Down-Regulation of C1GALT1 Enhances the Progression of Cholangiocarcinoma through Activation of AKT/ERK Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Gene Expression Analysis from the Database
2.3. Human CCA Tissues and Cell Lines
2.4. RNA Extraction and Quantitative Real-Time PCR (qPCR)
2.5. Immunohistochemistry
2.6. C1GALT1 Knockdown
2.7. Cell Viability
2.8. 5-Fluorouracil (5-FU) Treatment
2.9. Protein Collection and Western Blot Analysis
2.10. Lectin-Cytochemistry
2.11. Statistical Analysis
3. Results
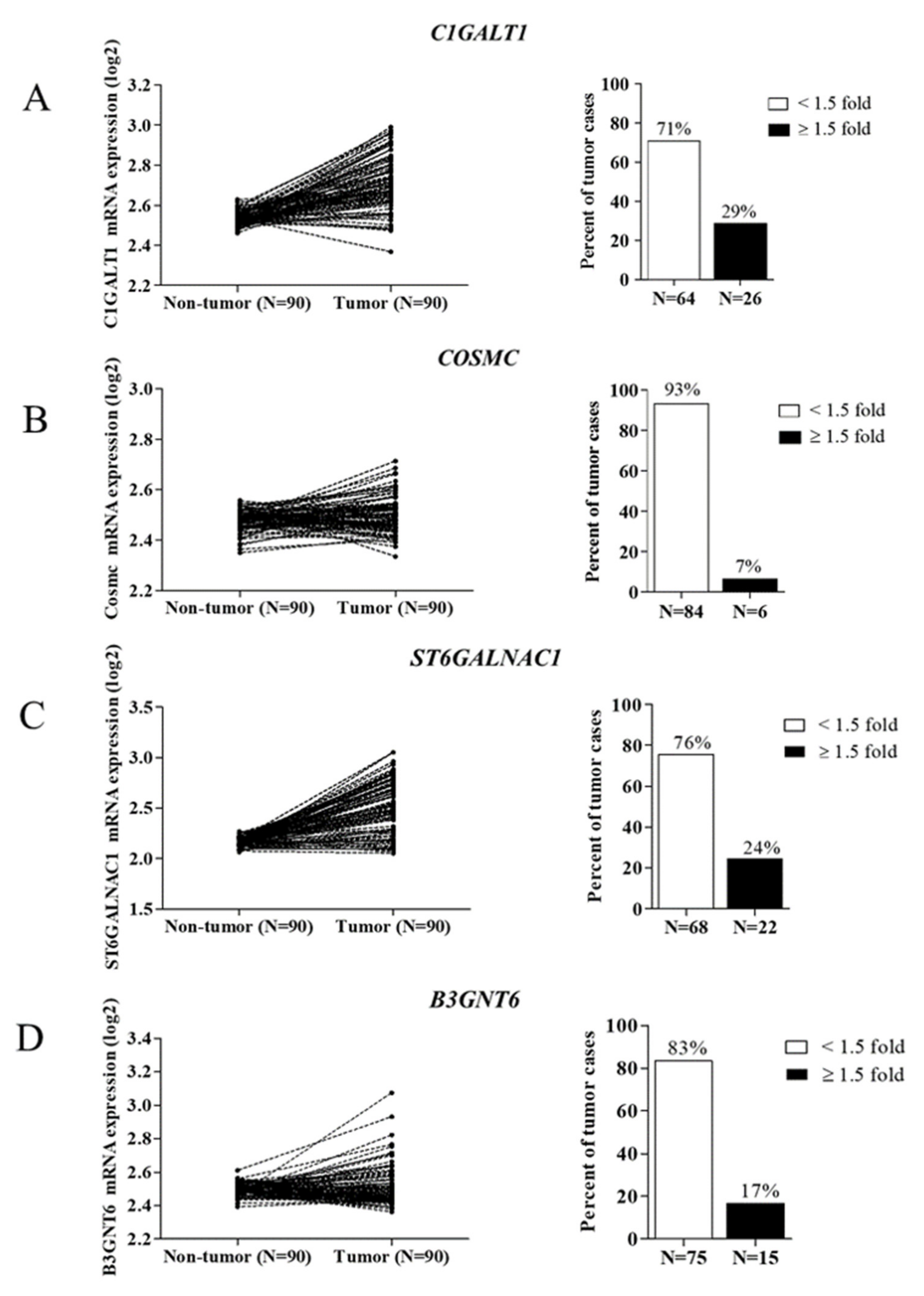
3.1. Expression of O-Linked Glycosyltransferases in CCA Tissues
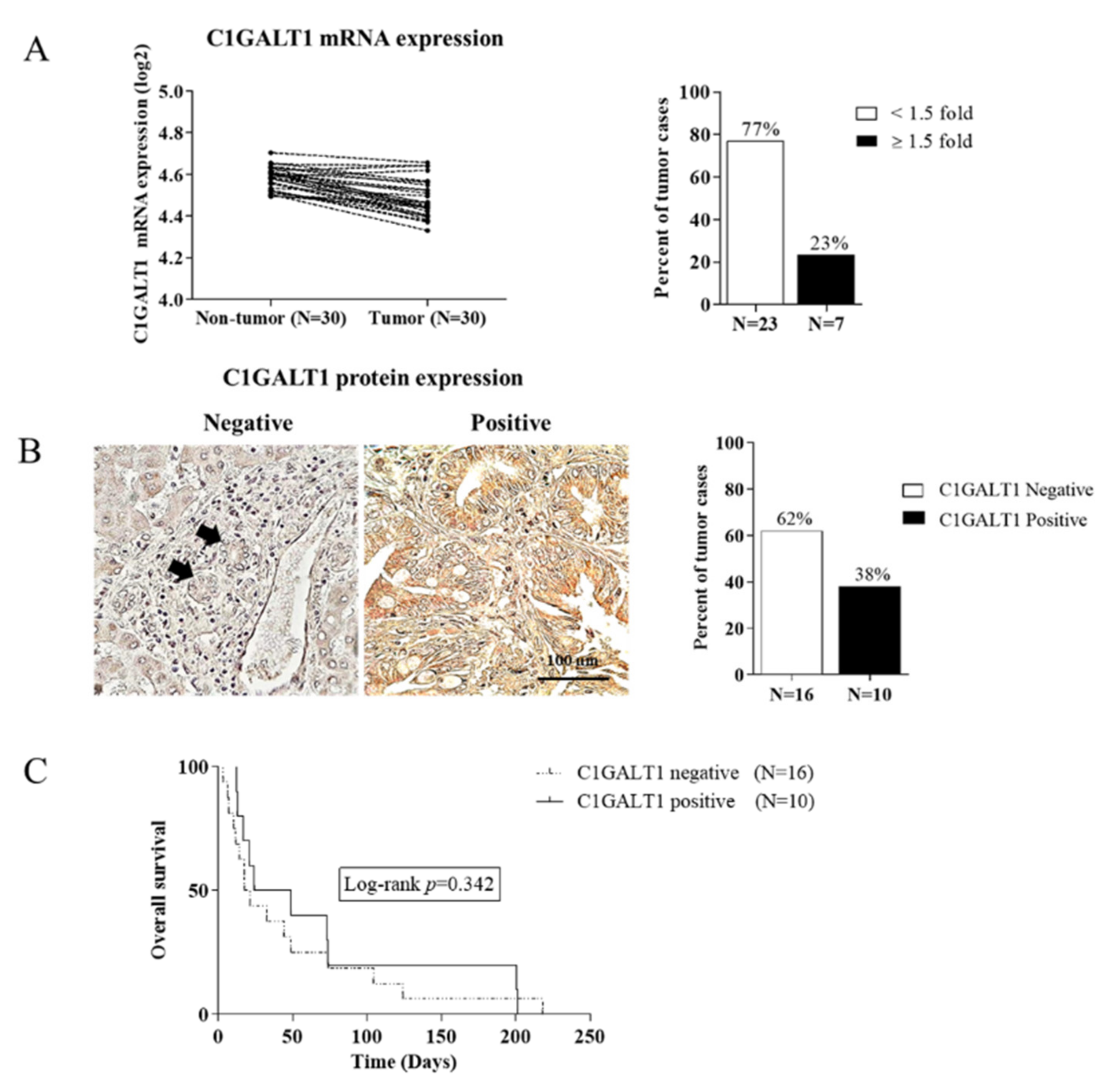
3.2. Down-Regulation of C1GALT1 in CCA Tissues
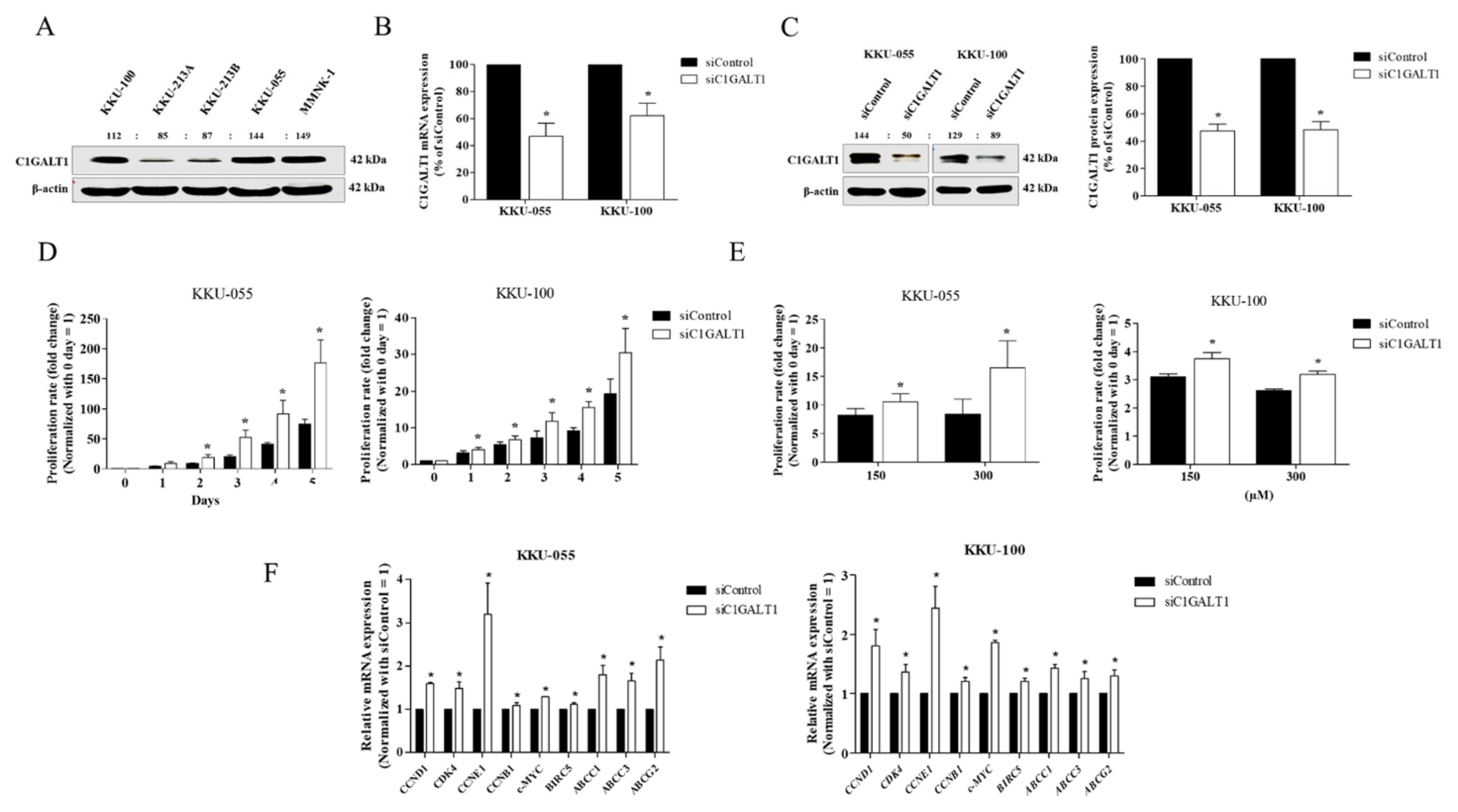
3.3. Silencing C1GALT1 Promotes CCA Cell Proliferation and 5-Fluorouracil (5-FU) Drug Resistance
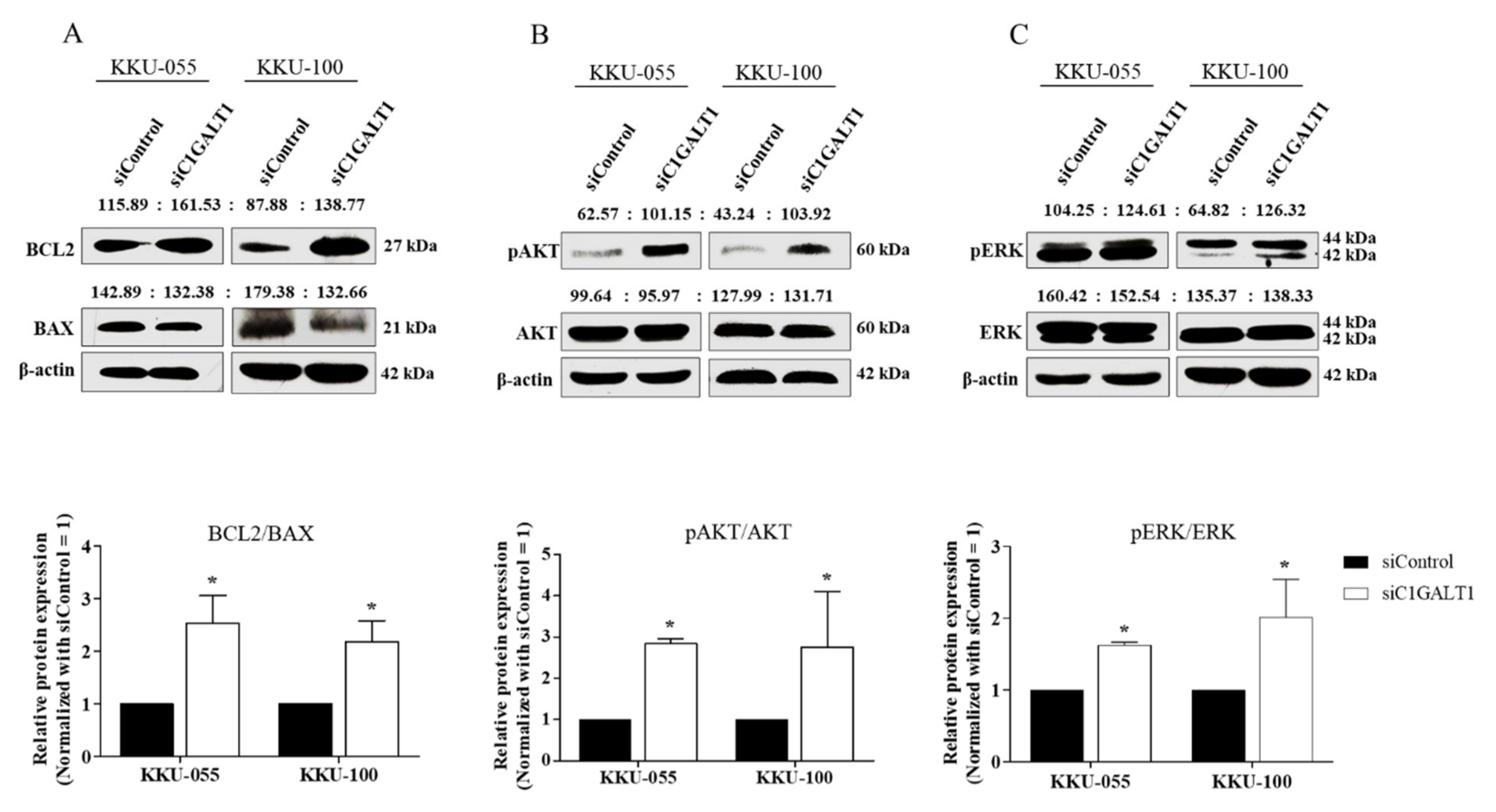
3.4. Silencing C1GALT1 Increases CCA Progression via the Activation of the AKT/ERK Signaling Pathway
3.5. Silencing C1GALT1 Is Associated with the Truncation of Core 1-Derived Mucin-Type O-Glycans
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Detarya, M.; Sawanyawisuth, K.; Aphivatanasiri, C.; Chuangchaiya, S.; Saranaruk, P.; Sukprasert, L.; Silsirivanit, A.; Araki, N.; Wongkham, S.; Wongkham, C. The O-GalNAcylating enzyme GALNT5 mediates carcinogenesis and progression of cholangiocarcinoma via activation of AKT/ERK signaling. Glycobiology 2020, 30, 312–324. [Google Scholar] [CrossRef]
- Silsirivanit, A. Glycans: Potential therapeutic targets for cholangiocarcinoma and their therapeutic and diagnostic implications. Expert Opin. Ther. Targets 2021, 25, 1–4. [Google Scholar] [CrossRef]
- Boonla, C.; Sripa, B.; Thuwajit, P.; Cha-On, U.; Puapairoj, A.; Miwa, M.; Wongkham, S. MUC1 and MUC5AC mucin expression in liver fluke-associated intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2005, 11, 4939–4946. [Google Scholar] [CrossRef] [PubMed]
- Mall, A.S.; Tyler, M.G.; Ho, S.B.; Krige, J.E.; Kahn, D.; Spearman, W.; Myer, L.; Govender, D. The expression of MUC mucin in cholangiocarcinoma. Pathol. Res. Pract. 2010, 206, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Talabnin, K.; Talabnin, C.; Ishihara, M.; Azadi, P.; Wongkham, S.; Sripa, B. Differential Expression of O-glycoprotein Glycans in Cholangiocarcinoma Cell Lines. Asian Pac. J. Cancer Prev. 2016, 17, 691–695. [Google Scholar] [CrossRef] [Green Version]
- Talabnin, K.; Talabnin, C.; Khiaowichit, J.; Sutatum, N.; Asavaritikrai, P.; Suksaweang, S.; Tongtawee, T.; Ishihara, M.; Azadi, P.; Sripa, B. High expression of tissue O-linked glycans is associated with a malignant phenotype of cholangiocarcinoma. J. Int. Med. Res. 2021, 49, 300060520976864. [Google Scholar] [CrossRef]
- Gupta, R.; Leon, F.; Rauth, S.; Batra, S.K.; Ponnusamy, M.P. A Systematic Review on the Implications of O-linked Glycan Branching and Truncating Enzymes on Cancer Progression and Metastasis. Cells 2020, 9, 446. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.H.; Huang, M.J.; Chen, C.H.; Shyu, M.K.; Huang, J.; Hung, J.S.; Huang, C.S.; Huang, M.C. Up-regulation of C1GALT1 promotes breast cancer cell growth through MUC1-C signaling pathway. Oncotarget 2015, 6, 6123–6135. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Jiang, Y.; Liu, J.; Liu, Z.; Gao, T.; An, G.; Wen, T. T-Synthase Deficiency Enhances Oncogenic Features in Human Colorectal Cancer Cells via Activation of Epithelial-Mesenchymal Transition. BioMed Res. Int. 2018, 2018, 9532389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.C.; Chen, S.T.; Kuo, T.C.; Lin, T.C.; Lin, M.C.; Huang, J.; Hung, J.S.; Hsu, C.L.; Juan, H.F.; Lee, P.H.; et al. C1GALT1 is associated with poor survival and promotes soluble Ephrin A1-mediated cell migration through activation of EPHA2 in gastric cancer. Oncogene 2020, 39, 2724–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.C.; Chien, P.H.; Wu, H.Y.; Chen, S.T.; Juan, H.F.; Lou, P.J.; Huang, M.C. C1GALT1 predicts poor prognosis and is a potential therapeutic target in head and neck cancer. Oncogene 2018, 37, 5780–5793. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Aryal, R.P.; Kudelka, M.R.; Wang, Y.; Cummings, R.D. The Cosmc connection to the Tn antigen in cancer. Cancer Biomark. 2014, 14, 63–81. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, P.; Dabelsteen, S.; Madsen, F.B.; Francavilla, C.; Kopp, K.L.; Steentoft, C.; Vakhrushev, S.Y.; Olsen, J.V.; Hansen, L.; Bennett, E.P.; et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl. Acad. Sci. USA 2014, 111, E4066–E4075. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ju, T.; Ding, X.; Xia, B.; Wang, W.; Xia, L.; He, M.; Cummings, R.D. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc. Natl. Acad. Sci. USA 2010, 107, 9228–9233. [Google Scholar] [CrossRef] [Green Version]
- Chugh, S.; Barkeer, S.; Rachagani, S.; Nimmakayala, R.K.; Perumal, N.; Pothuraju, R.; Atri, P.; Mahapatra, S.; Thapa, I.; Talmon, G.A.; et al. Disruption of C1galt1 Gene Promotes Development and Metastasis of Pancreatic Adenocarcinomas in Mice. Gastroenterology 2018, 155, 1608–1624. [Google Scholar] [CrossRef]
- Liu, F.; Fu, J.; Bergstrom, K.; Shan, X.; McDaniel, J.M.; McGee, S.; Bai, X.; Chen, W.; Xia, L. Core 1-derived mucin-type O-glycosylation protects against spontaneous gastritis and gastric cancer. J. Exp. Med. 2020, 217, e20182325. [Google Scholar] [CrossRef] [Green Version]
- Sagar, S.; Leiphrakpam, P.D.; Thomas, D.; McAndrews, K.L.; Caffrey, T.C.; Swanson, B.J.; Clausen, H.; Wandall, H.H.; Hollingsworth, M.A.; Radhakrishnan, P. MUC4 enhances gemcitabine resistance and malignant behaviour in pancreatic cancer cells expressing cancer-associated short O-glycans. Cancer Lett. 2021, 503, 91–102. [Google Scholar] [CrossRef]
- Chou, C.H.; Huang, M.J.; Liao, Y.Y.; Chen, C.H.; Huang, M.C. C1GALT1 Seems to Promote In Vitro Disease Progression in Ovarian Cancer. Int. J. Gynecol. Cancer 2017, 27, 863–871. [Google Scholar] [CrossRef]
- Wu, Y.M.; Liu, C.H.; Huang, M.J.; Lai, H.S.; Lee, P.H.; Hu, R.H.; Huang, M.C. C1GALT1 enhances proliferation of hepatocellular carcinoma cells via modulating MET glycosylation and dimerization. Cancer Res. 2013, 73, 5580–5590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, J.S.; Huang, J.; Lin, Y.C.; Huang, M.J.; Lee, P.H.; Lai, H.S.; Liang, J.T.; Huang, M.C. C1GALT1 overexpression promotes the invasive behavior of colon cancer cells through modifying O-glycosylation of FGFR2. Oncotarget 2014, 5, 2096–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.H.; Hu, R.H.; Huang, M.J.; Lai, I.R.; Chen, C.H.; Lai, H.S.; Wu, Y.M.; Huang, M.C. C1GALT1 promotes invasive phenotypes of hepatocellular carcinoma cells by modulating integrin β1 glycosylation and activity. PLoS ONE 2014, 9, e94995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Deng, X.; Qiu, L.; Peng, F.; Geng, S.; Shen, L.; Luo, Z. Knockdown of C1GalT1 inhibits radioresistance of human esophageal cancer cells through modifying β1-integrin glycosylation. J. Cancer 2018, 9, 2666–2677. [Google Scholar] [CrossRef]
- Sripa, B.; Seubwai, W.; Vaeteewoottacharn, K.; Sawanyawisuth, K.; Silsirivanit, A.; Kaewkong, W.; Muisuk, K.; Dana, P.; Phoomak, C.; Lert-Itthiporn, W.; et al. Functional and genetic characterization of three cell lines derived from a single tumor of an Opisthorchis viverrini-associated cholangiocarcinoma patient. Hum. Cell 2020, 33, 695–708. [Google Scholar] [CrossRef]
- Talabnin, C.; Talabnin, K.; Wongkham, S. Enhancement of piperlongumine chemosensitivity by silencing heme oxygenase-1 expression in cholangiocarcinoma cell lines. Oncol. Lett. 2020, 20, 2483–2492. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Talabnin, C.; Janthavon, P.; Thongsom, S.; Suginta, W.; Talabnin, K.; Wongkham, S. Ring finger protein 43 expression is associated with genetic alteration status and poor prognosis among patients with intrahepatic cholangiocarcinoma. Hum. Pathol. 2016, 52, 47–54. [Google Scholar] [CrossRef]
- Munkley, J.; Elliott, D.J. Hallmarks of glycosylation in cancer. Oncotarget 2016, 7, 35478–35489. [Google Scholar] [CrossRef] [Green Version]
- Inoue, M.; Ogawa, H.; Tanizawa, O.; Kobayashi, Y.; Tsujimoto, M.; Tsujimura, T. Immunodetection of sialyl-Tn antigen in normal, hyperplastic and cancerous tissues of the uterine endometrium. Virchows Arch. A Pathol. Anat. Histopathol. 1991, 418, 157–162. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Yuan, M.; Montgomery, C.K.; Kjeldsen, T.; Takahashi, H.K.; Bigbee, W.L.; Kim, Y.S. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989, 49, 197–204. [Google Scholar]
- Terasawa, K.; Furumoto, H.; Kamada, M.; Aono, T. Expression of Tn and sialyl-Tn antigens in the neoplastic transformation of uterine cervical epithelial cells. Cancer Res. 1996, 56, 2229–2232. [Google Scholar] [PubMed]
- Tsuchiya, A.; Kanno, M.; Kawaguchi, T.; Endo, Y.; Zhang, G.J.; Ohtake, T.; Kimijima, I.I. Prognostic Relevance of Tn Expression in Breast Cancer. Breast Cancer 1999, 6, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhao, H.; Wang, Y.; Cai, H.; Xiao, Y.; Zeng, Y.; Chen, H. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. HLA 2016, 88, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Barrow, H.; Tam, B.; Duckworth, C.A.; Rhodes, J.M.; Yu, L.G. Suppression of core 1 Gal-transferase is associated with reduction of TF and reciprocal increase of Tn, sialyl-Tn and Core 3 glycans in human colon cancer cells. PLoS ONE 2013, 8, e59792. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| C1GALT1 | 5′-GGG AAT CTG GGC GGC A-3′ | 5′-GGG ACT GGT GAC CTT TGC TT-3′ |
| COSMC | 5′-AAC GTG AGA GGA AAC CCG TG-3′ | 5′-AAA GCA TTT TTC CCG CGT CT-3′ |
| B3GNT6 | 5′-TCA ACC TCA CGC TCA AGC AC-3′ | 5′-CAG GAA GCG GAC TAC GTT GG-3′ |
| ST6GALNAC1 | 5′-CAG AGG CAC AAT CAT GGA AG-3′ | 5′-GCT GAC TTT TGG GAA TGA GC-3′ |
| C-Myc | 5′-CTG CTG TGG ACC CTA CTG-3′ | 5′-AAC TGC GTC TCT GCC AGG AC-3′ |
| BIRC5 | 5′-TGA GGA GAC ACC GCC CAC-3′ | 5′-CAA CAT CGA TTT CTT CCT CAT CTT C-3′ |
| ABCC1 | 5′-CTG GGC TTA TTT CGG ATC AA-3′ | 5′-TGA ATG GGT CCA GGT TCA TT-3′ |
| ABCC3 | 5′-CCT GCT CTC CTT CAT CAA TC-3′ | 5′-ATG TAG TGG TAA TAG TGT TGT AAG-3′ |
| ABCG2 | 5′-CAC CTT ATT GGC CTC AGG AA-3′ | 5′-CCT GCT TGG AAG GCT CTA TG-3′ |
| CCND1 | 5′-CCA CTT GAG CTT GTT CAC CA-3′ | 5′-AAC TAC CTG GAC CGC TTC CT-3′ |
| CDK4 | 5′-GTC GGC TTC AGA GTT TCC AC -3′ | 5′-TGC AGT CCA CAT ATG CAA CA-3′ |
| CCNE1 | 5′-GAA ATG GCC AAA ATC GAC AG -3′ | 5′-TCT TTG TCA GGT GTG GGG A -3′ |
| CCNB1 | 5′-GAC AAC TTG AGG AAG AGC AAG C -3′ | 5′-ATG GTC TCC TGC AAC AAC CT -3′ |
| β-actin | 5′- GAT CAG CAA GCA GGA GTA TGA CG -3′ | 5′-AAG GGT GTA ACG CAA CTA AGT CAT AG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khiaowichit, J.; Talabnin, C.; Dechsukhum, C.; Silsirivanit, A.; Talabnin, K. Down-Regulation of C1GALT1 Enhances the Progression of Cholangiocarcinoma through Activation of AKT/ERK Signaling Pathways. Life 2022, 12, 174. https://doi.org/10.3390/life12020174
Khiaowichit J, Talabnin C, Dechsukhum C, Silsirivanit A, Talabnin K. Down-Regulation of C1GALT1 Enhances the Progression of Cholangiocarcinoma through Activation of AKT/ERK Signaling Pathways. Life. 2022; 12(2):174. https://doi.org/10.3390/life12020174
Chicago/Turabian StyleKhiaowichit, Juthamas, Chutima Talabnin, Chavaboon Dechsukhum, Atit Silsirivanit, and Krajang Talabnin. 2022. "Down-Regulation of C1GALT1 Enhances the Progression of Cholangiocarcinoma through Activation of AKT/ERK Signaling Pathways" Life 12, no. 2: 174. https://doi.org/10.3390/life12020174
APA StyleKhiaowichit, J., Talabnin, C., Dechsukhum, C., Silsirivanit, A., & Talabnin, K. (2022). Down-Regulation of C1GALT1 Enhances the Progression of Cholangiocarcinoma through Activation of AKT/ERK Signaling Pathways. Life, 12(2), 174. https://doi.org/10.3390/life12020174






