt | 414.79 | 36.91 | 1.3 | 0.88 | 0.75 |
| MOL000032 | β-eudesmol | 222.41 | 26.09 | 1.32 | 1.38 | 0.1 |
| MOL000358 | β-sitosterol | 414.79 | 36.91 | 1.32 | 0.99 | 0.75 |
| MOL000088 | β-sitosterol 3-O-glucoside_qt | 414.79 | 36.91 | 1.3 | 0.91 | 0.75 |
| MOL000095 | Δ-7-stigmastenol | 416.81 | 25.32 | 1.31 | 0.98 | 0.75 |
| No. | Gene | Uniprot | Protein |
|---|---|---|---|
| 1 | ADRA2A | P08913 | Alpha-2A adrenergic receptor |
| 2 | ADRB1 | P08588 | Beta-1 adrenergic receptor |
| 3 | AR | P10275 | Androgen receptor |
| 4 | CALM2 | P0DP23 | Calmodulin 2 |
| 5 | CHRM2 | P08172 | Muscarinic acetylcholine receptor M2 |
| 6 | CHRNA2 | Q15822 | Neuronal acetylcholine receptor subunit alpha-2 |
| 7 | DPP4 | P27487 | Dipeptidyl peptidase IV |
| 8 | DRD1 | P21728 | Dopamine D1 receptor |
| 9 | ESR1 | P03372 | Estrogen receptor |
| 10 | GABRA3 | P34903 | Gamma-aminobutyric-acid receptor alpha-3 subunit |
| 11 | GABRA6 | Q16445 | Gamma-aminobutyric-acid receptor alpha-6 subunit |
| 12 | GSK3B | P49841 | Glycogen synthase kinase-3 beta |
| 13 | HTR2A | P28223 | 5-hydroxytryptamine 2A receptor |
| 14 | IL1B | P01584 | Interleukin-1 beta |
| 15 | IL6 | P05231 | Interleukin-6 |
| 16 | LTA4H | P09960 | Leukotriene A-4 hydrolase |
| 17 | MAOA | P21397 | Amine oxidase [flavin-containing] A |
| 18 | MAOB | P27338 | Amine oxidase [flavin-containing] B |
| 19 | NOS2 | P35228 | Nitric oxide synthase, inducible |
| 20 | NOS3 | P29474 | Nitric-oxide synthase, endothelial |
| 21 | NR3C2 | P08235 | Mineralocorticoid receptor |
| 22 | OPRM1 | P35372 | Mu-type opioid receptor |
| 23 | PTGS2 | P35354 | Prostaglandin G/H synthase 2 |
| 24 | SLC6A2 | P23975 | Sodium-dependent noradrenaline transporter |
| 25 | SLC6A3 | Q01959 | Sodium-dependent dopamine transporter |
| 26 | SLC6A4 | P31645 | Sodium-dependent serotonin transporter |
| 27 | TNF | P01375 | Tumor necrosis factor |
| 28 | VEGFA | P15692 | Vascular endothelial growth factor A |
| Compound | Closeness Centrality | Betweenness Centrality | Degree |
|---|---|---|---|
| Stigmasterol | 0.357262341 | 0.430232558 | 12 |
| 3β-acetoxyatractylone | 0.256199505 | 0.411111111 | 11 |
| Wogonin | 0.429541937 | 0.411111111 | 9 |
| β-sitosterol | 0.138956867 | 0.393617021 | 8 |
| Selina-4(14),7(11)-dien-8-one | 0.134796286 | 0.37755102 | 5 |
| Atractylenolide I | 0.107357357 | 0.246666667 | 4 |
| Patchoulene | 0.005315137 | 0.284615385 | 2 |
| Cyperene | 0 | 0.330357143 | 1 |
| Atractylenolide III | 0 | 0.220238095 | 1 |
| Atractylenolide II | 0 | 0.264285714 | 1 |
| Docking Score (kcal/mol) | |||||
|---|---|---|---|---|---|
| Compound | ESR1 | IL6 | NOS3 | SLC6A4 | TNF |
| 3β-acetoxyatractylone | - | - | −7.8 | −7.2 | - |
| Atractylenolide I | - | −6.6 | - | - | −7.7 |
| Wogonin | −6.7 | −6.3 | - | - | −6.4 |
| β-sitosterol | - | - | - | −6.8 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, L.T.H.; Nguyen, N.P.K.; Tran, K.N.; Shin, H.-M.; Yang, I.-J. Network Pharmacology and Experimental Validation to Investigate the Antidepressant Potential of Atractylodes lancea (Thunb.) DC. Life 2022, 12, 1925. https://doi.org/10.3390/life12111925
Nguyen LTH, Nguyen NPK, Tran KN, Shin H-M, Yang I-J. Network Pharmacology and Experimental Validation to Investigate the Antidepressant Potential of Atractylodes lancea (Thunb.) DC. Life. 2022; 12(11):1925. https://doi.org/10.3390/life12111925
Chicago/Turabian StyleNguyen, Ly Thi Huong, Nhi Phuc Khanh Nguyen, Khoa Nguyen Tran, Heung-Mook Shin, and In-Jun Yang. 2022. "Network Pharmacology and Experimental Validation to Investigate the Antidepressant Potential of Atractylodes lancea (Thunb.) DC." Life 12, no. 11: 1925. https://doi.org/10.3390/life12111925
APA StyleNguyen, L. T. H., Nguyen, N. P. K., Tran, K. N., Shin, H.-M., & Yang, I.-J. (2022). Network Pharmacology and Experimental Validation to Investigate the Antidepressant Potential of Atractylodes lancea (Thunb.) DC. Life, 12(11), 1925. https://doi.org/10.3390/life12111925








