Association of TYR SNP rs1042602 with Melanoma Risk and Prognosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
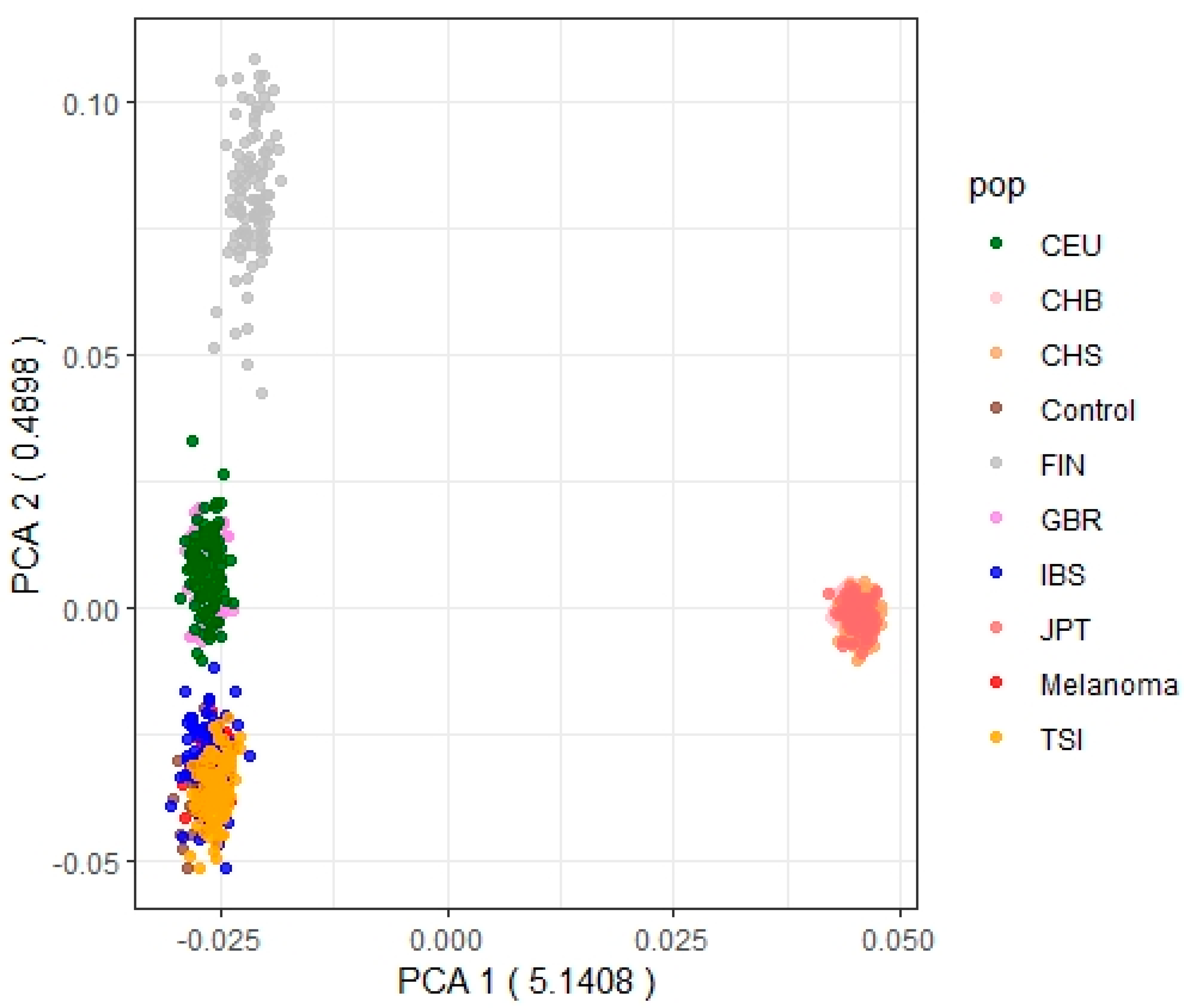
2.2. Patients and Samples
2.3. Exome Analysis
Comparison of Allele and Genotype Frequencies between Melanoma and Control Samples
2.4. SNPs Genotyping
2.5. Statistical Analysis of Association
2.6. Disease-Free Survival (DFS) Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur. J. Cancer 2022, 170, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershenwald, J.E.; Scolyer, R.A. Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Ann. Surg. Oncol. 2018, 25, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Rendleman, J.; Shang, S.; Dominianni, C.; Shields, J.F.; Scanlon, P.; Adaniel, C.; Desrichard, A.; Ma, M.; Shapiro, R.; Berman, R.; et al. Melanoma risk loci as determinants of melanoma recurrence and survival. J. Transl. Med. 2013, 11, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelsang, M.; Wilson, M.; Kirchhoff, T. Germline determinants of clinical outcome of cutaneous melanoma. Pigment Cell Melanoma Res. 2016, 29, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Rendleman, J.; Vogelsang, M.; Bapodra, A.; Adaniel, C.; Silva, I.; Moogk, D.; Martinez, C.N.; Fleming, N.; Shields, J.; Shapiro, R.; et al. Genetic associations of the interleukin locus at 1q32.1 with clinical outcomes of cutaneous melanoma. J. Med. Genet. 2015, 52, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Vogelsang, M.; Martinez, C.N.; Rendleman, J.; Bapodra, A.; Malecek, K.; Romanchuk, A.; Kazlow, E.; Shapiro, R.L.; Berman, R.S.; Krogsgaard, M.; et al. The Expression Quantitative Trait Loci in Immune Pathways and their Effect on Cutaneous Melanoma Prognosis. Clin. Cancer Res. 2016, 22, 3268–3280. [Google Scholar] [CrossRef] [Green Version]
- Aoude, L.G.; Bonazzi, V.F.; Brosda, S.; Patel, K.; Koufariotis, L.T.; Oey, H.; Nones, K.; Wood, S.; Pearson, J.V.; Lonie, J.M.; et al. Pathogenic germline variants are associated with poor survival in stage III/IV melanoma patients. Sci. Rep. 2020, 10, 17687. [Google Scholar] [CrossRef]
- López, S.; García, O.; Yurrebaso, I.; Flores, C.; Acosta-Herrera, M.; Chen, H.; Gardeazabal, J.; Careaga, J.M.; Boyano, M.D.; Sánchez, A.; et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a South European population. PLoS ONE 2014, 9, e104367. [Google Scholar] [CrossRef] [Green Version]
- García-Borrón, J.C.; Abdel-Malek, Z.; Jiménez-Cervantes, C. MC1R, the CAMP Pathway, and the Response to Solar UV: Extending the Horizon beyond Pigmentation. Pigment Cell Melanoma Res. 2014, 27, 699–720. [Google Scholar] [CrossRef]
- Ainger, S.A.; Jagirdar, K.; Lee, K.J.; Soyer, H.P.; Sturm, R.A. Skin Pigmentation Genetics for the Clinic. Dermatology 2017, 233, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.T.; Bishop, D.T.; MacGregor, S.; Machiela, M.J.; Stratigos, A.J.; Ghiorzo, P.; Brossard, M.; Calista, D.; Choi, J.; Fargnoli, M.C.; et al. Genome-wide association meta-analyses combining multiple risk phenotypes provide insights into the genetic architecture of cutaneous melanoma susceptibility. Nat. Genet. 2020, 52, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, G.J.; Oliveira, C.; Carvalho, B.S.; Torricelli, C.; Silva, J.K.; Gomez, G.V.B.; Rinck-Junior, J.A.; Oliveira, W.L.; Vazquez, V.L.; Serrano, S.V.; et al. Inherited variations in human pigmentation-related genes modulate cutaneous melanoma risk and clinicopathological features in Brazilian population. Sci. Rep. 2020, 10, 12129. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.; Lunter, G.; Marth, G.; Sherry, S.T.; et al. The Variant Call Format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Tian, J.; Xu, C.; Zhan, H.; Yang, Y. Exact MAX Tests in Case-Control Association Analysis (Manuscript). 2009. Available online: https://rdrr.io/cran/MaXact/man/maxact.html (accessed on 1 June 2022).
- Zheng, X.; Levine, D.; Shen, J.; Gogarten, S.; Laurie, C.; Weir, B. A High-performance Computing Toolset for Relatedness and Principal Component Analysis of SNP Data. Bioinformatics 2012, 28, 3326–3328. [Google Scholar] [CrossRef] [Green Version]
- Therneau, T. A Package for Survival Analysis in R. R Package Version 3.3-1. 2022. Available online: https://CRAN.R-project.org/package=survival (accessed on 1 June 2022).
- Chen, H.; Conomos, M.P.; Pham, D.T. GMMAT: Generalized LinearMixed Model Association Tests. R Package Version 1.3.2. 2021. Available online: https://rdrr.io/cran/GMMAT/ (accessed on 1 June 2022).
- Shriver, M.D.; Parra, E.J.; Dios, S.; Bonilla, C.; Norton, H.; Jovel, C.; Pfaff, C.; Jones, C.; Massac, A.; Cameron, N.; et al. Skin pigmentation, biogeographical ancestry and admixture mapping. Hum. Genet. 2003, 112, 387–399. [Google Scholar] [CrossRef]
- Stokowski, R.P.; Pant, P.V.; Dadd, T.; Fereday, A.; Hinds, D.A.; Jarman, C.; Filsell, W.; Ginger, R.S.; Green, M.R.; van der Ouderaa, F.J.; et al. A genomewide association study of skin pigmentation in a South Asian population. Am. J. Hum. Genet. 2007, 81, 1119–1132. [Google Scholar] [CrossRef] [Green Version]
- Jonnalagadda, M.; Norton, H.; Ozarkar, S.; Kulkarni, S.; Ashma, R. Association of genetic variants with skin pigmentation phenotype among populations of west Maharashtra, India. Am. J. Hum. Biol. 2016, 28, 610–618. [Google Scholar] [CrossRef]
- Adhikari, K.; Mendoza-Revilla, J.; Sohail, A.; Fuentes-Guajardo, M.; Lampert, J.; Chacón-Duque, J.C.; Hurtado, M.; Villegas, V.; Granja, V.; Acuña-Alonzo, V.; et al. A GWAS in Latin Americans highlights the convergent evolution of lighter skin pigmentation in Eurasia. Nat. Commun. 2019, 10, 358. [Google Scholar] [CrossRef]
- Lona-Durazo, F.; Hernandez-Pacheco, N.; Fan, S.; Zhang, T.; Choi, J.; Kovacs, M.A.; Loftus, S.K.; Le, P.; Edwards, M.; Fortes-Lima, C.A.; et al. Meta-analysis of GWA studies provides new insights on the genetic architecture of skin pigmentation in recently admixed populations. BMC Genet. 2019, 20, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, M.A.; Meyer, O.S.; Refn, M.; Morling, N.; Andersen, J.D.; Børsting, C. Analysis of Skin Pigmentation and Genetic Ancestry in Three Subpopulations from Pakistan: Punjabi, Pashtun, and Baloch. Genes 2021, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- Frudakis, T.; Thomas, M.; Gaskin, Z.; Venkateswarlu, K.; Chandra, K.S.; Ginjupalli, S.; Gunturi, S.; Natrajan, S.; Ponnuswamy, V.K.; Ponnuswamy, K.N. Sequences associated with human iris pigmentation. Genetics 2003, 165, 2071–2083. [Google Scholar] [CrossRef]
- Morgan, M.D.; Pairo-Castineira, E.; Rawlik, K.; Canela-Xandri, O.; Rees, J.; Sims, D.; Tenesa, A.; Jackson, I.J. Genome-wide study of hair colour in UK Biobank explains most of the SNP heritability. Nat. Commun. 2018, 9, 5271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulem, P.; Gudbjartsson, D.F.; Stacey, S.N.; Helgason, A.; Rafnar, T.; Magnusson, K.P.; Manolescu, A.; Karason, A.; Palsson, A.; Thorleifsson, G.; et al. Genetic determinants of hair.; eye and skin pigmentation in Europeans. Nat. Genet. 2007, 39, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Galván-Femenía, I.; Obón-Santacana, M.; Piñeyro, D.; Guindo-Martinez, M.; Duran, X.; Carreras, A.; Pluvinet, R.; Velasco, J.; Ramos, L.; Aussó, S.; et al. Multitrait genome association analysis identifies new susceptibility genes for human anthropometric variation in the GCAT cohort. J. Med. Genet. 2018, 55, 765–778. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.E.; Kricker, A.; Waxweiler, W.T.; Dillon, P.M.; Busman, K.J.; From, L.; Groben, P.A.; Armstrong, B.K.; Anton-Culver, H.; Gruber, S.B.; et al. Comparison of clinicopathologic features and survival of histopathologically amelanotic and pigmented melanomas: A population-based study. JAMA Dermatol. 2014, 150, 1306–1314. [Google Scholar] [CrossRef]
- Guo, W.; Yin, G.; Liu, H.; Duan, H.; Huang, Z.; Chen, X. Matched analysis of the prognosis of amelanotic and pigmented melanoma in head and neck. Acta Otolaryngol. 2020, 140, 785–788. [Google Scholar] [CrossRef]
- Ryu, G.W.; Choi, Y.D.; Jin, S.; Chung, I.J.; Shin, M.H.; Yun, S.J. Volar location and degree of pigmentation are associated with poor survival and first metastasis pattern in acral melanoma. Pigment Cell Melanoma Res. 2021, 34, 1094–1104. [Google Scholar] [CrossRef]
- Giebel, L.B.; Spritz, R.A. RFLP for MboI in the human tyrosinase (TYR) gene detected by PCR. Nucleic Acids Res. 1990, 18, 3103. [Google Scholar] [CrossRef]
- Ito, S. The IFPCS presidential lecture: A chemist’s view of melanogenesis. Pigment Cell Res. 2003, 16, 230–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaki, M.; Sengupta, M.; Mondal, M.; Bhattacharya, A.; Mallick, S.; Bhadra, R.; Indian Genome Variation Consortium; Ray, K. Molecular and functional studies of tyrosinase variants among Indian oculocutaneous albinism type 1 patients. J. Investig. Dermatol. 2011, 131, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Jagirdar, K.; Smit, D.J.; Ainger, S.A.; Lee, K.J.; Brown, D.L.; Chapman, B.; Zhao, Z.Z.; Montgomery, G.W.; Martin, N.G.; Stow, J.L.; et al. Molecular analysis of common polymorphisms within the human Tyrosinase locus and genetic association with pigmentation traits. Pigment Cell Melanoma Res. 2014, 27, 552–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoggart, C.J.; Parra, E.J.; Shriver, M.D.; Bonilla, C.; Kittles, R.A.; Clayton, D.G.; McKeigue, P.M. Control of confounding of genetic associations in stratified populations. Am. J. Hum. Genet. 2003, 72, 1492–1504. [Google Scholar] [CrossRef] [Green Version]
- Chaitanya, L.; Breslin, K.; Zuñiga, S.; Wirken, L.; Pośpiech, E.; Kukla-Bartoszek, M.; Sijen, T.; Knijff, P.; Liu, F.; Branicki, W.; et al. The HIrisPlex-S system for eye, hair and skin colour prediction from DNA: Introduction and forensic developmental validation. Forensic Sci. Int. Genet. 2018, 35, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Kalahroudi, V.G.; Kamalidehghan, B.; Kani, A.A.; Aryani, O.; Tondar, M.; Ahmadipour, F.; Chung, L.Y.; Houshmand, M. Two novel tyrosinase (TYR) gene mutations with pathogenic impact on oculocutaneous albinism type 1 (OCA1). PLoS ONE 2014, 9, e106656. [Google Scholar] [CrossRef] [Green Version]
- Grønskov, K.; Jespersgaard, C.; Bruun, G.H.; Harris, P.; Brøndum-Nielsen, K.; Andresen, B.S.; Rosenberg, T. A pathogenic haplotype, common in Europeans, causes autosomal recessive albinism and uncovers missing heritability in OCA1. Sci. Rep. 2019, 9, 645. [Google Scholar] [CrossRef] [Green Version]
- Mendez, R.; Iqbal, S.; Vishnopolska, S.; Martinez, C.; Dibner, G.; Aliano, R.; Zaiat, J.; Biagioli, G.; Fernandez, C.; Turjanski, A.; et al. Oculocutaneous albinism type 1B associated with a functionally significant tyrosinase gene polymorphism detected with Whole Exome Sequencing. Ophthalmic Genet. 2021, 42, 291–295. [Google Scholar] [CrossRef]
- Bishop, D.T.; Demenais, F.; Iles, M.M.; Harland, M.; Taylor, J.C.; Corda, E.; Randerson-Moor, J.; Aitken, J.F.; Avril, M.F.; Azizi, E.; et al. Genome-wide association study identifies three loci associated with melanoma risk. Nat. Genet. 2009, 41, 920–925. [Google Scholar] [CrossRef]
- Amos, C.I.; Wang, L.E.; Lee, J.E.; Gershenwald, J.E.; Chen, W.V.; Fang, S.; Kosoy, R.; Zhang, M.; Qureshi, A.A.; Vattathil, S.; et al. Genome-wide association study identifies novel loci predisposing to cutaneous melanoma. Hum. Mol. Genet. 2011, 20, 5012–5023. [Google Scholar] [CrossRef]
- Hernando, B.; Ibarrola-Villava, M.; Fernandez, L.P.; Peña-Chilet, M.; Llorca-Cardeñosa, M.; Oltra, S.S.; Alonso, S.; Boyano, M.D.; Martinez-Cadenas, C.; Ribas, G. Sex-specific genetic effects associated with pigmentation, sensitivity to sunlight, and melanoma in a population of Spanish origin. Biol. Sex Differ. 2016, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, D.L.; Zhao, Z.Z.; Sturm, R.A.; Hayward, N.K.; Martin, N.G.; Montgomery, G.W. Multiple pigmentation gene polymorphisms account for a substantial proportion of risk of cutaneous malignant melanoma. J. Investig. Dermatol. 2010, 130, 520–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castaneda-Garcia, C.; Iyer, V.; Nsengimana, J.; Trower, A.; Droop, A.; Brown, K.M.; Choi, J.; Zhang, T.; Harland, M.; Newton-Bishop, J.A.; et al. Defining novel causal SNPs and linked phenotypes at melanoma-associated loci. Hum. Mol. Genet. 2022, 31, ddac074. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kuo, C.; Morton, D.L.; Wang, H.J.; Hoon, D.S. Expression of differentiation melanoma-associated antigen genes is associated with favorable disease outcome in advanced-stage melanomas. Cancer Res. 2003, 63, 441–448. [Google Scholar] [PubMed]
- Parlar, A.; Sayitoglu, E.C.; Ozkazanc, D.; Georgoudaki, A.M.; Pamukcu, C.; Aras, M.; Josey, B.J.; Chrobok, M.; Branecki, S.; Zahedimaram, P.; et al. Engineering antigen-specific NK cell lines against the melanoma-associated antigen tyrosinase via TCR gene transfer. Eur. J. Immunol. 2019, 49, 1278–1290. [Google Scholar] [CrossRef]
- Fürst, K.; Steder, M.; Logotheti, S.; Angerilli, A.; Spitschak, A.; Marquardt, S.; Schumacher, T.; Engelmann, D.; Herchenröder, O.; Rupp, R.A.W.; et al. DNp73-induced degradation of tyrosinase links depigmentation with EMT-driven melanoma progression. Cancer Lett. 2019, 442, 299–309. [Google Scholar] [CrossRef]
- Kamo, H.; Kawahara, R.; Simizu, S. Tyrosinase suppresses vasculogenic mimicry in human melanoma cells. Oncol. Lett. 2022, 23, 169. [Google Scholar] [CrossRef]
- Millán-Esteban, D.; Peña-Chilet, M.; García-Casado, Z.; Manrique-Silva, E.; Requena, C.; Bañuls, J.; López-Guerrero, J.A.; Rodríguez-Hernández, A.; Traves, V.; Dopazo, J.; et al. Mutational Characterization of Cutaneous Melanoma Supports Divergent Pathways Model for Melanoma Development. Cancers 2021, 13, 5219. [Google Scholar] [CrossRef]




| All Patients | Women | Men | |
|---|---|---|---|
| N | 1025 | 540 | 485 |
| Age at diagnosis | Range 21–96 (median: 59) | Range 22–93 (median: 56) | Range 21–96 (median: 62) |
| Disease evolution | |||
| Disease free | 727 | 405 | 322 |
| Metastasis | 298 | 135 | 163 |
| Stage at diagnosis (AJCC 8th edition) | |||
| In situ | 155 | 87 | 68 |
| IA | 367 | 204 | 163 |
| IB | 136 | 75 | 61 |
| IIA | 77 | 41 | 36 |
| IIB | 55 | 31 | 24 |
| IIC | 59 | 26 | 33 |
| IIIA | 31 | 13 | 18 |
| IIIB | 22 | 10 | 12 |
| IIIC | 57 | 20 | 37 |
| IIID | 3 | 0 | 3 |
| IV | 13 | 7 | 6 |
| nd | 50 | 26 | 24 |
| Melanoma subtypes | |||
| SSM | 524 | 279 | 245 |
| NM | 153 | 76 | 77 |
| LM | 36 | 17 | 19 |
| LMM | 52 | 29 | 23 |
| ALM | 48 | 31 | 17 |
| Others | 17 | 9 | 8 |
| nd | 195 | 99 | 96 |
| Breslow Thickness (mm) | |||
| 0 | 158 | 89 | 69 |
| ≤1 | 403 | 223 | 180 |
| >1–2 | 176 | 91 | 85 |
| >2–4 | 132 | 72 | 60 |
| >4 | 116 | 46 | 70 |
| nd | 40 | 19 | 21 |
| Location | |||
| Head/Neck | 158 | 70 | 88 |
| Trunk | 424 | 165 | 259 |
| Upper limb | 125 | 77 | 48 |
| Lower limb | 230 | 173 | 57 |
| Hands/foot | 56 | 39 | 17 |
| Others | 19 | 11 | 8 |
| All Patients | Women | Men | |
|---|---|---|---|
| N | 664 | 360 | 304 |
| Age at diagnosis | Range 22–93 (median: 58) | Range 22–93 (median: 53) | Range 22–93 (median: 62) |
| Disease evolution | |||
| Disease free | 469 | 275 | 194 |
| Metastasis | 195 | 85 | 110 |
| Stage at diagnosis (AJCC 8th edition) | |||
| In situ | 91 | 53 | 38 |
| IA | 238 | 135 | 103 |
| IB | 91 | 56 | 35 |
| IIA | 61 | 33 | 28 |
| IIB | 38 | 22 | 16 |
| IIC | 42 | 19 | 23 |
| IIIA | 17 | 7 | 10 |
| IIIB | 11 | 5 | 6 |
| IIIC | 46 | 16 | 30 |
| IIID | 3 | 0 | 3 |
| IV | 13 | 7 | 6 |
| nd | 13 | 7 | 6 |
| Melanoma subtypes | |||
| SSM | 362 | 196 | 166 |
| NM | 100 | 53 | 47 |
| LM | 19 | 8 | 11 |
| LMM | 21 | 13 | 8 |
| ALM | 37 | 24 | 13 |
| Others | 29 | 17 | 13 |
| nd | 96 | 49 | 46 |
| Breslow Thickness (mm) | |||
| 0 | 88 | 49 | 39 |
| ≤1 | 257 | 146 | 111 |
| >1–2 | 116 | 64 | 52 |
| >2–4 | 92 | 52 | 40 |
| >4 | 85 | 33 | 52 |
| nd | 26 | 16 | 10 |
| Location | |||
| Head/Neck | 86 | 35 | 51 |
| Trunk | 265 | 105 | 160 |
| Upper limb | 84 | 52 | 32 |
| Lower limb | 165 | 124 | 41 |
| Hands/foot | 42 | 31 | 11 |
| Others | 12 | 8 | 4 |
| nd | 10 | 5 | 5 |
| Genotype N (%) | Chi2 Test | CATT Test | |||
|---|---|---|---|---|---|
| CC | CA | AA | p-Value | p-Value | |
| All patients | |||||
| Controls | 210 (27.2%) | 373 (48.2%) | 190 (24.6%) | 0.0044 | 0.0035 |
| Patients | 221 (21.6%) | 494 (48.2%) | 310 (30.2%) | ||
| Men | |||||
| Controls | 65 (31%) | 105 (50%) | 40 (19%) | 0.0015 | 0.0030 |
| Patients | 101 (20.8%) | 238 (49.1%) | 146 (30.1%) | ||
| Women | |||||
| Controls | 108 (24.7%) | 207 (47.2%) | 123 (28.1%) | 0.5915 | 0.2061 |
| Patients | 120 (22.2%) | 256 (47.4%) | 164 (30.4%) | ||
| Samples | Chi-Square Test (df = 2) (p-Value) | CATT Test: One-Sided (Theta = 1) (p-Value) | ||||
|---|---|---|---|---|---|---|
| ALL | WOMEN | MEN | ALL | WOMEN | MEN | |
| All stages | 0.1526 | 0.7118 | 0.0308 | 0.0384 | 0.5968 | 0.0054 |
| Stages I, II and III | 0.1188 | 0.4231 | 0.0287 | 0.0471 | 0.6761 | 0.0052 |
| Stage II | 0.4493 | 0.8737 | 0.1503 | 0.1541 | 0.6162 | 0.0716 |
| Genotype N (%) | Chi2 Test | CATT Test | |||
|---|---|---|---|---|---|
| CC | CA | AA | p-Value | p-Value | |
| All stages (n = 304) | |||||
| Disease-free | 45 (23.2%) | 91 (46.9%) | 58 (29.9%) | 0.0308 | 0.0054 |
| Metastatic | 12 (10.9%) | 60 (54.55%) | 38 (34.55%) | ||
| Stages I, II and III (n = 254) | |||||
| Disease-free | 34 (21.9%) | 72 (46.5%) | 49 (31.6%) | 0.0287 | 0.0052 |
| Metastatic | 9 (9.1%) | 53 (53.5%) | 37 (37.4%) | ||
| Stage II (n = 67) | |||||
| Disease-free | 6 (23.1%) | 12 (46.1%) | 8 (30.8%) | 0.1503 | 0.0716 |
| Metastatic | 3 (7.3%) | 26 (63.4%) | 12 (29.3%) | ||
| Samples | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Men and women (n = 1025) | CC | 0.62 (0.42–0.93) | 0.0202 | 0.64 (0.41–1.01) | 0.0530 |
| CA | 0.92 (0.69–1.22) | 0.5579 | 0.86 (0.63–1.18) | 0.3550 | |
| Men and women, stages I, II, III (n = 807) | CC | 0.59 (0.38–0.89) | 0.0145 | 0.62 (0.39–0.99) | 0.0446 |
| CA | 0.84 (0.63–1.14) | 0.2616 | 0.85 (0.62–1.18) | 0.3331 | |
| Men (n = 485) | CC | 0.49 (0.27–0.89) | 0.0183 | 0.48 (0.25–0.94) | 0.0320 |
| CA | 0.99 (0.68–1.44) | 0.9545 | 0.85 (0.55–1.30) | 0.4450 | |
| Men, stages I, II, III (n = 387) | CC | 0.43 (0.23–0.82) | 0.0099 | 0.44 (0.21–0.89) | 0.0219 |
| CA | 0.93 (0.63–1.38) | 0.7278 | 0.86 (0.56–1.32) | 0.4870 | |
| Men, 5 years follow-up (n = 304) | CC | 0.46 (0.24–0.89) | 0.0200 | 0.4 (0.20–0.83) | 0.0139 |
| CA | 0.95 (0.63–1.42) | 0.7940 | 0.68 (0.42–1.11) | 0.1218 | |
| Men, 5 years follow-up, stages I, II, III (n = 254) | CC | 0.4 (0.19–0.83) | 0.0136 | 0.36 (0.16–0.79) | 0.0107 |
| CA | 0.9 (0.59–1.37) | 0.6191 | 0.7 (0.43–1.13) | 0.1461 | |
| Samples | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Men and women (n = 1025) | 0.66 (0.46–0.94) | 0.0222 | 0.7 (0.47–1.06) | 0.0904 |
| Men and women, stages I, II, III (n = 807) | 0.65 (0.44–0.96) | 0.0308 | 0.68 (0.44–1.05) | 0.0789 |
| Men (n = 485) | 0.50 (0.29–0.85) | 0.0110 | 0.54 (0.29–0.99) | 0.0459 |
| Men, stages I, II, III (n = 387) | 0.45 (0.25–0.82) | 0.0086 | 0.48 (0.25–0.93) | 0.0290 |
| Men, 5 years follow-up (n = 304) | 0.48 (0.26–0.87) | 0.0160 | 0.51 (0.26–0.99) | 0.0480 |
| Men, 5 years follow-up, stages I, II, III (n = 254) | 0.43 (0.21–0.85) | 0.0147 | 0.45 (0.21–0.93) | 0.0318 |
| CC (%) | CA (%) | AA (%) | N | |
|---|---|---|---|---|
| Men and women | 19.4 | 49.2 | 31.4 | 651 |
| In situ | 24.2 | 46.1 | 29.7 | 91 |
| Stage I | 20.7 | 49.5 | 29.8 | 329 |
| Stage II | 16.3 | 51.1 | 32.6 | 141 |
| Stage III | 11.7 | 48.0 | 40.3 | 77 |
| Stage IV | 30.8 | 46.1 | 23.1 | 13 |
| Men | 18.8 | 49.0 | 32.2 | 298 |
| In situ | 26.3 | 50.0 | 23.7 | 38 |
| Stage I | 22.5 | 45.6 | 31.9 | 138 |
| Stage II | 13.4 | 56.7 | 29.9 | 67 |
| Stage III | 6.1 | 49.0 | 44.9 | 49 |
| Stage IV | 50.0 | 33.3 | 16.7 | 6 |
| Women | 19.8 | 49.3 | 30.9 | 353 |
| In situ | 22.6 | 43.4 | 34.0 | 53 |
| Stage I | 19.4 | 52.3 | 28.3 | 191 |
| Stage II | 18.9 | 46.0 | 35.1 | 74 |
| Stage III | 21.4 | 46.4 | 32.2 | 28 |
| Stage IV | 14.3 | 57.1 | 28.6 | 7 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | Coef | HR (95% CI) | p-Value | Coef | HR (95% CI) | p-Value |
| Men and women | ||||||
| Age | 0.0359 | 1.0365 (1.002–1.073) | 0.0404 | 0.0520 | 1.0534 (1.015–1.093) | 0.0061 |
| Sex | 0.9262 | 2.5249 (0.828–7.704) | 0.1040 | - | - | - |
| Breslow thickness | 0.0980 | 1.1029 (0.992–1.226) | 0.0693 | - | - | - |
| Ulceration | 0.8022 | 2.2305 (0.842–5.907) | 0.1060 | - | - | - |
| Melanoma subtypes | 0.0077 | 1.0078 (0.680–1.494) | 0.9690 | - | - | - |
| rs1042602 genotype | −1.1387 | 0.3202 (0.127–0.809) | 0.0160 | −1.5605 | 0.2100 (0.079–0.555) | 0.0017 |
| Men | ||||||
| Age | 0.0340 | 1.0346 (0.997–1.074) | 0.0719 | - | - | - |
| Breslow thickness | 0.0887 | 1.0928 (0.983–1.215) | 0.1 | - | - | - |
| Ulceration | 1.1984 | 3.3148 (1.075–10.22) | 0.037 | 1.7475 | 5.7401 (1.594–20.657) | 0.0075 |
| Melanoma subtypes | 0.1234 | 1.1313 (0.752–1.702) | 0.5540 | - | - | - |
| rs1042602 genotype | −1.3253 | 0.2657 (0.090–0.787) | 0.0168 | −1.8709 | 0.154 (0.044- 0.539) | 0.0034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevilla, A.; Sánchez-Diez, A.; Cobo, S.; Izagirre, N.; Martinez-Cadenas, C.; Martí, R.M.; Puértolas, T.; de Unamuno, B.; Bañuls, J.; Izu, R.; et al. Association of TYR SNP rs1042602 with Melanoma Risk and Prognosis. Life 2022, 12, 2004. https://doi.org/10.3390/life12122004
Sevilla A, Sánchez-Diez A, Cobo S, Izagirre N, Martinez-Cadenas C, Martí RM, Puértolas T, de Unamuno B, Bañuls J, Izu R, et al. Association of TYR SNP rs1042602 with Melanoma Risk and Prognosis. Life. 2022; 12(12):2004. https://doi.org/10.3390/life12122004
Chicago/Turabian StyleSevilla, Arrate, Ana Sánchez-Diez, Sofía Cobo, Neskuts Izagirre, Conrado Martinez-Cadenas, Rosa M. Martí, Teresa Puértolas, Blanca de Unamuno, José Bañuls, Rosa Izu, and et al. 2022. "Association of TYR SNP rs1042602 with Melanoma Risk and Prognosis" Life 12, no. 12: 2004. https://doi.org/10.3390/life12122004





