Immunomodulatory Effect of Lactobacillus reuteri (Limosilactobacillus reuteri) and Its Exopolysaccharides Investigated on Epithelial Cell Line IPEC-J2 Challenged with Salmonella Typhimurium
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Extraction and Purification of EPS
2.3. IPEC-J2 Cell Culture
2.4. Experimental Design—Exposure of IPEC-J2 Cells to the Different Treatments
2.4.1. Exposure of IPEC-J2 Cells to S. Typhimurium (ST), L. reuteri L26 (L26), or EPS
2.4.2. Exposure of IPEC-J2 Cells to L. reuteri L26 (L26) or EPS isolated from L. reuteri L26 (EPS) Followed by Challenge with S. Typhimurium
2.5. RNA Extraction and cDNA Synthesis
2.6. Gene Expression Analysis (qPCR)
2.7. Dot Blot Analysis
2.8. Statistical Analysis
3. Results
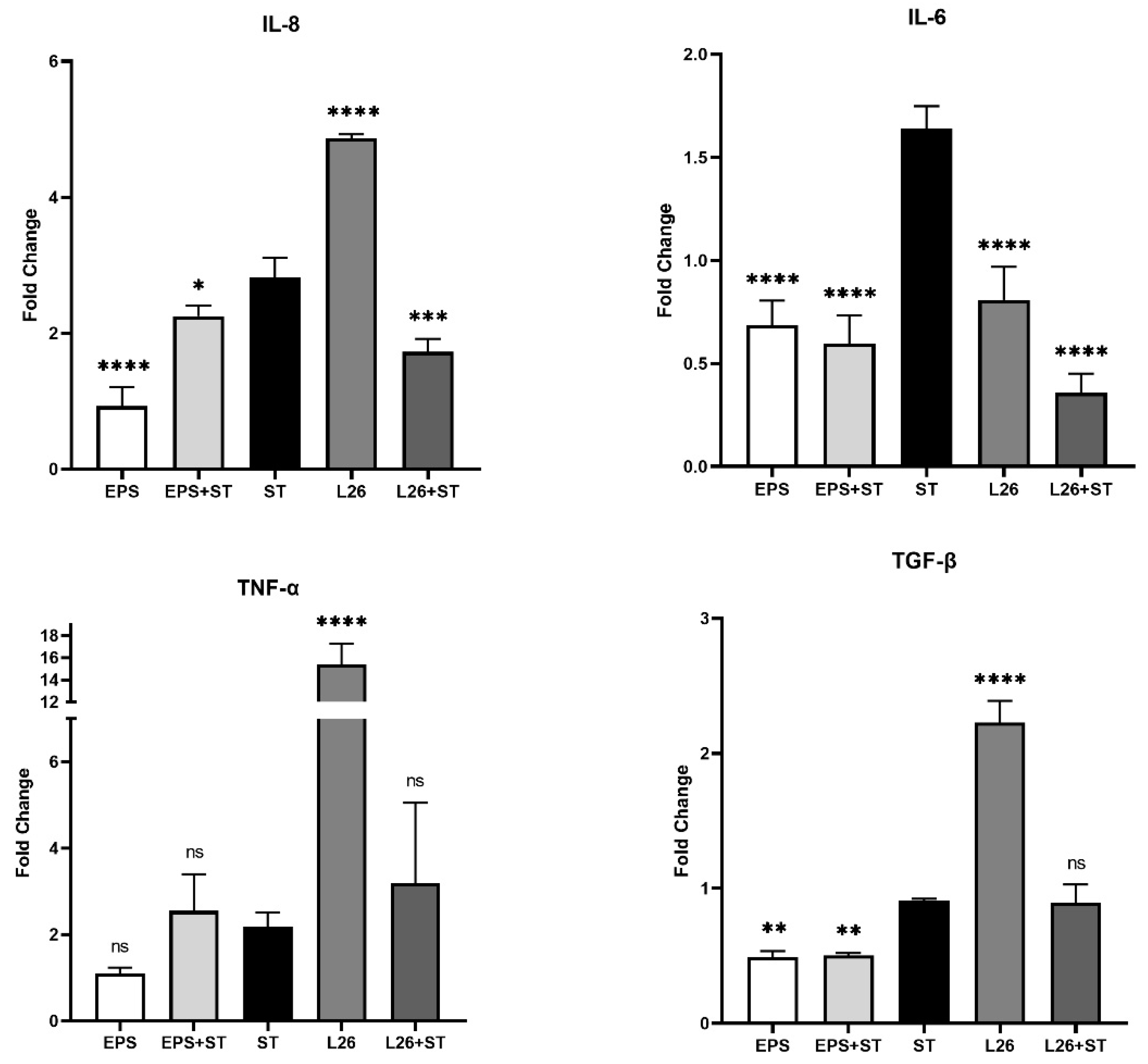
3.1. The Effect of EPS Isolated from L. reuteri L26 (EPS) and L. reuteri L26 (L26) on the Expression of Genes Encoding Pro-Inflammatory Cytokines in IPEC-J2 Cells Challenged with S. Typhimurium (ST)
3.2. The Effect of EPS Isolated from L. reuteri L26 (EPS) and L. reuteri L26 (L26) on Expression of Genes Related to TLR Cascade in IPEC-J2 Cells Challenged with S. Typhimurium (ST)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llewellyn, A.; Foey, A. Probiotic modulation of innate cell pathogen sensing and signaling events. Nutrients 2017, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Badel, S.; Bernardi, T.; Michaud, P. New perspectives for lactobacilli exopolysaccharides. Biotechnol. Adv. 2011, 29, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Genes and molecules of lactobacilli supporting probiotic action. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, M.M.P.; Akkerman, R.; Ferrari, M.; Walvoort, M.T.C.; de Vos, P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J. Funct. Foods 2021, 76, 104289. [Google Scholar] [CrossRef]
- Pérez-Ramos, A.; Nácher-Vázquez, M.; Notararigo, S.; López, P.; Mohedano, M.L. Chapter 22–Current and future applications of bacterial extracellular polysaccharides. In Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion; Warton, R.R., Preedy, R.V., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 329–344. [Google Scholar] [CrossRef]
- Abedfar, A.; Hossininezhad, M. Overview of the most important characterization of exopolysaccharides produced by probiotics bacteria and their biological function. IOSR J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 47–55. [Google Scholar] [CrossRef]
- Zhao, L.; Li, M.; Sun, K.; Su, S.; Geng, T.; Sun, H. Hippophae rhamnoides polysaccharides protect IPEC-J2 cells from LPS-induced inflammation, apoptosis and barrier dysfunction in vitro via inhibiting TLR4/NF-κB signaling pathway. Int. J. Biol. Macromol. 2020, 155, 1202–1215. [Google Scholar] [CrossRef]
- Fukata, M.; Arditi, M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013, 6, 451–463. [Google Scholar] [CrossRef]
- Leite, F.L.; Vasquez, E.; Gebhart, C.J.; Isaacson, R.E. The effects of Lawsonia intracellularis, Salmonella enterica serovar Typhimurium and co-infection on IL-8 and TNF-α expression in IPEC-J2 cells. Vet. Microbiol. 2019, 231, 76–79. [Google Scholar] [CrossRef]
- Kim, H.B.; Isaacson, R.E. Salmonella in swine: Microbiota interactions. Annu. Rev. Anim. Biosci. 2017, 5, 43–63. [Google Scholar] [CrossRef]
- Tkáčiková, Ľ.; Mochnáčová, E.; Tyagi, P.; Kiššová, Z.; Bhide, M. Comprehensive mapping of the cell response to E. coli infection in porcine intestinal epithelial cells pretreated with exopolysaccharide derived from Lactobacillus reuteri. Vet. Res. 2020, 51, 49. [Google Scholar] [CrossRef]
- Kšonžeková, P.; Bystrický, P.; Vlčková, S.; Pätoprstý, V.; Pulzová, L.; Mudroňová, D.; Kubašková, T.; Csank, T.; Tkáčiková, L. Exopolysaccharides of Lactobacillus reuteri: Their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr. Polym. 2016, 141, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Sung, J.; Stacey, G.; Masters, J.R. Detection of mycoplasma in cell cultures. Nat. Protoc. 2010, 5, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Loss, H.; Aschenbach, J.R.; Tedin, K.; Ebner, F.; Lodemann, U. The inflammatory response to enterotoxigenic E. coli and probiotic E. faecium in a coculture model of porcine intestinal epithelial and dendritic cells. Mediators Inflamm. 2018, 2018, 9368295. [Google Scholar] [CrossRef] [PubMed]
- Moue, M.; Tohno, M.; Shimazu, T.; Kido, T.; Aso, H.; Saito, T.; Kitazawa, H. Toll-like receptor 4 and cytokine expression involved in functional immune response in an originally established porcine intestinal epitheliocyte cell line. Biochim. Biophys. Acta–Gen. Subj. 2008, 1780, 134–144. [Google Scholar] [CrossRef]
- Chytilová, M.; Kšonžeková, P.; Solár, P.; Tkáčiková, Ľ. Štúdium imunomodulačného účinku probiotického kmeňa L. plantarum–Biocenol™ LP96 in vitro. In ČSM. 26. Kongres Československé Společnosti Mikrobiologické s Mezinárodní Účastí; Československá spoločnosť mikrobiologická: Brno, Czech Republic, 2008; ISBN 978-80-260-4507-6. [Google Scholar]
- Alvarado, J.; Taylor, P.; Del Castillo, J.R.; Thomas, L.E. Interferon gamma bound to extracellular matrix changes the hyporesponsiveness to LPS in crypt but not villous intestinal epithelial cells. Immunol. Lett. 2005, 99, 109–112. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Hoffmann, M.; Szcesny, S.; Blaut, M.; Haller, D. Innate mechanisms for Bifidobacterium lactis to activate transient pro-inflammatory host responses in intestinal epithelial cells after the colonization of germ-free rats. Immunology 2005, 115, 441–450. [Google Scholar] [CrossRef]
- Arce, C.; Ramírez-Boo, M.; Lucena, C.; Garrido, J.J. Innate immune activation of swine intestinal epithelial cell lines (IPEC-J2 and IPI-2I) in response to LPS from Salmonella typhimurium. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 161–174. [Google Scholar] [CrossRef]
- Yu, S.; Gao, N. Compartmentalizing intestinal epithelial cell toll-like receptors for immune surveillance. Cell. Mol. Life Sci. 2015, 176, 139–148. [Google Scholar] [CrossRef]
- Laiño, J.; Villena, J.; Kanmani, P.; Kitazawa, H. Immunoregulatory effects triggered by lactic acid bacteria exopolysaccharides: New insights into molecular interactions with host cells. Microorganisms 2016, 4, 27. [Google Scholar] [CrossRef]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor pathway establishes commensal gut colonization. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- Wells, J.M.; Rossi, O.; Meijerink, M.; van Baarlen, P. Epithelial crosstalk at the microbiota-mucosal interface. Proc. Natl. Acad. Sci. USA 2011, 108, 4607–4614. [Google Scholar] [CrossRef]
- Li, W.F.; Huang, Y.; Li, Y.L.; Huang, Q.; Cui, Z.W.; Yu, D.Y.; Rajput, I.R.; Hu, C.H. Effect of oral administration of Enterococcus faecium Ef1 on innate immunity of sucking piglets. Pak. Vet. J. 2013, 33, 9–13. Available online: http://www.pvj.com.pk/pdf-files/33_1/9-13.pdf (accessed on 16 January 2013).
- Lee, Y.D.; Hong, Y.F.; Jeon, B.; Jung, B.J.; Chung, D.K.; Kim, H. Differential cytokine regulatory effect of three Lactobacillus strains isolated from fermented foods. J. Microbiol. Biotechnol. 2016, 26, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- de Moreno de Leblanc, A.; Chaves, S.; Carmuega, E.; Weill, R.; Antóine, J.; Perdigón, G. Effect of long-term continuous consumption of fermented milk containing probiotic bacteria on mucosal immunity and the activity of peritoneal macrophages. Immunobiology 2007, 213, 97–108. [Google Scholar] [CrossRef]
- Haller, D.; Bode, C.; Hammes, W.P.; Pfeifer, A.M.A.; Schiffrin, E.J.; Blum, S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 2000, 47, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Y.L.; Huang, Q.; Cui, Z.W.; Yu, D.Y.; Rajput, I.R.; Hu, C.H.; Li, W.F. Effect of orally administered Enterococcus faecium EF1 on intestinal cytokines and chemokines production of suckling piglets. Pak. Vet. J. 2012, 32, 2074–7764. Available online: http://www.pvj.com.pk/pdf-files/32_1/81-84.pdf (accessed on 17 December 2011).
- Zoumpopoulou, G.; Foligne, B.; Christodoulou, K.; Grangette, C.; Pot, B.; Tsakalidou, E. Lactobacillus fermentum ACA-DC 179 displays probiotic potential in vitro and protects against trinitrobenzene sulfonic acid (TNBS)-induced colitis and Salmonella infection in murine models. Int. J. Food Microbiol. 2008, 121, 18–26. [Google Scholar] [CrossRef]
- Rocha-Ramírez, L.M.; Pérez-Solano, R.A.; Castañón-Alonso, S.L.; Moreno Guerrero, S.S.; Ramírez Pacheco, A.; García Garibay, M.; Eslava, C. Probiotic Lactobacillus strains stimulate the inflammatory response and activate human macrophages. J. Immunol. Res. 2017, 2017, 4607491. [Google Scholar] [CrossRef]
- Skjolaas, K.A.; Burkey, T.E.; Dritz, S.S.; Minton, J.E. Effects of Salmonella enterica serovar Typhimurium, or serovar Choleraesuis, Lactobacillus reuteri and Bacillus licheniformis on chemokine and cytokine expression in the swine jejunal epithelial cell line, IPEC-J2. Vet. Immunol. Immunopathol. 2007, 115, 299–308. [Google Scholar] [CrossRef]
- Kanmani, P.; Kim, H. Beneficial effect of immunobiotic strains on attenuation of Salmonella induced inflammatory response in human intestinal epithelial cells. PLoS ONE 2020, 15, e0229647. [Google Scholar] [CrossRef]
- Karimi, S.; Jonsson, H.; Lundh, T.; Roos, S. Lactobacillus reuteri strains protect epithelial barrier integrity of IPEC-J2 monolayers from the detrimental effect of enterotoxigenic Escherichia coli. Physiol. Rep. 2018, 6, e13514. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Pallone, F.; MacDonald, T.T. Smad7 in TGF-β-mediated negative regulation of gut inflammation. Trends Immunol. 2004, 25, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.F.; Lin, I.C.; Liu, P.F.; Cheng, M.F.; Liu, Y.C.; Hsieh, Y.D.; Chen, J.J.; Chen, C.L.; Chang, H.W.; Shu, C.W. Lactobacillus acidophilus attenuates Salmonella-induced intestinal inflammation via TGF-β signaling. BMC Microbiol. 2015, 15, 203. [Google Scholar] [CrossRef] [PubMed]



| Type of Cell Treatment | Experimental Design | Abbreviation |
|---|---|---|
| Control | IPEC-J2 cells were without any treatment. | _ |
| ST infection | IPEC-J2 cells were challenged with Salmonella infection for 1 h. After this incubation period, for the purpose of killing the remaining extracellular bacteria gentamicin was added, and the cells were further incubated for the next 3 h in the presence of 100 ug/mL of gentamicin. | ST |
| EPS/L26 treatment | IPEC-J2 cells were treated with EPS 100 ug/mL for 4 h. | EPS |
| IPEC-J2 cells were treated with L. reuteri L26 for 5 h. | L26 | |
| EPS/L26 pretreatment | IPEC-J2 cells were pretreated with EPS 100 ug/mL for 4 h, and subsequently challenged with Salmonella infection for 1 h. After this incubation period, for the purpose of killing the remaining extracellular bacteria gentamicin was added, and the cells were further incubated for the next 3 h in the presence of 100 ug/mL of gentamicin. | EPS + ST |
| IPEC-J2 cells were pretreated with L.reuteri L26 for 5 h, and subsequently challenged with Salmonella infection for 1 h. After this incubation period, for the purpose of killing the remaining extracellular bacteria gentamicin was added, and the cells were further incubated for the next 3 h in the presence of 100 ug/mL of gentamicin. | L26 + ST |
| Genes | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) | Tm (°C) | Reference |
|---|---|---|---|---|
| GAPDH | ACT CAC TCT TCT ACC TTT GAT GCT | TGT TGC TGT AGC CAA ATT CA | 60 | [14] |
| TLR4 | CTC TGC CTT CAC TAC AGA GA | CTG AGT CGT CTC CAG AAG AT | 60 | [15] |
| TLR5 | TTT CTG GCA ATG GCT GGA CA | TGG AGG TTG TCA AGT CCA TG | 60 | [15] |
| IL-6 | TGG ATA AGC TGC AGT CAC AG | ATT ATC CGA ATG GCC CTC AG | 60 | [15] |
| IL-8 | CGC ATT CCA CAC CTT TCC ACC CC | TCC TTG GGG TCC AGG CAG ACC | 60 | [16] |
| TNF-α | CGA CTC AGT GCC GAG ATC AA | CCT GCC CAG ATT CAG CAA AG | 60 | [12] |
| TGF-β | CAC GTG GAG CTA TAC CAG AA | TCC GGT GAC ATC AAA GGA CA | 60 | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiššová, Z.; Tkáčiková, Ľ.; Mudroňová, D.; Bhide, M.R. Immunomodulatory Effect of Lactobacillus reuteri (Limosilactobacillus reuteri) and Its Exopolysaccharides Investigated on Epithelial Cell Line IPEC-J2 Challenged with Salmonella Typhimurium. Life 2022, 12, 1955. https://doi.org/10.3390/life12121955
Kiššová Z, Tkáčiková Ľ, Mudroňová D, Bhide MR. Immunomodulatory Effect of Lactobacillus reuteri (Limosilactobacillus reuteri) and Its Exopolysaccharides Investigated on Epithelial Cell Line IPEC-J2 Challenged with Salmonella Typhimurium. Life. 2022; 12(12):1955. https://doi.org/10.3390/life12121955
Chicago/Turabian StyleKiššová, Zuzana, Ľudmila Tkáčiková, Dagmar Mudroňová, and Mangesh R. Bhide. 2022. "Immunomodulatory Effect of Lactobacillus reuteri (Limosilactobacillus reuteri) and Its Exopolysaccharides Investigated on Epithelial Cell Line IPEC-J2 Challenged with Salmonella Typhimurium" Life 12, no. 12: 1955. https://doi.org/10.3390/life12121955
APA StyleKiššová, Z., Tkáčiková, Ľ., Mudroňová, D., & Bhide, M. R. (2022). Immunomodulatory Effect of Lactobacillus reuteri (Limosilactobacillus reuteri) and Its Exopolysaccharides Investigated on Epithelial Cell Line IPEC-J2 Challenged with Salmonella Typhimurium. Life, 12(12), 1955. https://doi.org/10.3390/life12121955







