Dual Effect of Low-Molecular-Weight Bioregulators of Bacterial Origin in Experimental Model of Asthma
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. OVA-Induced Allergic Asthma in Mice
2.3. The Number and Analysis of Cells in BAL
2.4. Collecting Blood
2.5. ELISA
2.6. Histology
2.7. Statistical Analysis
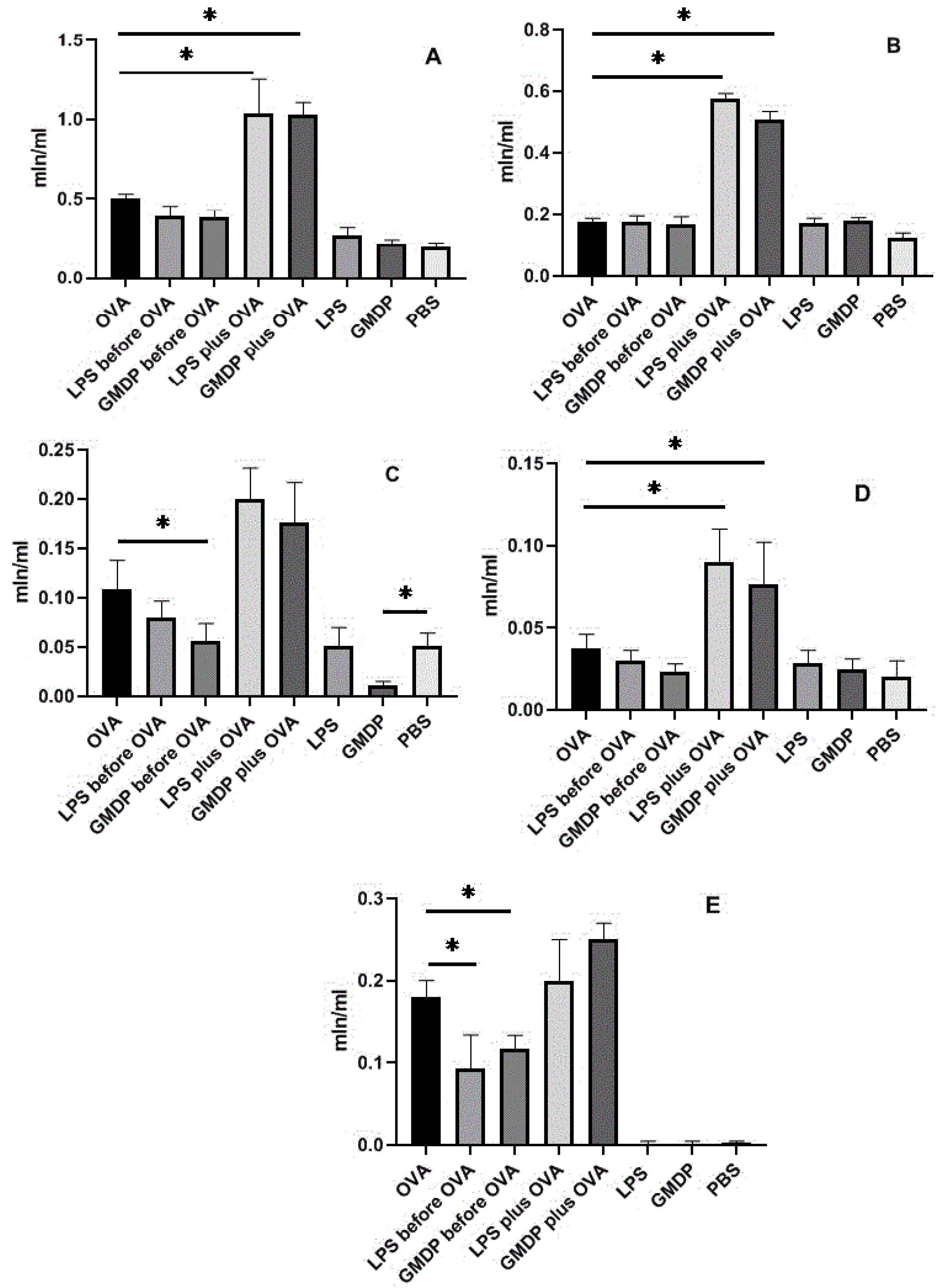
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. Available online: https://www.thelancet.com/gbd/summaries (accessed on 12 December 2021). [CrossRef]
- Mattiuzzi, C.; Lippi, G. Worldwide asthma epidemiology: Insights from the Global Health Data Exchange database. Int. Forum Allergy Rhinol. 2020, 10, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Y.; Yu, C.H.; Yu, W.Y.; Ying, H.Z. Gut-Lung Microbiota in Chronic Pulmonary Diseases: Evolution, Pathogenesis, and Therapeutics. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 9278441. [Google Scholar] [CrossRef] [PubMed]
- Hilty, M.; Burke, C.; Pedro, H.; Cardenas, P.; Bush, A.; Bossley, C.; Davies, J.; Ervine, A.; Poulter, L.; Pachter, L.; et al. Disordered microbial communities in asthmatic airways. PLoS ONE 2010, 5, e8578. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Borisova OYu Kolesnikova, N.V.; Lezhava, N.L.; Kozlov, I.G.; Gudima, G.O. The effect of muramyl peptide on the microbial composition of the microflora of the oral cavity. Immunologiya 2019, 40, 34–40. (In Russian) [Google Scholar] [CrossRef]
- Invernizzi, R.; Lloyd, C.M.; Molyneaux, P.L. Respiratory microbiome and epithelial interactions shape immunity in the lungs. Immunology 2020, 160, 171–182. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Kolesnikova, N.V.; Gudima, G.O.; Lezhava, N.L.; Karaulov, A.V. Dynamics of immunological and microbiological indicators of oral fluid in caries therapy. Immunologiya 2021, 42, 386–394. [Google Scholar] [CrossRef]
- Bassis, C.M.; Erb-Downward, J.R.; Dickson, R.P.; Freeman, C.M.; Schmidt, T.M.; Young, V.B.; Beck, J.M.; Curtis, J.L.; Huffnagle, G.B. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 2015, 6, e00037-15. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Bjorksten, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef]
- Feuille, E.J.; Nowak-Wegrzyn, A. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Pediatrics 2012, 130, S47–S48. [Google Scholar] [CrossRef]
- Russell, S.L.; Gold, M.J.; Willing, B.P.; McNagny, K.M.; Finlay, B.B. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes 2013, 4, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Ananya, F.N.; Ahammed, M.R.; Fahem, M.M.; Kafle, S.; Viswanathan, M.; Desai, D.; Akku, R.; Khan, F.; Hernandez, T.E.; Bala, S.K.; et al. Association of Intestinal Microbial Dysbiosis with Chronic Obstructive Pulmonary Disease. Cureus 2021, 13, e19343. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.L.; Gold, M.J.; Hartmann, M.; Willing, B.P.; Thorson, L.; Wlodarska, M.; Gill, N.; Blanchet, M.; Mohn, W.W.; McNagny, K.M.; et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012, 13, 440–447. [Google Scholar] [CrossRef]
- Arrieta, M.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Logotheti, M.; Agioutantis, P.; Katsaounou, P.; Loutrari, H. Microbiome Research and Multi-Omics Integration for Personalized Medicine in Asthma. J. Pers. Med. 2021, 11, 1299. [Google Scholar] [CrossRef]
- Reijmerink, N.E.; Bottema, R.W.; Kerkhof, M.; Gerritsen, J.; Stelma, F.F.; Thijs, C.; van Schayck, C.P.; Smit, H.A.; Brunekreef, B.; Koppelman, G.H.; et al. TLR-related pathway analysis: Novel gene-gene interactions in the development of asthma and atopy. Allergy 2010, 65, 199–207. [Google Scholar] [CrossRef]
- Guryanova, S.V. Integrated approaches in diagnostics and therapy of allergic diseases. RUDN J. Med. 2018, 22, 75–85. [Google Scholar] [CrossRef][Green Version]
- sbv IMPROVER Project Team; Challenge Best Performers. Enhancement of COPD biological networks using a web-based collaboration interface. F1000Reserch 2015, 4, 32. [Google Scholar] [CrossRef]
- sbv IMPROVER Project Team; Challenge Best Performers. Community-Reviewed Biological Network Models for Toxicology and Drug Discovery Applications. Gene Regul. Syst. Bio. 2016, 10, 51–66. [Google Scholar] [CrossRef]
- Chen, L.W.; Chen, P.H.; Hsu, C.M. Commensal microflora contribute to host defense against Escherichia coli pneumonia through Toll-like receptors. Shock 2011, 36, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.B. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infect. Immun. 2014, 82, 4596–4606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.F.; Yin, K.S. Toll-like receptors: Function and roles in asthma. Chin. Med. J. 2004, 117, 1709–1715. [Google Scholar] [PubMed]
- Lin, K.W.; Li, J.; Finn, P.W. Emerging pathways in asthma: Innate and adaptive interactions. Biochim. Biophys. Acta. 2011, 1810, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Ulevitch, R.J. Therapeutics targeting the innate immune system. Nat. Rev. Immunol. 2004, 4, 512–520. [Google Scholar] [CrossRef]
- Juárez, E.; Carranza, C.; Hernández-Sánchez, F.; León-Contreras, J.C.; Hernández-Pando, R.; Escobedo, D.; Torres, M.; Sada, E. NOD2 enhances the innate response of alveolar macrophages to Mycobacterium tuberculosis in humans. Eur. J. Immunol. 2012, 42, 880–889. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Kufer, T.A.; Banks, D.J.; Philpott, D.J. Innate immune sensing of microbes by Nod proteins. Ann. N. Y. Acad. Sci. 2006, 1072, 19–27. [Google Scholar] [CrossRef]
- Debeuf, N.; Haspeslagh, E.; van Helden, M.; Hammad, H.; Lambrecht, B.N. Mouse Models of Asthma. Curr. Protoc. Mouse Biol. 2016, 6, 169–184. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Z. Establishment of different experimental asthma models in mice. Exp. Ther. Med. 2018, 15, 2492–2498. [Google Scholar] [CrossRef]
- Casaro, M.; Souza, V.R.; Oliveira, F.A.; Ferreira, C.M. OVA-Induced Allergic Airway Inflammation Mouse Model. Methods Mol. Biol. 2019, 1916, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Mehta, A.K.; Magalhaes, J.G.; Ziegler, S.F.; Dong, C.; Philpott, D.J.; Croft, M. Innate signals from Nod2 block respiratory tolerance and program T(H)2-driven allergic inflammation. J. Allergy Clin. Immunol. 2010, 126, 1284–1293.e10. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.R.; Khuman, V.; Beladiya, J.V.; Chaudagar, K.K.; Mehta, A.A. An experimental model of asthma in rats using ovalbumin and lipopolysaccharide allergens. Heliyon 2019, 5, e02864. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Fu, Z.; Liu, B. Lipopolysaccharide Exposure Alleviates Asthma in Mice by Regulating Th1/Th2 and Treg/Th17 Balance. Med. Sci. Monit. 2018, 24, 3220–3229. [Google Scholar] [CrossRef] [PubMed]
- Gorshkova, R.P.; Isakov, V.V.; Nazarenko, E.L.; Ovodov, Y.S.; Guryanova, S.V.; Dmitriev, B.A. Structure of the O-specific polysaccharide of the lipopolysaccharide from Yersinia kristensenii O:25.35. Carbohydr. Res. 1993, 241, 201–208. [Google Scholar] [CrossRef]
- Eldridge, M.W.; Peden, D.B. Allergen provocation augments endotoxin-induced nasal inflammation in subjects with atopic asthma. J. Allergy Clin. Immunol. 2000, 105, 475–481. [Google Scholar] [CrossRef]
- Chen, G.L.; Jiang, H.; Zou, F. Upregulation of transient receptor potential canonical channels contributes to endotoxin-induced pulmonary arterial stenosis. Med. Sci. Monit. 2016, 22, 2679–2684. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, L.; Li, J.; Jin, Y.; He, L. Association of HLA-DRB1 gene polymorphism with risk of asthma: A meta-analysis. Med. Sci. Monit. Basic Res. 2016, 22, 80–86. [Google Scholar] [CrossRef]
- Stein, M.M.; Hrusch, C.L.; Gozdz, J.; Igartua, C.; Pivniouk, V.; Murray, S.E.; Ledford, J.G.; Santos, M.M.d.; Anderson, R.L.; Metwali, N.; et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N. Engl. J. Med. 2016, 375, 411–421. [Google Scholar] [CrossRef]
- Morcos, M.M.; Morcos, W.M.; Ibrahim, M.A.; Shaheen, M.A. Environmental exposure to endotoxin in rural and urban Egyptian school children and its relation to asthma and atopy. Minerva Pediatr. 2011, 63, 19–26. [Google Scholar]
- Holt, P.G. Key factors in the development of asthma: Atopy. Am. J. Respir. Crit. Care Med. 2000, 161, S172–S175. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Macaubas, C.; Prescott, S.L.; Sly, P.D. Microbial stimulation as an aetiologic factor in atopic disease. Allergy 1999, 54, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Gereda, J.E.; Leung, D.Y.; Thatayatikom, A.; Streib, J.E.; Price, M.R.; Klinnert, M.D.; Liu, A.H. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet 2000, 355, 1680–1683. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Nino, G.; Rodriguez-Martinez, C.E.; Gutierrez, M.J. Early Microbial-Immune Interactions and Innate Immune Training of the Respiratory System during Health and Disease. Children 2021, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Deckers, J.; Marsland, B.J.; von Mutius, E. Protection against allergies: Microbes, immunity, and the farming effect. Eur. J. Immunol. 2021, 51, 2387–2398. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Shembade, N.; Harhaj, E.W. Regulation of NF-κB signaling by the A20 deubiquitinase. Cell Mol. Immunol. 2012, 9, 123–130. [Google Scholar] [CrossRef]
- Renner, F.; Schmitz, M.L. Autoregulatory feedback loops terminating the NF-kappaB response. Trends Biochem. Sci. 2009, 34, 128–135. [Google Scholar] [CrossRef]
- Guryanova, S.; Udzhukhu, V.; Kubylinsky, A. Pathogenetic Therapy of Psoriasis by Muramyl Peptide. Front. Immunol. 2019, 10, 1275. [Google Scholar] [CrossRef]
- Meshcheryakova, E.; Guryanova, S.; Makarov, E.; Alekseeva, L.; Andronova, T.; Ivanov, V. Prevention of experimental septic shock by pretreatment of mice with muramyl peptides. Int. Immunopharmacol. 2001, 1, 1857–1865. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Li, X.Q.; Hu, W.P.; Cu, S.C.; Dai, J.J.; Gao, Y.N.; Zhang, Y.T.; Bai, X.Y.; Shi, D.Y. Self-developed NF-κB inhibitor 270 protects against LPS-induced acute kidney injury and lung injury through improving inflammation. Biomed. Pharmacother. 2022, 147, 112615. [Google Scholar] [CrossRef] [PubMed]
- Husebye, H.; Halaas, Ø.; Stenmark, H.; Tunheim, G.; Sandanger, Ø.; Bogen, B.; Brech, A.; Latz, E.; Espevik, T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006, 25, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Normand, S.; Waldschmitt, N.; Neerincx, A.; Martinez-Torres, R.J.; Chauvin, C.; Couturier-Maillard, A.; Boulard, O.; Cobret, L.; Awad, F.; Huot, L.; et al. Proteasomal degradation of NOD2 by NLRP12 in monocytes promotes bacterial tolerance and colonization by enteropathogens. Nat. Commun. 2018, 9, 5338. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, I.V.; Khaidukov, S.V.; Nguyen, T.D.L.; Rongina, A.N.; Guryanova, S.V. Glucosaminylmuramyl dipeptide modulate experimental transformed phenotype of neutrophilic granulocytes of healthy persons. Russ. J. Immunol. 2017, 11, 737–740. (In Russian) [Google Scholar]
- Guryanova, S.V.; Kudryashova, N.A.; Kataeva, A.A.; Orozbekova, B.T.; Kolesnikova, N.V.; Chuchalin, A.G. Novel approaches to increase resistance to acute respiratory infections. RUDN J. Med. 2021, 25, 181–195. [Google Scholar] [CrossRef]
- Kolesnikova, N.V.; Kozlov, I.G.; Guryanova, S.V.; Kokov, E.A.; Andronova, T.M. Clinical and immunological efficiency of muramyl dipeptide in the treatment of atopic diseases. Med. Immunol. 2016, 1, 15–20. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Khaitov, R.M. Strategies for Using Muramyl Peptides—Modulators of Innate Immunity of Bacterial Origin—in Medicine. Front. Immunol. 2021, 12, 607178. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Khaitov, R.M. Glucosaminylmuramyldipeptide—GMDP: Effect on mucosal immunity (on the issue of immunotherapy and immunoprophylaxis). Immunologiya 2020, 41, 174–183. [Google Scholar] [CrossRef]
- Pandey, M.K. Molecular basis for downregulation of C5a-mediated inflammation by IgG1 immune complexes in allergy and asthma. Curr. Allergy Asthma. Rep. 2013, 13, 596–606. [Google Scholar] [CrossRef]
- Matucci, A.; Vultaggio, A.; Maggi, E.; Kasujee, I. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir. Res. 2018, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Kozlov, I.G.; Meshcheryakova, E.A.; Alekseeva, L.G.; Andronova, T.M. Glucosaminylmuramyl Dipeptide Normalizes Th1 / Th2 Balance in Atopic Bronchial Asthma. Immunology 2009, 5, 305–308. (In Russian) [Google Scholar]
- Rechkina, E.A.; Denisova, G.F.; Masalova, O.V.; Lideman, L.F.; Denisov, D.A.; Lesnova, E.I.; Ataullakhanov, R.I.; Gur’ianova, S.V.; Kushch, A.A. Epitope mapping of antigenic determinants of hepatitis C virus proteins by phage display. Mol. Biol. 2006, 40, 357–368. (In Russian) [Google Scholar] [CrossRef]
- Guryanova, S.V.; Andronova, T.M.; Safonova, N.G. Use of N-acetylglucosaminyl-N-acetylmuramil-L-alanyl-D-isoglutamine as an Adjuvant in In Vitro Immunization in Order to Obtain Monoclonal Antibodies to the IL-1 β Peptide (163–171). Biotechnology 1991, 6, 23–25. (In Russian) [Google Scholar]
- Fallon, P.G.; Schwartz, C. The high and lows of type 2 asthma and mouse models. J. Allergy Clin. Immunol. 2020, 145, 496–498. [Google Scholar] [CrossRef]
- Hamada, K.; Oishi, K.; Murata, Y.; Hirano, T.; Matsunaga, K. Feasibility of Discontinuing Biologics in Severe Asthma: An Algorithmic Approach. J. Asthma Allergy 2021, 14, 1463–1471. [Google Scholar] [CrossRef]


| Day | Animal Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 Asthma (OVA) | 2 LPS before OVA | 3 GMDP before OVA | 4 LPS and OVA | 5 GMDP and OVA | 6 LPS | 7 GMDP | 8 Phosphate Buffer (PBS) | |
| −5 | LPS i/p | GMDP i/p | LPS i/p | GMDP i/p | PBS i/p | |||
| −4 | LPS i/p | GMDP i/p | LPS i/p | GMDP i/p | PBS i/p | |||
| −3 | LPS i/p | GMDP i/p | LPS i/p | GMDP i/p | PBS i/p | |||
| −2 | LPS i/p | GMDP i/p | LPS i/p | GMDP i/p | PBS i/p | |||
| −1 | LPS i/p | GMDP i/p | LPS i/p | GMDP i/p | PBS i/p | |||
| 0 | OVA i/p | OVA+LPS i/p | OVA+GMDP i/p | PBS i/p | ||||
| 1 | ||||||||
| … | ||||||||
| 13 | ||||||||
| 14 | OVA i/p | OVA+LPS i/p | OVA+LPS i/p | PBS i/p | ||||
| 15 | ||||||||
| 16 | ||||||||
| 17 | ||||||||
| 18 | ||||||||
| 19 | ||||||||
| 20 | ||||||||
| 21 | OVA i/p | OVA+LPS i/p | OVA+LPS i/p | PBS i/p | ||||
| 22 | ||||||||
| 23 | ||||||||
| 24 | ||||||||
| 25 | ||||||||
| 26 | ||||||||
| 27 | OVA i/n | PBS i/n | PBS i/n | PBS i/n | ||||
| 28 | OVA i/n | PBS i/n | PBS i/n | PBS i/n | ||||
| 29 | ||||||||
| 30 | ||||||||
| 31 | ||||||||
| 32 | ||||||||
| 33 | ||||||||
| 34 | ||||||||
| 35 | OVA i/n | PBS i/n | PBS i/n | PBS i/n | ||||
| 36 | ||||||||
| 37 | BAL, lung and sera collecting | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guryanova, S.V.; Gigani, O.B.; Gudima, G.O.; Kataeva, A.M.; Kolesnikova, N.V. Dual Effect of Low-Molecular-Weight Bioregulators of Bacterial Origin in Experimental Model of Asthma. Life 2022, 12, 192. https://doi.org/10.3390/life12020192
Guryanova SV, Gigani OB, Gudima GO, Kataeva AM, Kolesnikova NV. Dual Effect of Low-Molecular-Weight Bioregulators of Bacterial Origin in Experimental Model of Asthma. Life. 2022; 12(2):192. https://doi.org/10.3390/life12020192
Chicago/Turabian StyleGuryanova, Svetlana V., Olga B. Gigani, Georgii O. Gudima, Anastasiya M. Kataeva, and Natalya V. Kolesnikova. 2022. "Dual Effect of Low-Molecular-Weight Bioregulators of Bacterial Origin in Experimental Model of Asthma" Life 12, no. 2: 192. https://doi.org/10.3390/life12020192
APA StyleGuryanova, S. V., Gigani, O. B., Gudima, G. O., Kataeva, A. M., & Kolesnikova, N. V. (2022). Dual Effect of Low-Molecular-Weight Bioregulators of Bacterial Origin in Experimental Model of Asthma. Life, 12(2), 192. https://doi.org/10.3390/life12020192






