The Therapeutic Role of SGLT-2 Inhibitors in Acute Heart Failure: From Pathophysiologic Mechanisms to Clinical Evidence with Pooled Analysis of Relevant Studies across Safety and Efficacy Endpoints of Interest
Abstract
1. The Entity of Acute Heart Failure
2. Mechanisms Targeted by SGLT-2 Inhibitors
2.1. Volume Regulation
2.2. Cardiac Function and Remodeling
2.3. Blood Pressure
2.4. Arterial Stiffness
2.5. Myocardial Energetics
2.6. Myocardial Flow Reserve
2.7. Myocardial Fibrosis
2.8. Arrhythmic Burden
2.9. Hemoconcentration
3. Effect of SGLT-2 Inhibitors in the Acute HF Setting on Surrogate Endpoints: Evidence from Randomized Controlled Trials and Observational Studies
3.1. Search Strategy and Study Selection
3.2. Crude Outcomes of Interest
3.3. Results
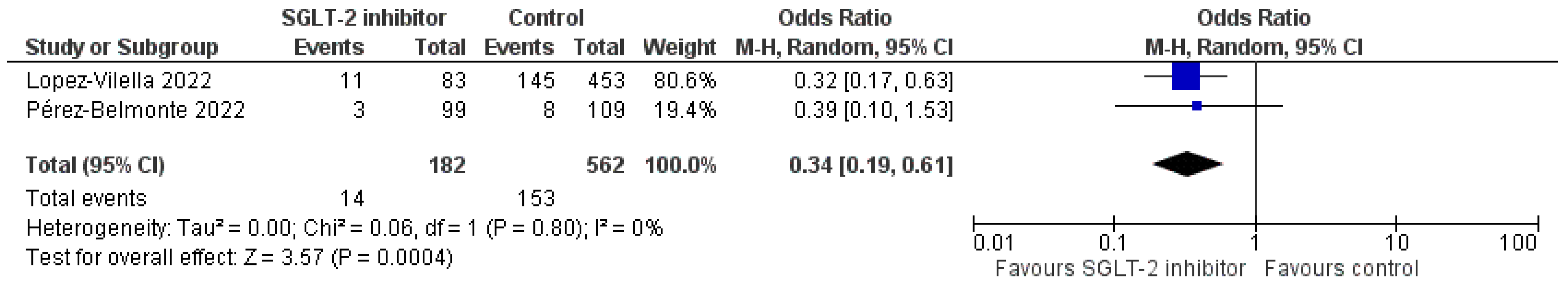
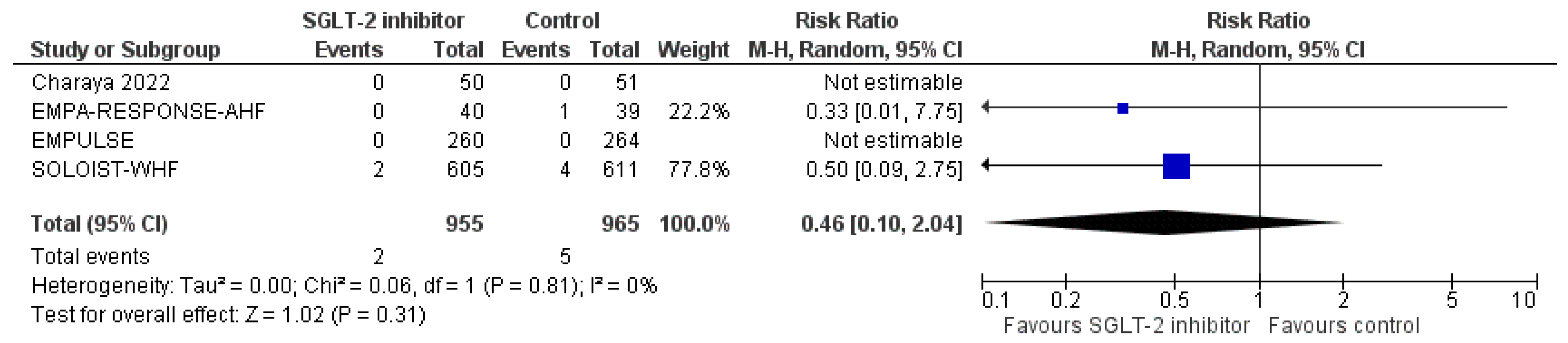
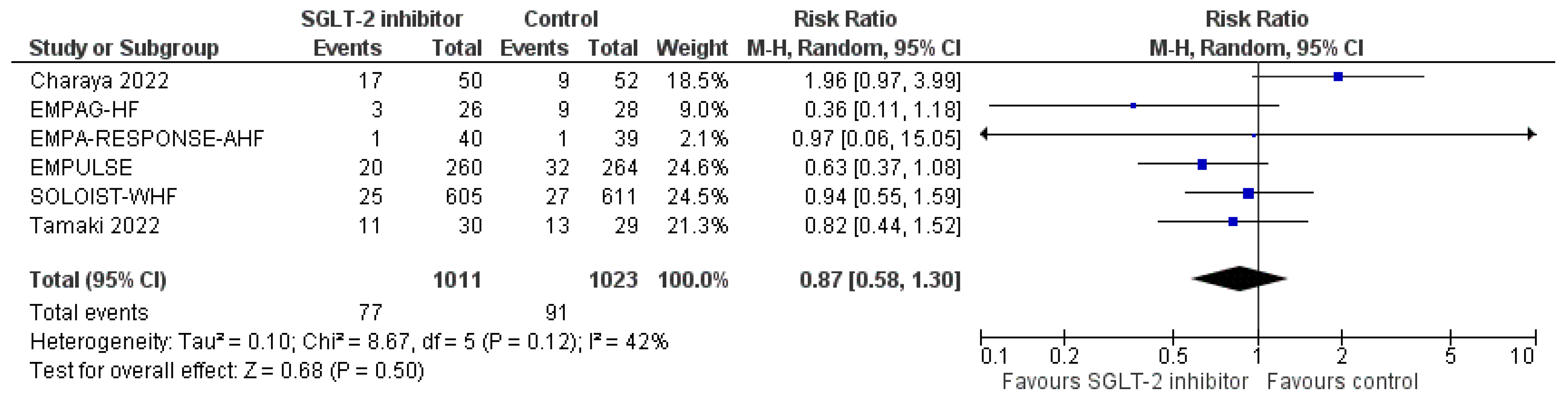
3.4. Pooled Analysis of RCTs
3.5. Pooled Analysis of Observational Studies
3.6. Other Efficacy Endpoints of Interest
3.6.1. Urine Output
3.6.2. Body Weight
3.6.3. N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) Levels
3.6.4. Renal Function
3.6.5. Hematocrit Levels
3.6.6. Change in Visual Analogue Scale (VAS) Dyspnoea Score
3.6.7. Change in Quality-of-Life Indices
3.6.8. Loop Diuretics Dosage and Diuretic Efficacy
3.6.9. Utilization Rates of Inotropes
3.6.10. Usage Rates of LV Assist Devices
3.6.11. Safety Endpoints
4. Areas for Further Research and Concluding Remarks
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arrigo, M.; Jessup, M.; Mullens, W.; Reza, N.; Shah, A.M.; Sliwa, K.; Mebazaa, A. Acute heart failure. Nat. Reviews Dis. Prim. 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Kurmani, S.; Squire, I. Acute Heart Failure: Definition, Classification and Epidemiology. Curr. Heart Fail. Rep. 2017, 14, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Abdo, A.S. Hospital Management of Acute Decompensated Heart Failure. Am. J. Med. Sci. 2017, 353, 265–274. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Pang, P.S. Acute heart failure syndromes. J. Am. Coll. Cardiol. 2009, 53, 557–573. [Google Scholar] [CrossRef]
- Metra, M.; Cotter, G.; El-Khorazaty, J.; Davison, B.A.; Milo, O.; Carubelli, V.; Bourge, R.C.; Cleland, J.G.; Jondeau, G.; Krum, H.; et al. Acute heart failure in the elderly: Differences in clinical characteristics, outcomes, and prognostic factors in the VERITAS Study. J. Card. Fail. 2015, 21, 179–188. [Google Scholar] [CrossRef]
- Metra, M.; Mentz, R.J.; Chiswell, K.; Bloomfield, D.M.; Cleland, J.G.; Cotter, G.; Davison, B.A.; Dittrich, H.C.; Fiuzat, M.; Givertz, M.M.; et al. Acute heart failure in elderly patients: Worse outcomes and differential utility of standard prognostic variables. Insights from the PROTECT trial. Eur. J. Heart Fail. 2015, 17, 109–118. [Google Scholar] [CrossRef]
- Pokorney, S.D.; Al-Khatib, S.M.; Sun, J.L.; Schulte, P.; O’Connor, C.M.; Teerlink, J.R.; Armstrong, P.W.; Ezekowitz, J.A.; Starling, R.C.; Voors, A.A.; et al. Sudden cardiac death after acute heart failure hospital admission: Insights from ASCEND-HF. Eur. J. Heart Fail. 2018, 20, 525–532. [Google Scholar] [CrossRef]
- Loungani, R.S.; Teerlink, J.R.; Metra, M.; Allen, L.A.; Butler, J.; Carson, P.E.; Chen, C.W.; Cotter, G.; Davison, B.A.; Eapen, Z.J.; et al. Cause of Death in Patients With Acute Heart Failure: Insights From RELAX-AHF-2. JACC. Heart Fail. 2020, 8, 999–1008. [Google Scholar] [CrossRef]
- Ng, K.T.; Yap, J. Continuous infusion vs. intermittent bolus injection of furosemide in acute decompensated heart failure: Systematic review and meta-analysis of andomized controlled trials. Anaesthesia 2018, 73, 238–247. [Google Scholar] [CrossRef]
- Chan, J.; Kot, T.; Ng, M.; Harky, A. Continuous Infusion Versus Intermittent Boluses of Furosemide in Acute Heart Failure: A Systematic Review and Meta-Analysis. J. Card. Fail. 2020, 26, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Spates, T.; Sun, J.L.; Kittipibul, V.; Testani, J.M.; Starling, R.C.; Tang, W.; Hernandez, A.F.; Felker, G.M.; O’Connor, C.M.; et al. Early diuretic strategies and the association with In-hospital and Post-discharge outcomes in acute heart failure. Am. Heart J. 2021, 239, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Testani, J.; Collins, S. Diuretic Resistance in Heart Failure. Curr. Heart Fail. Rep. 2019, 16, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; DeWald, T.A.; Hernandez, A.F. Combination of loop diuretics with thiazide-type diuretics in heart failure. J. Am. Coll. Cardiol. 2010, 56, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Cox, Z.L.; Hung, R.; Lenihan, D.J.; Testani, J.M. Diuretic Strategies for Loop Diuretic Resistance in Acute Heart Failure: The 3T Trial. JACC. Heart Fail. 2020, 8, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Mentz, R.J.; Cole, R.T.; Adams, K.F.; Egnaczyk, G.F.; Fiuzat, M.; Patel, C.B.; Echols, M.; Khouri, M.G.; Tauras, J.M.; et al. Efficacy and Safety of Tolvaptan in Patients Hospitalized With Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1399–1406. [Google Scholar] [CrossRef]
- Orso, D.; Tavazzi, G.; Corradi, F.; Mearelli, F.; Federici, N.; Peric, D.; D’Andrea, N.; Santori, G.; Mojoli, F.; Forfori, F.; et al. Comparison of diuretic strategies in diuretic-resistant acute heart failure: A systematic review and network meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2971–2980. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Vaduganathan, M. SGLT-2 Inhibitors in Heart Failure: Volume or Value? J. Am. Coll. Cardiol. 2021, 77, 1393–1396. [Google Scholar] [CrossRef]
- McMurray, J.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.; Boulton, D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, R.A.; Muskiet, M.; van Baar, M.; Hesp, A.C.; Greasley, P.J.; Karlsson, C.; Hammarstedt, A.; Arya, N.; van Raalte, D.H.; Heerspink, H. Natriuretic Effect of Two Weeks of Dapagliflozin Treatment in Patients With Type 2 Diabetes and Preserved Kidney Function During Standardized Sodium Intake: Results of the DAPASALT Trial. Diabetes Care 2021, 44, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Omar, M.; Kistorp, C.; Tuxen, C.; Gustafsson, I.; Køber, L.; Gustafsson, F.; Faber, J.; Malik, M.E.; Fosbøl, E.L.; et al. Effects of empagliflozin on estimated extracellular volume, estimated plasma volume, and measured glomerular filtration rate in patients with heart failure (Empire HF Renal): A prespecified substudy of a double-blind, andomized, placebo-controlled trial. Lancet. Diabetes Endocrinol. 2021, 9, 106–116. [Google Scholar] [CrossRef]
- Hoshika, Y.; Kubota, Y.; Mozawa, K.; Tara, S.; Tokita, Y.; Yodogawa, K.; Iwasaki, Y.K.; Yamamoto, T.; Takano, H.; Tsukada, Y.; et al. Effect of Empagliflozin Versus Placebo on Plasma Volume Status in Patients with Acute Myocardial Infarction and Type 2 Diabetes Mellitus. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2021, 12, 2241–2248. [Google Scholar] [CrossRef]
- Fukuoka, S.; Dohi, K.; Takeuchi, T.; Moriwaki, K.; Ishiyama, M.; Omori, T.; Fujimoto, N.; Ito, M. Mechanisms and prediction of short-term natriuretic effect of sodium-glucose cotransporter 2 inhibitor in heart failure patients coexisting type 2 diabetes mellitus. Heart Vessels 2020, 35, 1218–1226. [Google Scholar] [CrossRef]
- Kaze, A.D.; Zhuo, M.; Kim, S.C.; Patorno, E.; Paik, J.M. Association of SGLT2 inhibitors with cardiovascular, kidney, and safety outcomes among patients with diabetic kidney disease: A meta-analysis. Cardiovasc. Diabetol. 2022, 21, 47. [Google Scholar] [CrossRef]
- Shi, F.H.; Li, H.; Shen, L.; Xu, L.; Ge, H.; Gu, Z.C.; Lin, H.W.; Pu, J. Beneficial Effect of Sodium-Glucose Co-transporter 2 Inhibitors on Left Ventricular Function. J. Clin. Endocrinol. Metab. 2022, 107, 1191–1203. [Google Scholar] [CrossRef]
- Theofilis, P.; Antonopoulos, A.S.; Katsimichas, T.; Oikonomou, E.; Siasos, G.; Aggeli, C.; Tsioufis, K.; Tousoulis, D. The impact of SGLT2 inhibition on imaging markers of cardiac function: A systematic review and meta-analysis. Pharmacol. Res. 2022, 180, 106243. [Google Scholar] [CrossRef]
- Zhang, D.P.; Xu, L.; Wang, L.F.; Wang, H.J.; Jiang, F. Effects of antidiabetic drugs on left ventricular function/dysfunction: A systematic review and network meta-analysis. Cardiovasc. Diabetol. 2020, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; San Antonio, R.; Garcia-Ropero, A.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Vargas-Delgado, A.P.; Flores-Umanzor, E.J.; Sanz, J.; et al. Empagliflozin Ameliorates Diastolic Dysfunction and Left Ventricular Fibrosis/Stiffness in Nondiabetic Heart Failure: A Multimodality Study. JACC. Cardiovasc. Imaging 2021, 14, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC. Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Verma, S. Potential Mechanisms of Sodium-Glucose Co-Transporter 2 Inhibitor-Related Cardiovascular Benefits. Am. J. Cardiol. 2019, 124 (Suppl. 1), S36–S44. [Google Scholar] [CrossRef] [PubMed]
- Lytvyn, Y.; Bjornstad, P.; Udell, J.A.; Lovshin, J.A.; Cherney, D.Z.I. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation 2017, 136, 1643–1658. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Rezaie, P.; Gao, H.K.; Kengne, A.P. Effect of Sodium-Glucose Cotransport-2 Inhibitors on Blood Pressure in People With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of 43 Randomized Control Trials With 22 528 Patients. J. Am. Heart Assoc. 2017, 6, e004007. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.L.; Buckley, L.F.; Kelly, M.S.; Bucheit, J.D.; Parod, E.D.; Brown, R.; Carbone, S.; Abbate, A.; Dixon, D.L. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on 24-Hour Ambulatory Blood Pressure: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e005686. [Google Scholar] [CrossRef]
- Li, M.; Yi, T.; Fan, F.; Qiu, L.; Wang, Z.; Weng, H.; Ma, W.; Zhang, Y.; Huo, Y. Effect of sodium-glucose cotransporter-2 inhibitors on blood pressure in patients with heart failure: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2022, 21, 139. [Google Scholar] [CrossRef]
- Adam, C.A.; Anghel, R.; Marcu, D.; Mitu, O.; Roca, M.; Mitu, F. Impact of Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors on Arterial Stiffness and Vascular Aging-What Do We Know So Far? (A Narrative Review). Life 2022, 12, 803. [Google Scholar] [CrossRef]
- Patoulias, D.; Papadopoulos, C.; Kassimis, G.; Fragakis, N.; Vassilikos, V.; Karagiannis, A.; Doumas, M. Effect of sodium-glucose co-transporter-2 inhibitors on arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. Vasc. Med. 2022, 27, 433–439. [Google Scholar] [CrossRef]
- Karalliedde, J.; Fountoulakis, N.; Stathi, D.; Corcillo, A.; Flaquer, M.; Panagiotou, A.; Maltese, G.; Mangelis, A.; Ayis, S.; Gnudi, L. Does Dapagliflozin influence arterial stiffness and levels of circulating anti-aging hormone soluble Klotho in people with type 2 diabetes and kidney disease? Results of a randomized parallel group clinical trial. Front. Cardiovasc. Med. 2022, 9, 992327. [Google Scholar] [CrossRef]
- Requena-Ibáñez, J.A.; Santos-Gallego, C.G.; Rodriguez-Cordero, A.; Vargas-Delgado, A.P.; Mancini, D.; Sartori, S.; Atallah-Lajam, F.; Giannarelli, C.; Macaluso, F.; Lala, A.; et al. Mechanistic Insights of Empagliflozin in Nondiabetic Patients With HfrEF: From the EMPA-TROPISM Study. JACC. Heart Fail. 2021, 9, 578–589. [Google Scholar] [CrossRef]
- Lauritsen, K.M.; Nielsen, B.; Tolbod, L.P.; Johannsen, M.; Hansen, J.; Hansen, T.K.; Wiggers, H.; Møller, N.; Gormsen, L.C.; Søndergaard, E. SGLT2 Inhibition Does Not Affect Myocardial Fatty Acid Oxidation or Uptake, but Reduces Myocardial Glucose Uptake and Blood Flow in Individuals With Type 2 Diabetes: A Randomized Double-Blind, Placebo-Controlled Crossover Trial. Diabetes 2021, 70, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Leccisotti, L.; Cinti, F.; Sorice, G.P.; D’Amario, D.; Lorusso, M.; Guzzardi, M.A.; Mezza, T.; Gugliandolo, S.; Cocchi, C.; Capece, U.; et al. Dapagliflozin improves myocardial flow reserve in patients with type 2 diabetes: The DAPAHEART Trial: A preliminary report. Cardiovasc. Diabetol. 2022, 21, 173. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; San Antonio, R.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Fu, Z.; Jones, P.; Kwee, L.C.; Windsor, S.L.; Ilkayeva, O.; Newgard, C.B.; Margulies, K.B.; Husain, M.; Inzucchi, S.E.; et al. Metabolomic Profiling of the Effects of Dapagliflozin in Heart Failure With Reduced Ejection Fraction: DEFINE-HF. Circulation 2022, 146, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, M.; Schou, M.; Hasbak, P.; Kjær, A.; Wolsk, E.; Zerahn, B.; Wiberg, M.; Brandt-Jacobsen, N.H.; Gæde, P.; Rossing, P.; et al. Effects of Empagliflozin on Myocardial Flow Reserve in Patients With Type 2 Diabetes Mellitus: The SIMPLE Trial. J. Am. Heart Assoc. 2021, 10, e020418. [Google Scholar] [CrossRef]
- Wang, H.; Ding, L.; Tian, L.; Tian, Y.; Liao, L.; Zhao, J. Empagliflozin reduces diffuse myocardial fibrosis by extracellular volume mapping: A meta-analysis of clinical studies. Front. Endocrinol. 2022, 13, 917761. [Google Scholar] [CrossRef]
- Fu, H.; Nie, S.; Luo, P.; Ruan, Y.; Zhang, Z.; Miao, H.; Li, X.; Wen, S.; Bai, R. Galectin-3 and acute heart failure: Genetic polymorphisms, plasma level, myocardial fibrosis and 1-year outcomes. Biomark. Med. 2020, 14, 943–954. [Google Scholar] [CrossRef]
- Benza, R.L.; Tallaj, J.A.; Felker, G.M.; Zabel, K.M.; Kao, W.; Bourge, R.C.; Pearce, D.; Leimberger, J.D.; Borzak, S.; O’connor, C.M.; et al. The impact of arrhythmias in acute heart failure. J. Card. Fail. 2004, 10, 279–284. [Google Scholar] [CrossRef]
- Li, W.J.; Chen, X.Q.; Xu, L.L.; Li, Y.Q.; Luo, B.H. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: A systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc. Diabetol. 2020, 19, 130. [Google Scholar] [CrossRef]
- Sfairopoulos, D.; Zhang, N.; Wang, Y.; Chen, Z.; Letsas, K.P.; Tse, G.; Li, G.; Lip, G.; Liu, T.; Korantzopoulos, P. Association between sodium-glucose cotransporter-2 inhibitors and risk of sudden cardiac death or ventricular arrhythmias: A meta-analysis of randomized controlled trials. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2022, 24, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A.; Gragnano, F.; Paolisso, P.; Bergamaschi, L.; Gallinoro, E.; Sardu, C.; Mileva, N.; Foà, A.; Armillotta, M.; Sansonetti, A.; et al. In-hospital arrhythmic burden reduction in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: Insights from the SGLT2-I AMI PROTECT study. Front. Cardiovasc. Med. 2022, 9, 1012220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, T.; Huang, Y.; Zhan, Q.; Huang, X.; Zeng, Q.; Xu, D. The top tertile of hematocrit change during hospitalization is associated with lower risk of mortality in acute heart failure patients. BMC Cardiovasc. Disord. 2017, 17, 235. [Google Scholar] [CrossRef] [PubMed]
- Zulastri, M.; Hafidz, M.I.; Ismail, M.D.; Zuhdi, A. Hematocrit change as a predictor of readmission for decompensated heart failure: A retrospective single centre study. Rev. Cardiovasc. Med. 2021, 22, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Tapoi, L.; Ureche, C.; Tanriover, C.; Cevik, E.; Demiray, A.; Afsar, B.; Cherney, D.; Covic, A. Effect of sodium-glucose cotransporter 2 inhibitors on hemoglobin and hematocrit levels in type 2 diabetes: A systematic review and meta-analysis. Int. Urol. Nephrol. 2022, 54, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Guo, K.; Deng, J.; Zhong, Y.; Yang, L. Effects of SGLT2 inhibitors on haematocrit and haemoglobin levels and the associated cardiorenal benefits in T2DM patients: A meta-analysis. J. Cell. Mol. Med. 2022, 26, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Abuaysheh, S.; Hejna, J.; Green, K.; Batra, M.; Makdissi, A.; Chaudhuri, A.; Dandona, P. Dapagliflozin Suppresses Hepcidin And Increases Erythropoiesis. J. Clin. Endocrinol. Metab. 2020, 105, dgaa057. [Google Scholar] [CrossRef]
- Schulze, P.C.; Bogoviku, J.; Westphal, J.; Aftanski, P.; Haertel, F.; Grund, S.; von Haehling, S.; Schumacher, U.; Möbius-Winkler, S.; Busch, M. Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients With Acute Decompensated Heart Failure (EMPAG-HF). Circulation 2022, 146, 289–298. [Google Scholar] [CrossRef]
- Charaya, K.; Shchekochikhin, D.; Andreev, D.; Dyachuk, I.; Tarasenko, S.; Poltavskaya, M.; Mesitskaya, D.; Bogdanova, A.; Ananicheva, N.; Kuzub, A. Impact of dapagliflozin treatment on renal function and diuretics use in acute heart failure: A pilot study. Open Heart 2022, 9, e001936. [Google Scholar] [CrossRef]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Yamada, T.; Watanabe, T.; Morita, T.; Furukawa, Y.; Kawasaki, M.; Kikuchi, A.; Kawai, T.; Seo, M.; Abe, M.; et al. Effect of Empagliflozin as an Add-On Therapy on Decongestion and Renal Function in Patients With Diabetes Hospitalized for Acute Decompensated Heart Failure: A Prospective Randomized Controlled Study. Circulation. Heart Fail. 2021, 14, e007048. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.; Elvan, A.; van Eck, J.; Heerspink, H.; Voors, A.A. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur. J. Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Pérez-Belmonte, L.M.; Sanz-Cánovas, J.; Millán-Gómez, M.; Osuna-Sánchez, J.; López-Sampalo, A.; Ricci, M.; Jiménez-Navarro, M.; López-Carmona, M.D.; Bernal-López, M.R.; Barbancho, M.A.; et al. Clinical benefits of empagliflozin in very old patients with type 2 diabetes hospitalized for acute heart failure. J. Am. Geriatr. Soc. 2022, 70, 862–871. [Google Scholar] [CrossRef]
- López-Vilella, R.; Trenado, V.D.; Cervera, B.G.; Sánchez-Lázaro, I.; Bonet, L.A. Sodium-glucose cotransporter 2 inhibitors reduce cardiovascular events in acute heart failure. A real-world analysis. Eur. J. Intern. Med. 2022, 104, 128–130. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Docherty, K.F.; Claggett, B.L.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.; Martinez, F.; et al. SGLT-2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five andomized controlled trials. Lancet 2022, 400, 757–767. [Google Scholar] [CrossRef]
- Ahmad, Y.; Madhavan, M.V.; Stone, G.W.; Francis, D.P.; Makkar, R.; Bhatt, D.L.; Howard, J.P. Sodium-glucose cotransporter 2 inhibitors in patients with heart failure: A systematic review and meta-analysis of randomized trials. Eur. Heart Journal. Qual. Care Clin. Outcomes 2022, 8, 383–390. [Google Scholar] [CrossRef]
- Shrestha, D.B.; Budhathoki, P.; Sedhai, Y.R.; Karki, P.; Gurung, S.; Raut, S.; Damonte, J.I.; Del Buono, M.G.; Mojadidi, M.K.; Elgendy, I.Y.; et al. Sodium-glucose cotransporter-2 Inhibitors in Heart Failure: An Updated Systematic Review and Meta-analysis of 13 Randomized Clinical Trials Including 14,618 Patients With Heart Failure. J. Cardiovasc. Pharmacol. 2021, 78, 501–514. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, W.; Huang, W.; Wang, L.; Huang, S. Benefit of sodium-glucose cotransporter-2 inhibitors on survival outcome is related to the type of heart failure: A meta-analysis. Diabetes Res. Clin. Pract. 2022, 187, 109871. [Google Scholar] [CrossRef]
- Clemenza, F.; Citarrella, R.; Patti, A.; Rizzo, M. Obesity and HfpEF. J. Clin. Med. 2022, 11, 3858. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Cianflone, D.; Maranta, F. Treatment of diabetes and heart failure: Facts and hopes. Int. J. Cardiol. 2022, 359, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Al-Busaidi, N.; Rizvi, A.A. Dapagliflozin therapy in type-2 diabetes: Current knowledge and future perspectives. Expert Opin. Pharmacother. 2015, 16, 281–284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patoulias, D.; Papadopoulos, C.; Zografou, I.; Doumas, M. Acute heart failure, type 2 diabetes and loop diuretic use: Any adjunct role for sodium-glucose cotransporter-2 inhibitors? J. Cardiovasc. Med. Hagerstown Md. 2020, 21, 343. [Google Scholar] [CrossRef]
- Omar, M.; Jensen, J.; Frederiksen, P.H.; Kistorp, C.; Videbæk, L.; Poulsen, M.K.; Möller, S.; Ali, M.; Gustafsson, F.; Køber, L.; et al. Effect of Empagliflozin on Hemodynamics in Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2020, 76, 2740–2751. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Stein, P.K. Heart rate variability in risk stratification of cardiac patients. Prog. Cardiovasc. Dis. 2013, 56, 153–159. [Google Scholar] [CrossRef]
- Patoulias, D.; Katsimardou, A.; Fragakis, N.; Papadopoulos, C.; Doumas, M. Effect of SGLT-2 inhibitors on cardiac autonomic function in type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Acta Diabetol. 2022. Advance online publication. [Google Scholar] [CrossRef]
- Gronda, E.; Vanoli, E.; Iacoviello, M.; Caldarola, P.; Gabrielli, D.; Tavazzi, L. The Benefit of Sodium-Glucose Co-Transporter Inhibition in Heart Failure: The Role of the Kidney. Int. J. Mol. Sci. 2022, 23, 11987. [Google Scholar] [CrossRef]
- Zanchi, A.; Burnier, M.; Muller, M.E.; Ghajarzadeh-Wurzner, A.; Maillard, M.; Loncle, N.; Milani, B.; Dufour, N.; Bonny, O.; Pruijm, M. Acute and Chronic Effects of SGLT2 Inhibitor Empagliflozin on Renal Oxygenation and Blood Pressure Control in Nondiabetic Normotensive Subjects: A Randomized, Placebo-Controlled Trial. J. Am. Heart Assoc. 2020, 9, e016173. [Google Scholar] [CrossRef]
- Miftode, R.S.; Petriș, A.O.; Onofrei Aursulesei, V.; Cianga, C.; Costache, I.I.; Mitu, O.; Miftode, I.L.; Șerban, I.L. The Novel Perspectives Opened by ST2 in the Pandemic: A Review of Its Role in the Diagnosis and Prognosis of Patients with Heart Failure and COVID-19. Diagnostics 2021, 11, 175. [Google Scholar] [CrossRef]
- Sun, Y.; Pavey, H.; Wilkinson, I.; Fisk, M. Role of the IL-33/ST2 axis in cardiovascular disease: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0259026. [Google Scholar] [CrossRef] [PubMed]
- Gürgöze, M.T.; van Vark, L.C.; Baart, S.J.; Kardys, I.; Akkerhuis, K.M.; Manintveld, O.C.; Postmus, D.; Hillege, H.L.; Lesman-Leegte, I.; Asselbergs, F.W.; et al. Multimarker Analysis of Serially Measured GDF-15, NT-proBNP, ST2, GAL-3, cTnI, Creatinine, and Prognosis in Acute Heart Failure. Circulation. Heart Fail. 2022, e009526, Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Murthy, A.C.; Lee, Y.H.; Muscelli, E.; Weiss, S.; Ostroff, R.M.; Sattar, N.; Williams, S.A.; Ganz, P. Mechanisms of Sodium-Glucose Cotransporter 2 Inhibition: Insights From Large-Scale Proteomics. Diabetes Care 2020, 43, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Li, J.; Neuen, B.L.; Arnott, C.; Neal, B.; Perkovic, V.; Mahaffey, K.W.; Shaw, W.; Canovatchel, W.; Hansen, M.K.; et al. Association Between Circulating GDF-15 and Cardio-Renal Outcomes and Effect of Canagliflozin: Results From the CANVAS Trial. J. Am. Heart Assoc. 2021, 10, e021661. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.; Jensen, J.; Kistorp, C.; Højlund, K.; Videbæk, L.; Tuxen, C.; Larsen, J.H.; Andersen, C.F.; Gustafsson, F.; Køber, L.; et al. The effect of empagliflozin on growth differentiation factor 15 in patients with heart failure: A randomized controlled trial (Empire HF Biomarker). Cardiovasc. Diabetol. 2022, 21, 34. [Google Scholar] [CrossRef]

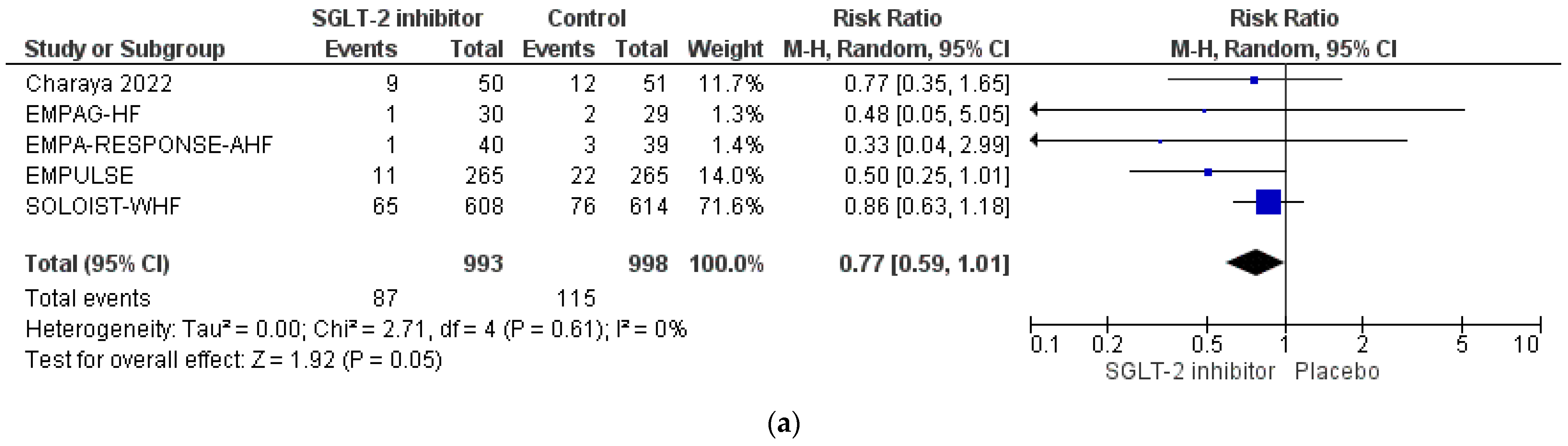
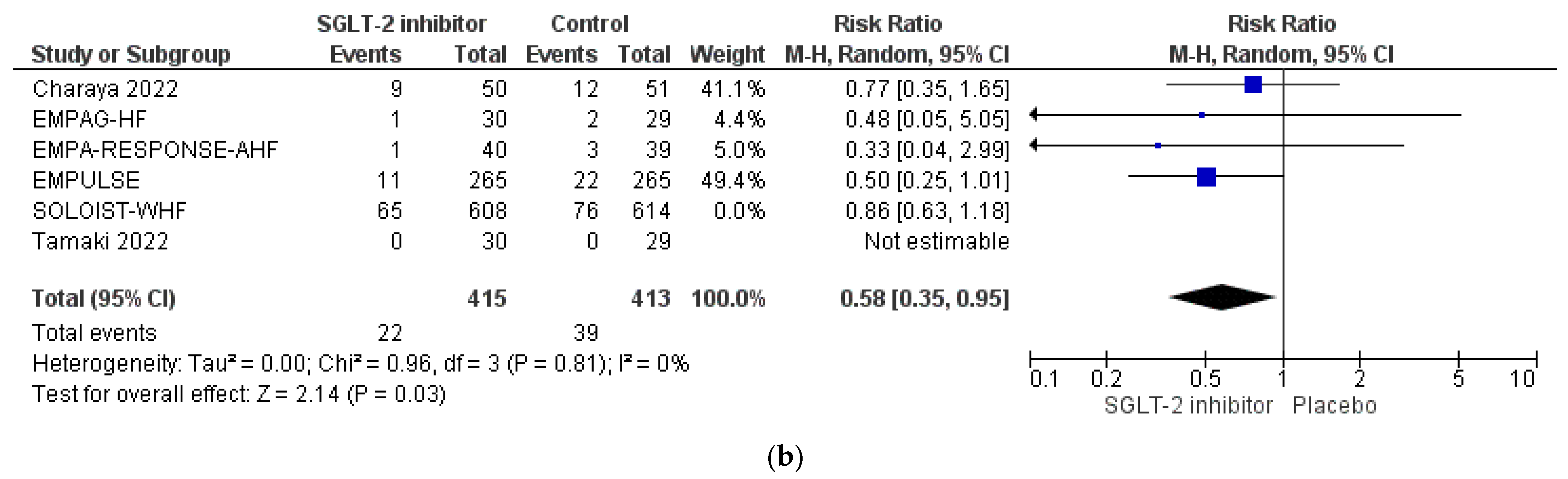
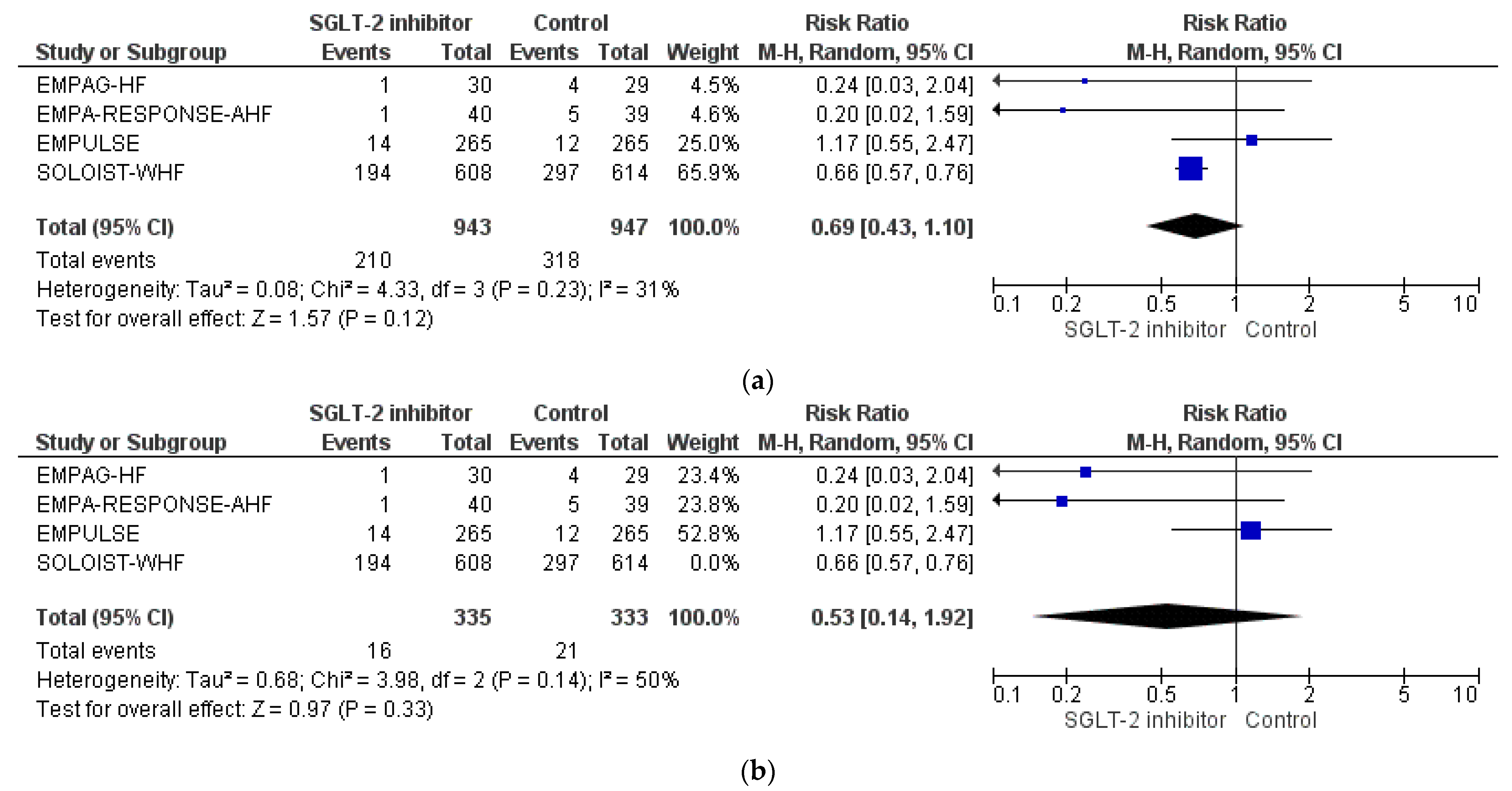
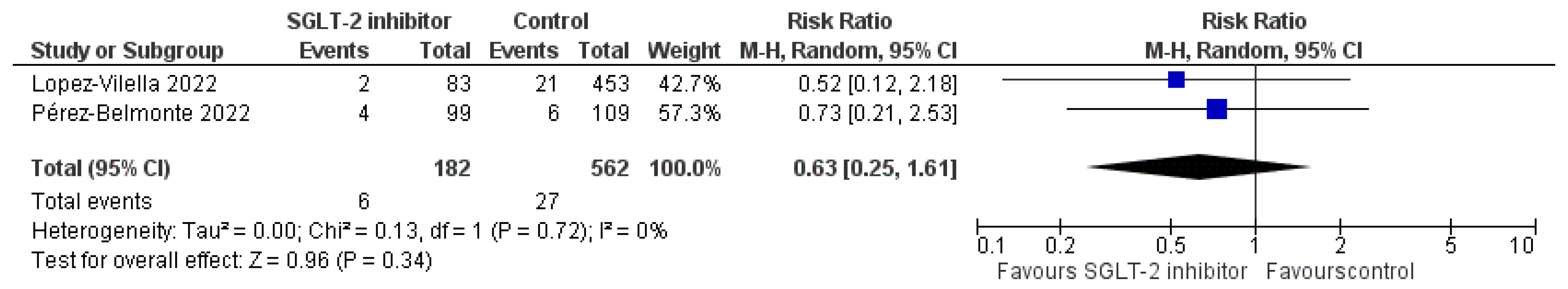

| EMPAG-HF [52] | Charaya et al. [53] | EMPULSE [54] | Tamaki et al. [55] | EMPA-RESPONSE-AHF [56] | Perez-Belmonte et al. [58] | Lopez-Villela et al. [59] | |
|---|---|---|---|---|---|---|---|
| All-cause mortality | - | - | - | Not reported | - | - | - |
| Worsening HF | - | Not reported | - | Not reported | - | - | + |
| Increase in urine output | + | Not reported | Not reported | + | + | + | Not reported |
| Weight loss | - | + | Not reported | - | - | Not reported | Not reported |
| Reduction in NT-proBNP levels | + | Not reported | + | + | - | + | Not reported |
| Hemoconcentration | Not reported | Not reported | Not reported | + | Not reported | Not reported | Not reported |
| Improvement in diuretic response/reduction in cumulative diuretic dosage | + | + | + | - | - | - | Not reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patoulias, D.; Fragakis, N.; Rizzo, M. The Therapeutic Role of SGLT-2 Inhibitors in Acute Heart Failure: From Pathophysiologic Mechanisms to Clinical Evidence with Pooled Analysis of Relevant Studies across Safety and Efficacy Endpoints of Interest. Life 2022, 12, 2062. https://doi.org/10.3390/life12122062
Patoulias D, Fragakis N, Rizzo M. The Therapeutic Role of SGLT-2 Inhibitors in Acute Heart Failure: From Pathophysiologic Mechanisms to Clinical Evidence with Pooled Analysis of Relevant Studies across Safety and Efficacy Endpoints of Interest. Life. 2022; 12(12):2062. https://doi.org/10.3390/life12122062
Chicago/Turabian StylePatoulias, Dimitrios, Nikolaos Fragakis, and Manfredi Rizzo. 2022. "The Therapeutic Role of SGLT-2 Inhibitors in Acute Heart Failure: From Pathophysiologic Mechanisms to Clinical Evidence with Pooled Analysis of Relevant Studies across Safety and Efficacy Endpoints of Interest" Life 12, no. 12: 2062. https://doi.org/10.3390/life12122062
APA StylePatoulias, D., Fragakis, N., & Rizzo, M. (2022). The Therapeutic Role of SGLT-2 Inhibitors in Acute Heart Failure: From Pathophysiologic Mechanisms to Clinical Evidence with Pooled Analysis of Relevant Studies across Safety and Efficacy Endpoints of Interest. Life, 12(12), 2062. https://doi.org/10.3390/life12122062







