Multi-Planar VMAT Plans for High-Grade Glioma and Glioblastoma Targeting the Hypothalamic-Pituitary Axis Sparing
Abstract
:1. Advances in Knowledge
2. Introduction
3. Materials and Methodology
3.1. Patient Selection
3.2. Image Collection and Processing
3.3. Ethics Approval
3.4. Treatment Design and Planning
3.5. Inter-Planner Variability
3.6. Treatment Plan Optimization
3.7. Plan Qualities and Dose-Volumetric Parameters
3.8. Statistical Analysis
4. Results
4.1. Patient Demographics
4.2. Dose-Volumetric Parameters of the PTV and Total Monitor Units
4.3. Centrally Located OAR Dose-Volumetric Parameters
4.4. Dose-Volumetric Parameters of Ipsilaterally Located OARs
4.5. Dose-Volumetric Parameters of Contralaterally Located OARs
4.6. Percentage of Normal Brain Tissue Volume Receiving a Low Dose (V30Gy or V15Gy)
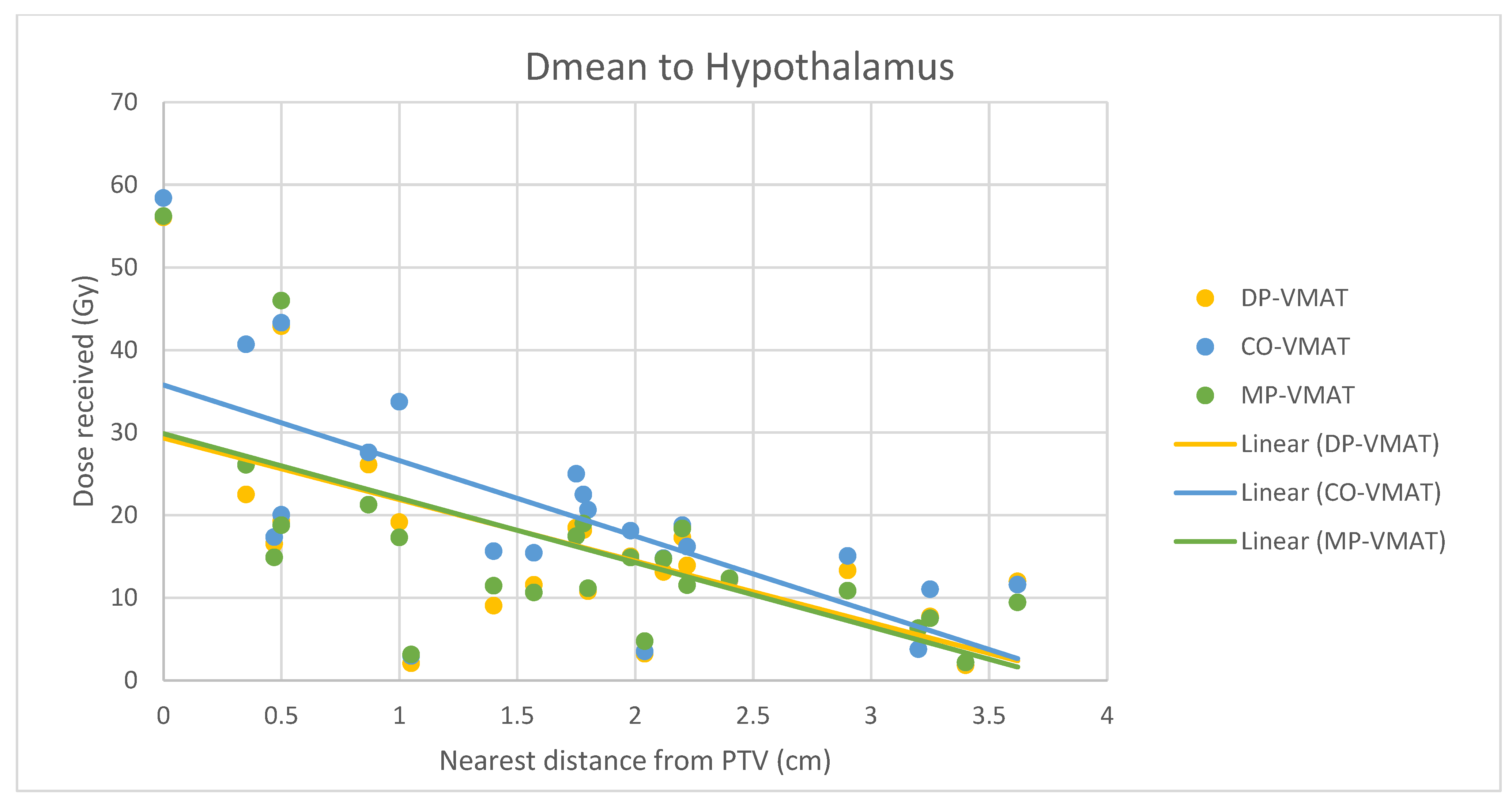
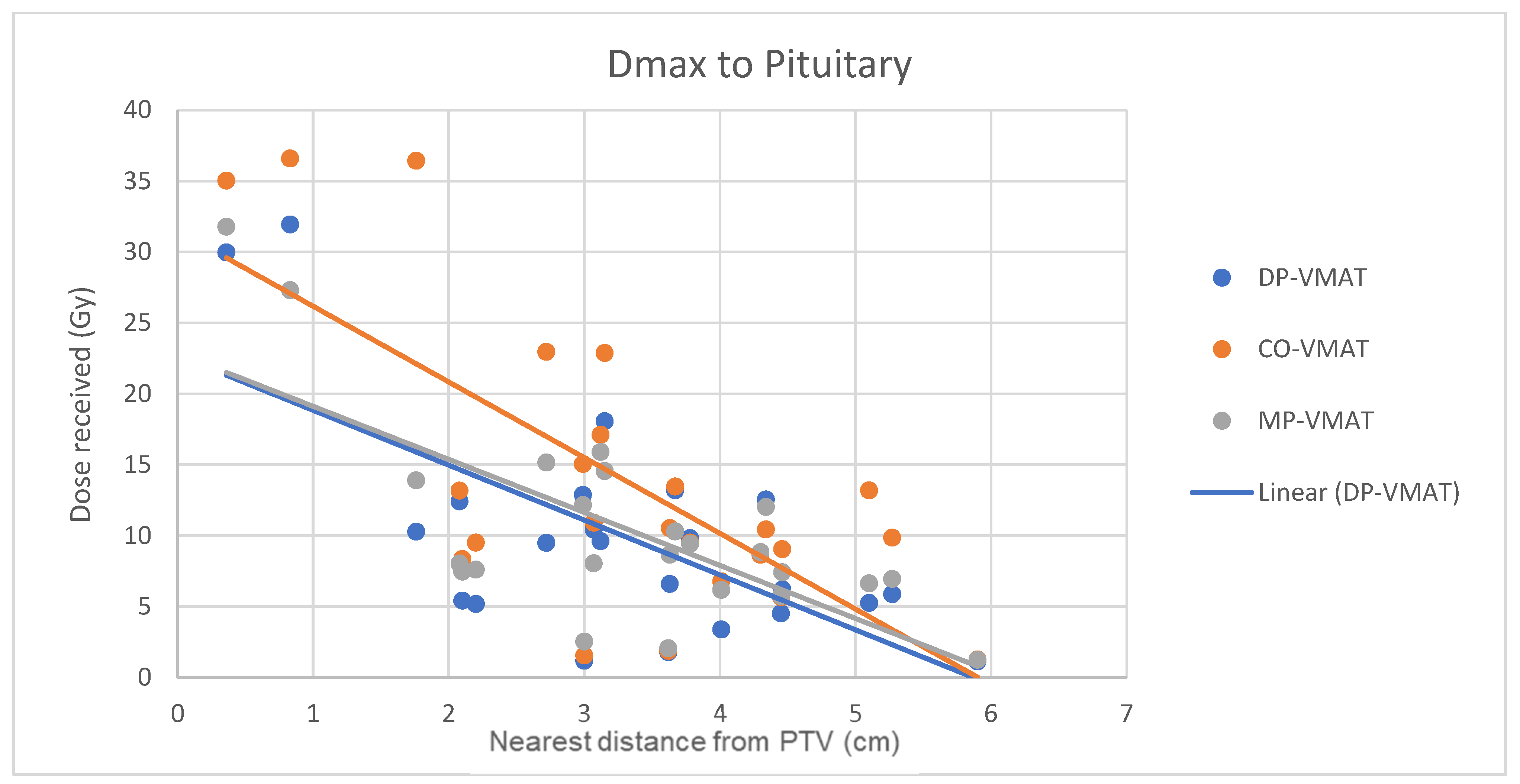
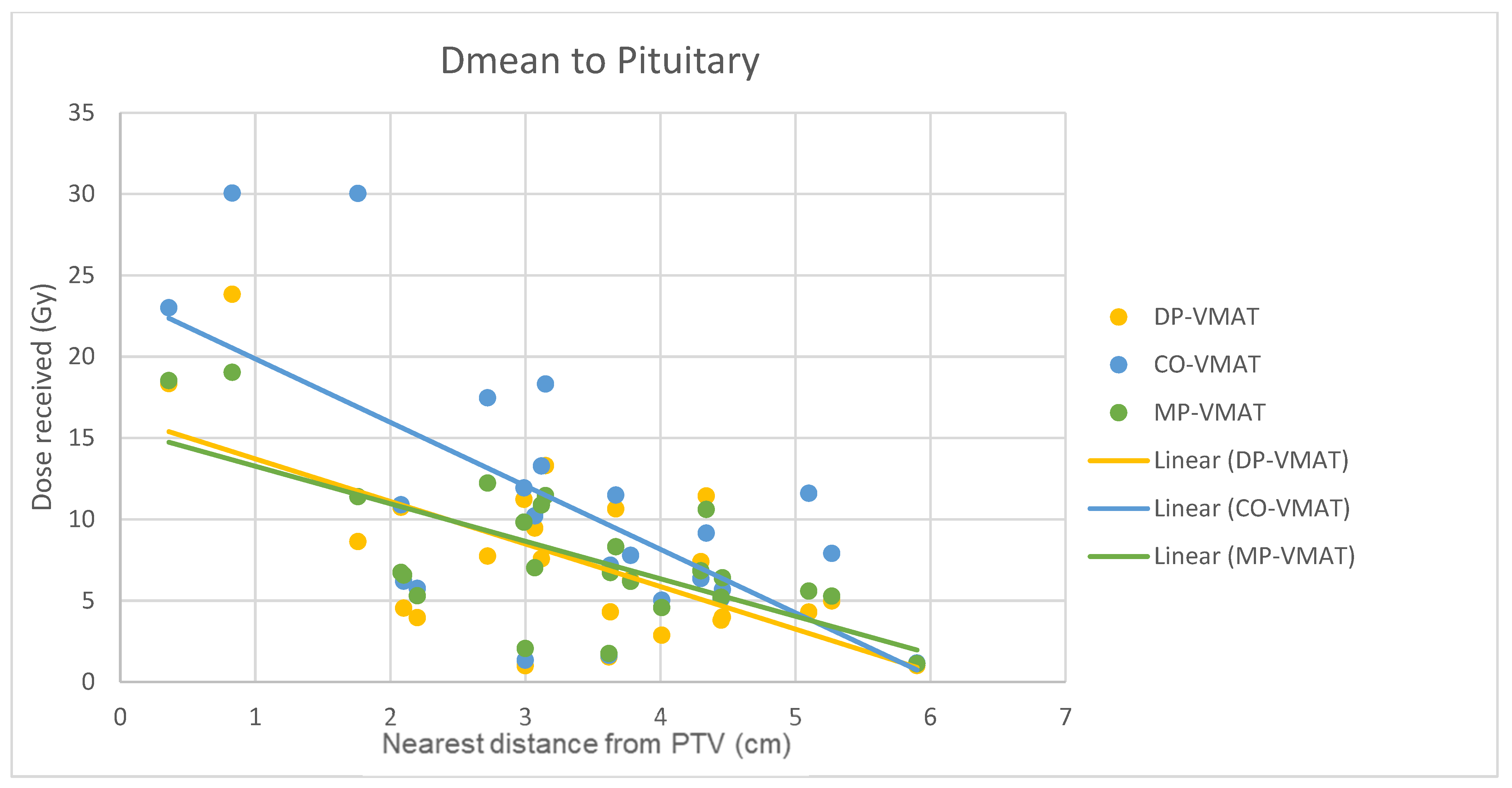
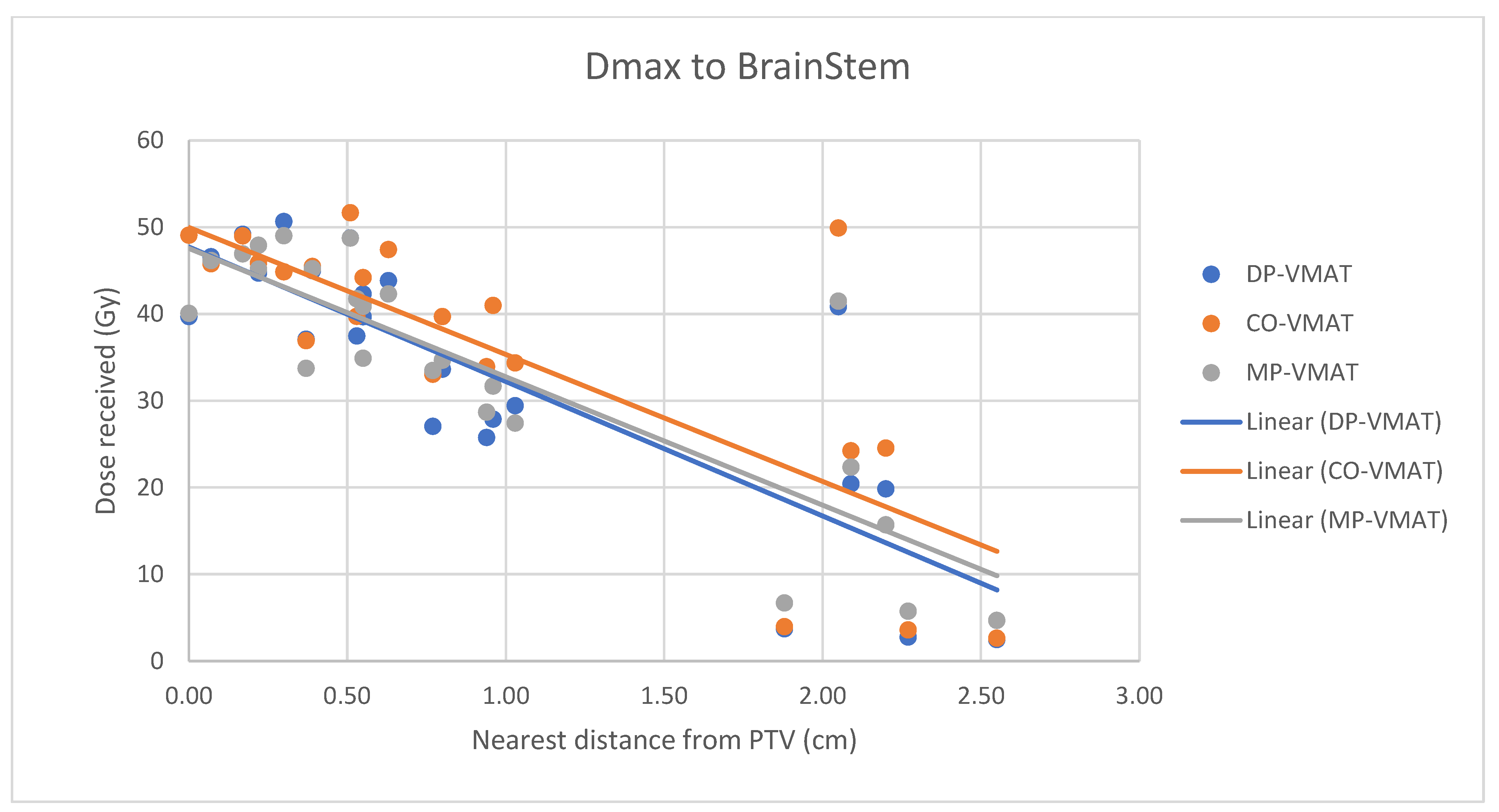
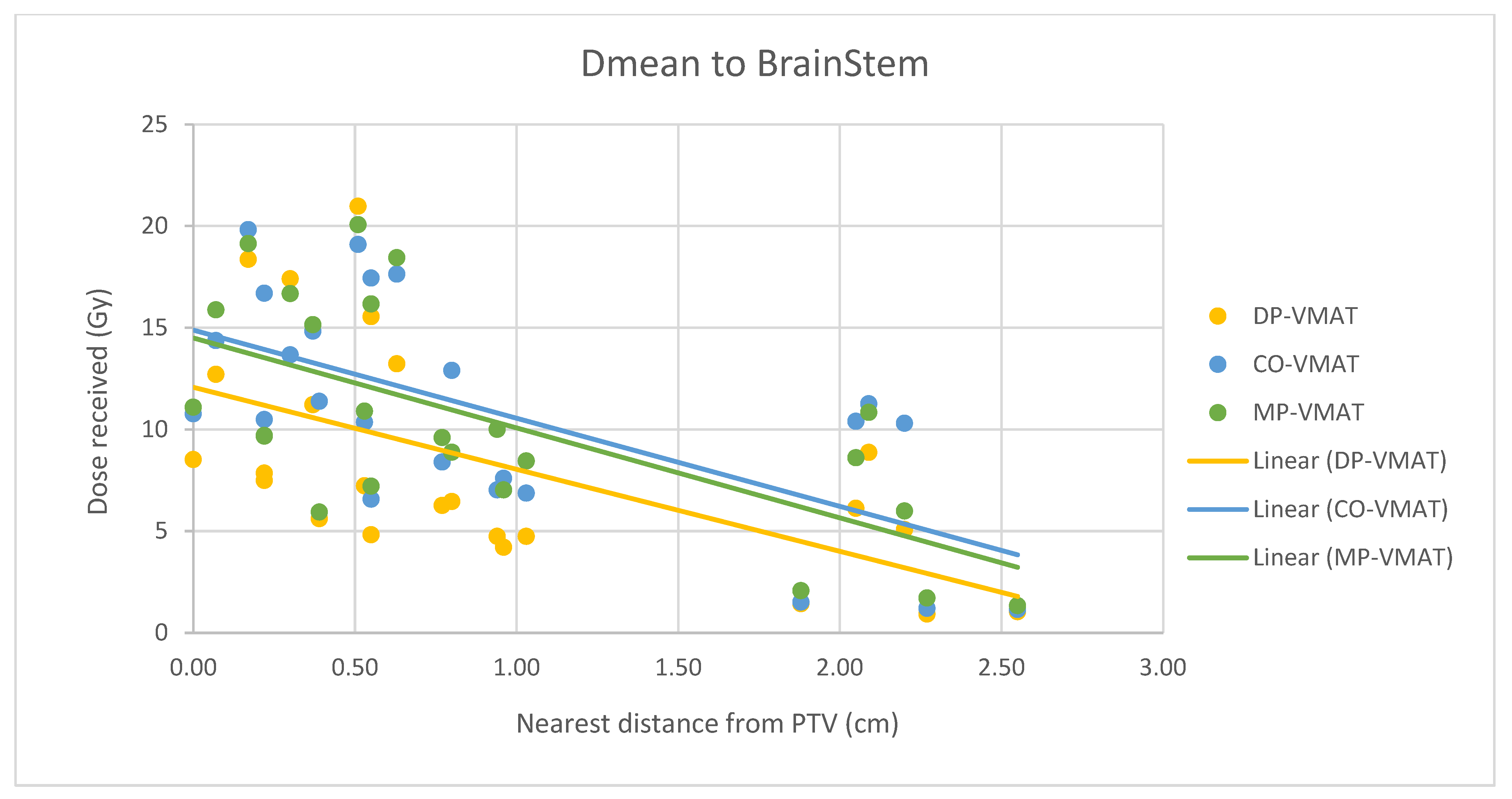
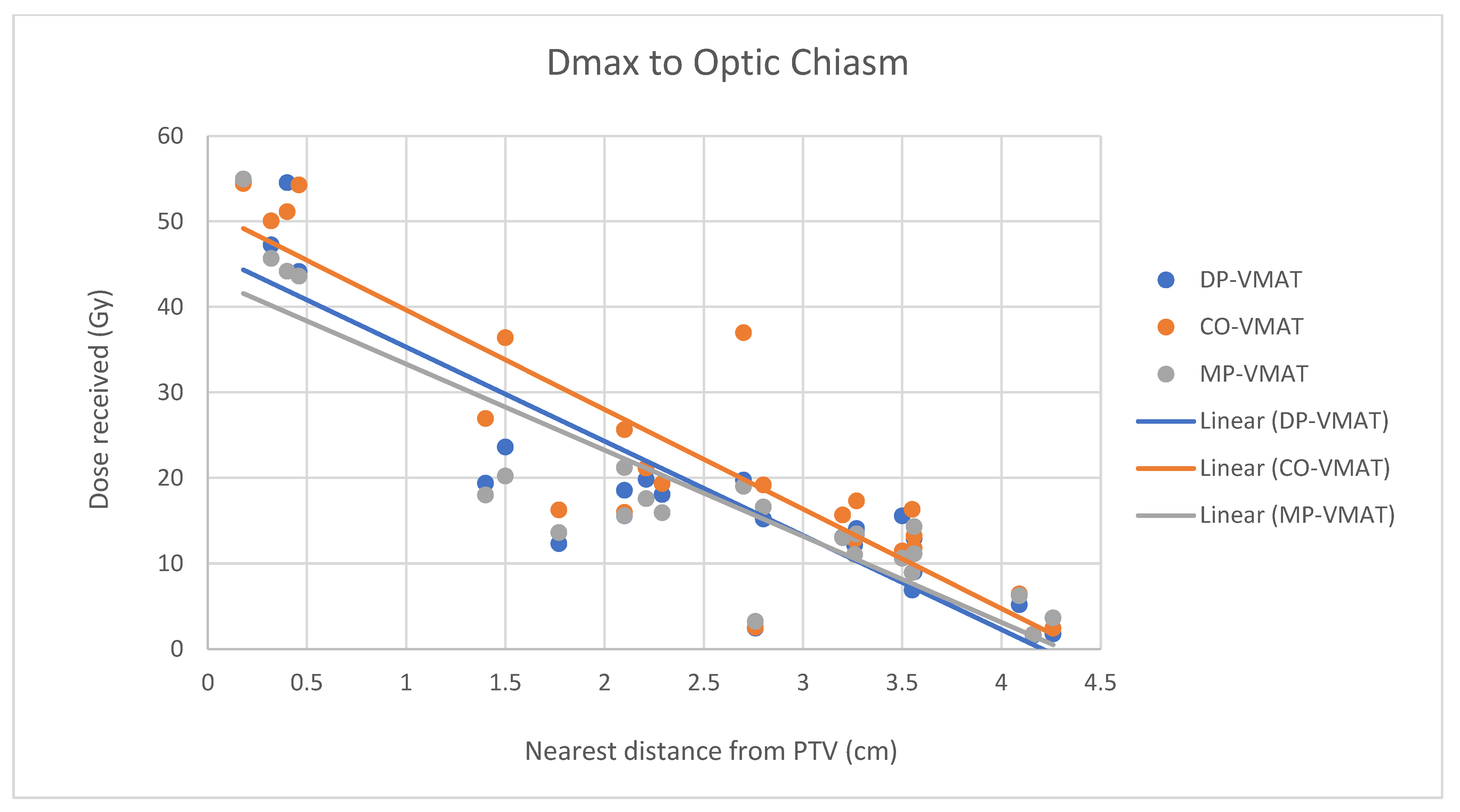
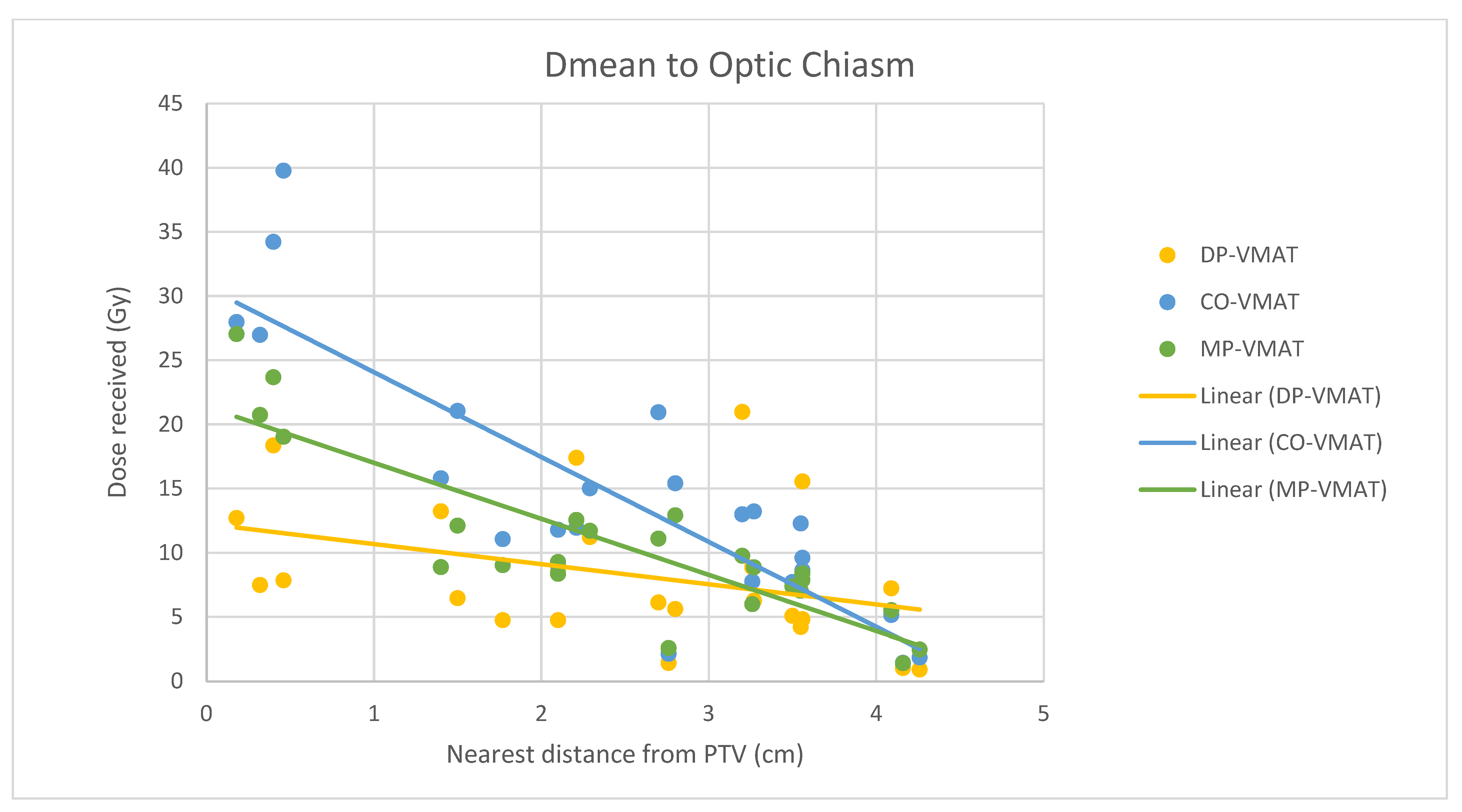
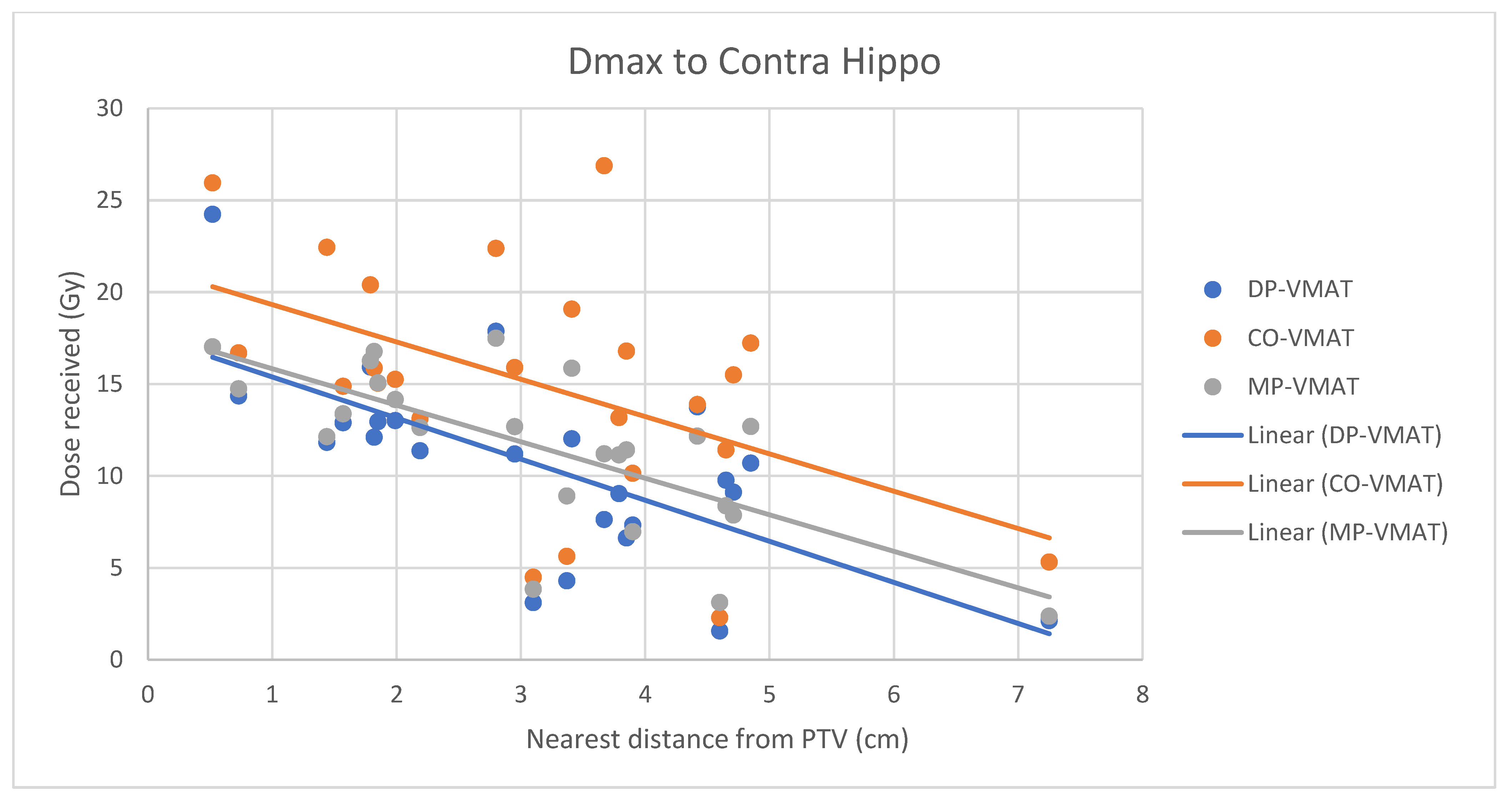
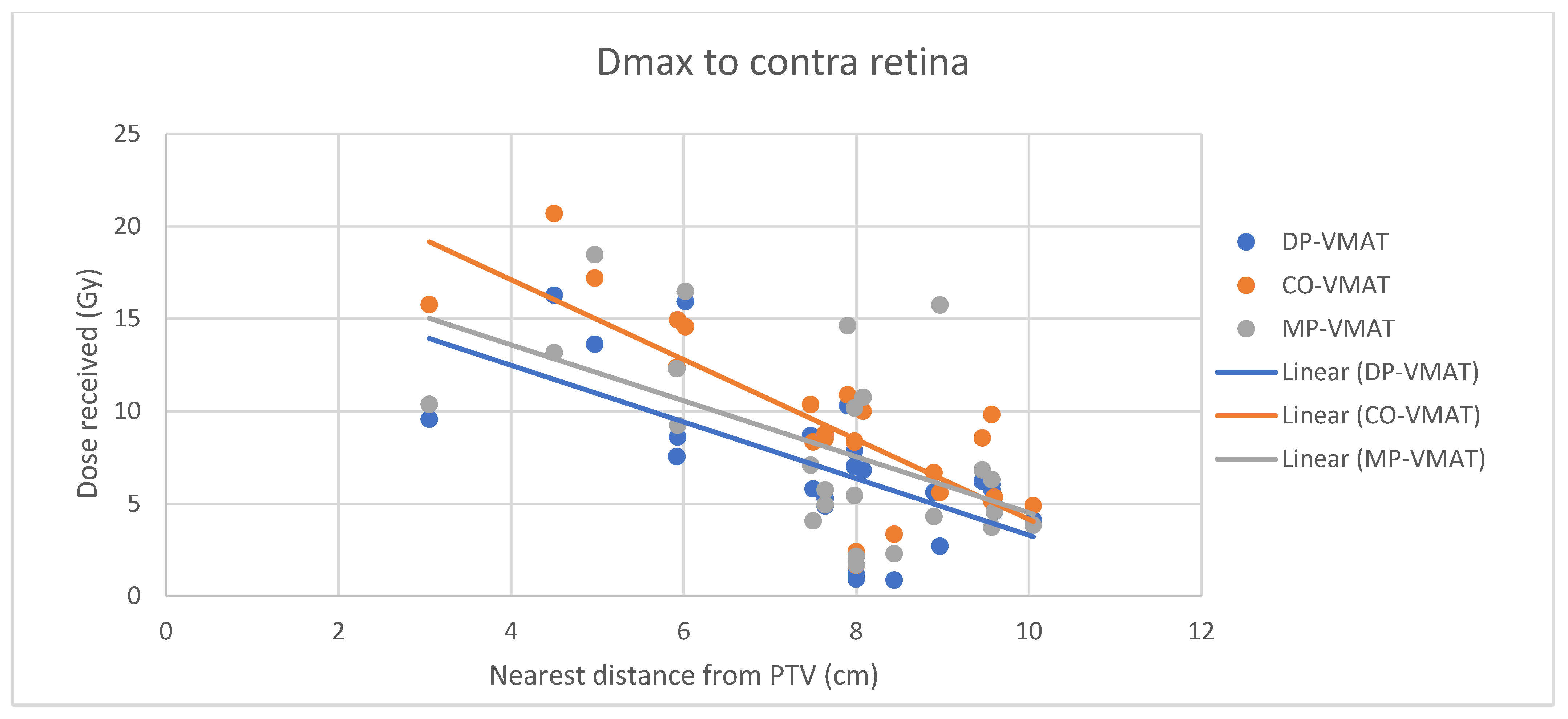
4.7. Correlation Analysis for PTV-OAR Distance and the Dose Received by the Corresponding OAR
- The correlation between the Dmax or Dmean of centrally located organs and their distance to the PTV
- 2.
- Correlation between the Dmax or Dmean of the peripherally located organs and their distance to the PTV
5. Discussion
5.1. Significance of the Study
5.2. Hypothalamus and Pituitary Sparing
5.3. Optic Chiasm, Retinae and Lens Sparing
5.4. Hippocampus Sparing
5.5. Normal Brain Tissue Sparing
5.6. Treatment Safety
5.7. Treatment Delivery Time
5.8. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Brain and Other CNS Cancer Collaborators. Global, regional, and national burden of brain and other CNS cancer, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 376–393. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Diaz, A.Z.; Choi, M. Radiation-Associated Toxicities in the Treatment of High-Grade Gliomas. Semin. Oncol. 2014, 41, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Douw, L.; Klein, M.; Fagel, S.S.; van den Heuvel, J.; Taphoorn, M.J.; Aaronson, N.K.; Postma, T.J.; Vandertop, W.P.; Mooij, J.J.; Boerman, R.H.; et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: Long-term follow-up. Lancet Neurol. 2009, 8, 810–818. [Google Scholar] [CrossRef]
- Høyer, M.; Muren, L.P.; Glimelius, B. The evolution of radiotherapy techniques in the management of prostate cancer. Acta Oncol. 2015, 54, 821–824. [Google Scholar] [CrossRef] [Green Version]
- Kiljunen, T.; Kangasmäki, A.; Aaltonen, A.; Kairemo, K.; Partanen, K.; Joensuu, G.; Alanko, T.; Vaalavirta, L.; Joensuu, T. VMAT technique enables concomitant radiotherapy of prostate cancer and pelvic bone metastases. Acta Oncol. 2015, 54, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Miura, H.; Ozawa, S.; Kusaba, H.; Doi, Y.; Kenjo, M.; Yamada, K.; Nagata, Y. Characterization of robust optimization for VMAT plan for liver cancer. Rep. Pract. Oncol. Radiother. 2020, 25, 376–381. [Google Scholar] [CrossRef]
- Moon, Y.M.; Jeon, W.; Yu, T.; Bae, S.I.; Kim, J.Y.; Kang, J.-K.; Choi, C.W. Which Is Better for Liver SBRT: Dosimetric Comparison Between DCAT and VMAT for Liver Tumors. Front. Oncol. 2020, 10, 1170. [Google Scholar] [CrossRef]
- Cheung, E.Y.W.; Kwong, V.H.Y.; Chan, F.Y.C.; Cheng, D.Y.T.; Cheng, J.K.Y.; Yung, S.H.Y.; Chan, K.T.K.; Cheung, K.T.Y.; Cheung, T.S.W.; Yiu, J.C.L. Modified VMAT Plans for Locally Advanced Centrally Located Non-Small Cell Lung Cancer (NSCLC). Life 2021, 11, 1085. [Google Scholar] [CrossRef]
- Awad, R.; Fogarty, G.; Hong, A.; Kelly, P.; Ng, D.; Santos, D.; Haydu, L. Hippocampal avoidance with volumetric modulated arc therapy in melanoma brain metastases–the first Australian experience. Radiat. Oncol. 2013, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinkham, M.B.; Bertrand, K.C.; Olson, S.; Zarate, D.; Oram, J.; Pullar, A.; Foote, M.C. Hippocampal-sparing radiotherapy: The new standard of care for World Health Organization grade II and III gliomas? J. Clin. Neurosci. 2014, 21, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Lanaspèze, C.; Varela Cagetti, L.; Vieillevigne, L. Hippocampal sparing whole brain radiotherapy with volumetric modulated arc therapy. Phys. Med. 2016, 32, 358–359. [Google Scholar] [CrossRef]
- Cheung, E.Y.W.; Lee, K.H.Y.; Lau, W.T.L.; Lau, A.P.Y.; Wat, P.Y. Non-coplanar VMAT plans for postoperative primary brain tumour to reduce dose to hippocampus, temporal lobe and cochlea: A planning study. BJR Open 2021, 3, 20210009. [Google Scholar] [CrossRef]
- Bas Ayata, H.; Ceylan, C.; Kılıç, A.; Güden, M.; Engin, K. Comparison of Multiple Treatment Planning Techniques for High-Grade Glioma Tumors Near to Critical Organs. Oncol. Res. Treat. 2018, 41, 514–519. [Google Scholar] [CrossRef]
- Constine, L.S.; Woolf, P.D.; Cann, D.; Mick, G.; McCormick, K.; Raubertas, R.F.; Rubin, P. Hypothalamic-Pituitary Dysfunction after Radiation for Brain Tumors. N. Engl. J. Med. 1993, 328, 87–94. [Google Scholar] [CrossRef]
- Toogood, A.A. Endocrine consequences of brain irradiation. Growth Horm. IGF Res. 2004, 14, 118–124. [Google Scholar] [CrossRef]
- Darzy, K.H.; Shalet, S.M. Radiation-Induced Growth Hormone Deficiency. Horm. Res. Paediatr. 2003, 59, 1–11. [Google Scholar] [CrossRef]
- Darzy, K.H.; Shalet, S.M. Hypopituitarism As a Consequence of Brain Tumours and Radiotherapy. Pituitary 2005, 8, 203–211. [Google Scholar] [CrossRef]
- Darzy, K.H. Radiation-induced hypopituitarism after cancer therapy: Who, how and when to test. Nat. Clin. Pract. Endocrinol. Metab. 2009, 5, 88–99. [Google Scholar] [CrossRef]
- Samaan, N.A.; Vieto, R.; Schultz, P.N.; Maor, M.; Meoz, R.T.; Sampiere, V.A.; Cangir, A.; Ried, H.L.; Jesse, R.H. Hypothalamic, pituitary and thyroid dysfunction after radiotherapy to the head and neck. Int. J. Radiat. Oncol. 1982, 8, 1857–1867. [Google Scholar] [CrossRef]
- Darzy, K.H. Radiation-induced hypopituitarism. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Partoune, E.; Virzi, M.; Vander Veken, L.; Renard, L.; Maiter, D. Occurrence of pituitary hormone deficits in relation to both pituitary and hypothalamic doses after radiotherapy for skull base meningioma. Clin. Endocrinol. (Oxf.) 2021, 95, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Hermann, B.P.; Mehta, M.P.; Tomé, W.A. Hippocampal Dosimetry Predicts Neurocognitive Function Impairment After Fractionated Stereotactic Radiotherapy for Benign or Low-Grade Adult Brain Tumors. Int. J. Radiat. Oncol. 2012, 83, e487–e493. [Google Scholar] [CrossRef] [Green Version]
- Nelms, B.E.; Robinson, G.; Markham, J.; Velasco, K.; Boyd, S.; Narayan, S.; Wheeler, J.; Sobczak, M.L. Variation in external beam treatment plan quality: An inter-institutional study of planners and planning systems. Pract. Radiat. Oncol. 2012, 2, 296–305. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.; Won, M.; Blumenthal, D.T.; Vogelbaum, M.A.; Aldape, K.D.; Colman, H.; Chakravarti, A.; Jeraj, R.; Armstrong, T.S.; et al. RTOG 0825: Phase III double-blind placebo-controlled trial evaluating bevacizumab (Bev) in patients (Pts) with newly diagnosed glioblastoma (GBM). J. Clin. Oncol. 2013, 31 (Suppl. S18). [Google Scholar] [CrossRef]
- Ali, A.N.; Zhang, P.; Yung, W.K.A.; Chen, Y.; Movsas, B.; Urtasun, R.C.; Jones, C.U.; Choi, K.N.; Michalski, J.M.; Fischbach, A.J.; et al. NRG oncology RTOG 9006: A phase III randomized trial of hyperfractionated radiotherapy (RT) and BCNU versus standard RT and BCNU for malignant glioma patients. J. Neurooncol. 2018, 137, 39–47. [Google Scholar] [CrossRef]
- Kataria, T.; Sharma, K.; Subramani, V.; Karrthick, K.; Bisht, S. Homogeneity Index: An objective tool for assessment of conformal radiation treatments. J. Med. Phys. 2012, 37, 207. [Google Scholar] [CrossRef]
- Van’t Riet, A.; Mak, A.C.A.; Moerland, M.A.; Elders, L.H.; van der Zee, W. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: Application to the prostate. Int. J. Radiat. Oncol. 1997, 37, 731–736. [Google Scholar] [CrossRef]
- Danesh-Meyer, H.V. Radiation-induced optic neuropathy. J. Clin. Neurosci. 2008, 15, 95–100. [Google Scholar] [CrossRef]
- Emami, B.; Lyman, J.; Brown, A.; Coia, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Lawrence, Y.R.; Li, X.A.; Naqa, I.; Hahn, C.A.; Marks, L.B.; Merchant, T.E.; Dicker, A.P. Radiation dose-volume effects in the brain. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S20–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Ten Haken, R.K.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. Use of normal tissue complication probability models in the clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S10–S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Hilverda, K.; Bosma, I.; Heimans, J.J.; Postma, T.J.; Peter Vandertop, W.; Slotman, B.J.; Buter, J.; Reijneveld, J.C.; Klein, M. Cognitive functioning in glioblastoma patients during radiotherapy and temozolomide treatment: Initial findings. J. Neurooncol. 2010, 97, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Wefel, J.S.; Armstrong, T.S.; Pugh, S.L.; Gilbert, M.R.; Wendland, M.M.; Brachman, D.G.; Roof, K.S.; Brown, P.D.; Crocker, I.R.; Robins, H.I.; et al. Neurocognitive, symptom, and health-related quality of life outcomes of a randomized trial of bevacizumab for newly diagnosed glioblastoma (NRG/RTOG 0825). Neuro-Oncology 2021, 23, 1125–1138. [Google Scholar] [CrossRef]
- Ramanan, S.; Kooshki, M.; Zhao, W.; Hsu, F.-C.; Riddle, D.R.; Robbins, M.E. The PPARα Agonist Fenofibrate Preserves Hippocampal Neurogenesis and Inhibits Microglial Activation After Whole-Brain Irradiation. Int. J. Radiat. Oncol. 2009, 75, 870–877. [Google Scholar] [CrossRef] [Green Version]
- Audet, C.; Poffenbarger, B.A.; Chang, P.; Jackson, P.S.; Lundahl, R.E.; Ryu, S.I.; Ray, G.R. Evaluation of volumetric modulated arc therapy for cranial radiosurgery using multiple noncoplanar arcs: Volumetric modulated arc therapy for cranial radiosurgery. Med. Phys. 2011, 38, 5863–5872. [Google Scholar] [CrossRef]
- Smyth, G.; Evans, P.M.; Bamber, J.C.; Bedford, J.L. Recent developments in non-coplanar radiotherapy. Br. J. Radiol. 2019, 92, 20180908. [Google Scholar] [CrossRef]
- Smyth, G.; Evans, P.M.; Bamber, J.C.; Mandeville, H.C.; Welsh, L.C.; Saran, F.H.; Bedford, J.L. Non-coplanar trajectories to improve organ at risk sparing in volumetric modulated arc therapy for primary brain tumors. Radiother. Oncol. 2016, 121, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Fralick, D.; Zheng, J.Z.; Wang, B.; Tu, X.M.; Feng, C. The Differences and Similarities Between Two-Sample T-Test and Paired T-Test. Shanghai Arch. Psychiatry 2017, 29, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Uto, M.; Mizowaki, T.; Ogura, K.; Hiraoka, M. Non-coplanar volumetric-modulated arc therapy (VMAT) for craniopharyngiomas reduces radiation doses to the bilateral hippocampus: A planning study comparing dynamic conformal arc therapy, coplanar VMAT, and non-coplanar VMAT. Radiat. Oncol. 2016, 11, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, K.; Sun, H.; Gao, L.; Lin, T.; Sui, J.; Ni, X. A comparative study of identical VMAT about two adjacent targets with and without fixed-jaw technique. Radiat. Oncol. 2019, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Snyder, K.C.; Wen, N.; Huang, Y.; Kim, J.; Zhao, B.; Siddiqui, S.; Chetty, I.J.; Ryu, S. Use of jaw tracking in intensity modulated and volumetric modulated arc radiation therapy for spine stereotactic radiosurgery. Pract. Radiat. Oncol. 2015, 5, e155–e162. [Google Scholar] [CrossRef] [PubMed]
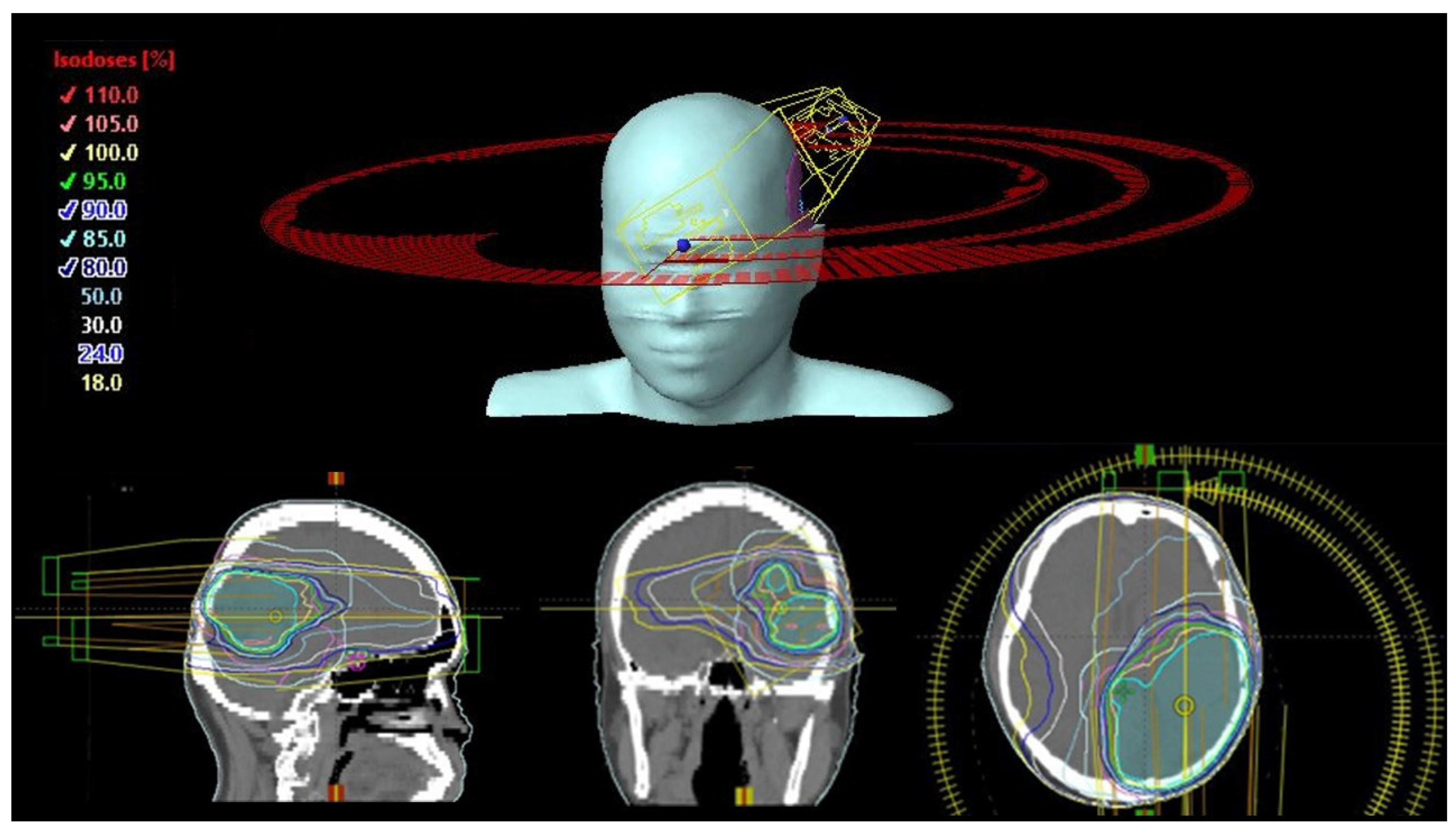






| Critical Organ or Organ-at Risk | Dose Constraints |
|---|---|
| Brainstem | Maximum dose < 60 Gy |
| Hypothalamus | Maximum dose < 30 Gy |
| Pituitary gland | Maximum dose < 30 Gy |
| Optic Chiasm | Maximum dose < 56 Gy |
| Optic Nerve | Maximum dose < 55 Gy |
| Retinae | Maximum dose < 50 Gy |
| Lens | Maximum dose < 7 Gy |
| Hippocampus | Maximum dose < 17 Gy |
| Structure | Dose Constraint | Weight |
|---|---|---|
| PTV | Maximum dose < 66 Gy | 100 |
| Minimum dose < 60 Gy | 100 | |
| Eye Balls | Maximum dose < 7 Gy | 5 |
| Mean dose < 35 Gy | ||
| Lenses | Maximum dose < 5 Gy | 5 |
| Hippocampus | Maximum dose < 11 Gy | 5 |
| D40% < 9 Gy | 10 | |
| Hypothalamus | N/A | N/A |
| Pituitary | N/A | N/A |
| Cochlear | N/A | N/A |
| PTV DV Parameters | CO-VMAT | DP-VMAT | MP-VMAT | DP-VMAT-CO-VMAT | MP-VMAT-CO-VMAT | ||
|---|---|---|---|---|---|---|---|
| Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | Diff | p | Diff | p | |
| Maximum Dose (Gy) | 65.63 ± 0.90 | 65.61 ± 0.66 | 65.05 ± 0.67 | −0.02 | 0.69 | −0.56 | 0.32 |
| Mean Dose (Gy) | 61.54 ± 0.26 | 61.54 ± 0.23 | 61.50 ± 0.26 | 0.00 | 0.82 | 0.04 | 0.38 |
| Minimum Dose (Gy) | 48.06 ± 3.74 | 49.75 ± 5.79 | 49.44 ± 5.66 | 1.68 | 0.83 | 1.38 | 0.14 |
| D98% (Gy) | 60.27 ± 0.55 | 60.33 ± 0.55 | 60.27 ± 0.31 | 0.06 | 0.14 | 0.00 | 0.88 |
| D95% (Gy) | 60.75 ± 0.297 | 60.77 ± 0.27 | 60.70 ± 0.17 | 0.03 | 0.35 | −0.05 | 0.33 |
| D50% (Gy) | 61.56 ± 0.308 | 61.54 ± 0.27 | 61.51 ± 0.30 | −0.02 | 0.36 | 0.05 | 0.23 |
| D2% (Gy) | 62.73 ± 0.311 | 62.80 ± 0.28 | 62.63 ± 0.39 | −0.08 | 0.40 | 0.097 | 0.20 |
| Homogeneity Index | 0.0410 ± 0.0117 | 0.0413 ± 0.01153 | 0.0394 ± 0.00883 | 0.0003 | 0.65 | 0.0016 | 0.40 |
| Conformity Index | 1.1146 ± 0.083 | 1.1092 ± 0.07734 | 1.0996 ± 0.07135 | 0.0054 | 0.38 | 0.015 | 0.08 |
| Centrally Located OARs | DV Parameters | CO-VMAT | DP-VMAT | MP-VMAT | DP-VMAT-CO-VMAT | MP-VMAT-CO-VMAT | ||
|---|---|---|---|---|---|---|---|---|
| Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | Diff | p | Diff | p | ||
| Hypothalamus | Max Dose (Gy) | 26.18 ± 16.55 | 23.54 ± 16.05 | 23.20 ± 15.79 | −2.65 * | 0.04 | −2.98 * | 0.003 |
| Mean Dose (Gy) | 19.61 ± 13.5 | 16.19 ± 12.10 | 16.09 ± 12.34 | −3.42 * | <0.001 | −3.51 * | 0.001 | |
| Pituitary | Max Dose (Gy) | 13.74 ± 10.18 | 9.83 ± 7.75 | 10.47 ± 7.09 | −3.91 * | <0.001 | −3.33 * | 0.001 |
| Mean Dose (Gy) | 10.77 ± 7.96 | 7.62 ± 5.47 | 7.9 ± 4.50 | −3.15 * | <0.001 | −2.87 * | 0.003 | |
| Brain Stem | Max Dose (Gy) | 36.54 ± 14.7 | 33.48 ± 14.72 | 33.96 ± 13.87 | −3.05 * | 0.001 | −2.57 * | 0.013 |
| Mean Dose (Gy) | 10.90 ± 5.33 | 8.36 ± 5.47 | 10.44 ± 5.35 | −2.54 * | <0.001 | −0.46 | 0.864 | |
| Optic Chiasm | Max Dose (Gy) | 22.48 ± 16.37 | 19.07 ± 15.5 | 18.48 ± 14.19 | −3.41 * | 0.002 | −4.00 * | <0.001 |
| Mean Dose (Gy) | 14.32 ± 9.86 | 10.57 ± 7.04 | 10.57 ± 6.39 | −3.75 * | <0.001 | −3.75 * | <0.001 | |
| Ipsilaterally Located OARs | DV Parameters | CO-VMAT | DP-VMAT | MP-VMAT | DP-VMAT-CO-VMAT | MP-VMAT-CO-VMAT | ||
|---|---|---|---|---|---|---|---|---|
| Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | Diff | p | Diff | p | ||
| Hippocampus | Max Dose (Gy) | 54.24 ± 17.64 | 54.89 ± 16.57 | 55.35 ± 15.69 | 0.66 | 0.361 | 1.12 | 0.162 |
| Mean Dose (Gy) | 41.61 ± 18.94 | 41.91 ± 12.10 | 41.88 ± 17.63 | 0.30 | 0.511 | −0.28 | 0.53 | |
| D40% (Gy) | 45.73 ± 21.02 | 45.89 ± 19.77 | 46.27 ± 19.61 | 0.16 | 0.864 | 0.53 | 0.475 | |
| Optic Nerve | Max Dose (Gy) | 17.95 ± 16.79 | 16.20 ± 15.62 | 16.40 ± 15.43 | −1.75 * | 0.012 | −1.55 | 0.076 |
| Mean Dose (Gy) | 11.91 ± 10.69 | 11.10 ± 11.12 | 11.73 ± 10.97 | −0.81 * | 0.034 | −0.18 | 0.732 | |
| Lens | Max Dose (Gy) | 5.43 ± 2.68 | 4.98 ± 2.24 | 4.99 ± 1.94 | −0.45 | 0.304 | −0.44 | 0.407 |
| Mean Dose (Gy) | 4.15 ± 2.06 | 3.79 ± 1.82 | 3.87 ± 1.42 | −0.50 | 0.376 | −0.27 | 0.493 | |
| Retina | Max Dose (Gy) | 17.52 ± 11.14 | 16.82 ± 10.41 | 16.51 ± 10.15 | −0.70 | 0.153 | −1.01 | 0.23 |
| Mean Dose (Gy) | 6.94 ± 3.84 | 6.61 ± 3.57 | 6.815 ± 3.57 | 0.33 | 0.137 | −0.13 | 0.79 | |
| Contra-Laterally Located OARs | DV Parameters | CO-VMAT | DP-VMAT | MP-VMAT | DP-VMAT-CO-VMAT | MP-VMAT-CO-VMAT | ||
|---|---|---|---|---|---|---|---|---|
| Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | Diff | p | Diff | p | ||
| Hippocampus | Max Dose (Gy) | 14.99 ± 6.35 | 10.62 ± 5.17 | 11.59 ± 4.35 | −4.37 * | <0.001 | −3.39 * | <0.001 |
| Mean Dose (Gy) | 10.32 ± 4.54 | 6.22 ± 2.77 | 7.21 ± 2.67 | −4.10 * | <0.001 | −3.11 * | <0.001 | |
| D40% (Gy) | 10.84 ± 4.77 | 6.42 ± 2.86 | 7.51 ± 3.04 | −4.42 * | <0.001 | −3.34 * | <0.001 | |
| Optic Nerve | Max Dose (Gy) | 9.10 ± 6.65 | 6.33 ± 5.21 | 7.11 ± 4.59 | −2.77 * | <0.001 | −1.98 * | 0.009 |
| Mean Dose (Gy) | 6.86 ± 4.73 | 4.49 ± 3.18 | 5.38 ± 3.26 | −2.37 * | <0.001 | −1.48 * | 0.006 | |
| Lens | Max Dose (Gy) | 4.30 ± 1.98 | 3.18 ± 1.65 | 4.05 ± 1.99 | −1.13 * | <0.001 | −0.25 | 0.265 |
| Mean Dose (Gy) | 3.56 ± 1.67 | 2.50 ± 1.30 | 3.29 ± 1.53 | −1.06 * | <0.001 | −0.26 | 0.284 | |
| Retina | Max Dose (Gy) | 9.27 ± 4.78 | 6.96 ± 4.11 | 8.09 ± 4.93 | −2.35 * | <0.001 | −1.18 * | 0.046 |
| Mean Dose (Gy) | 4.68 ± 2.33 | 3.25 ± 1.77 | 4.14 ± 2.15 | −1.43 * | <0.001 | −0.54 * | 0.025 | |
| Percentage of Normal Brain Tissue | CO-VMAT | DP-VMAT | MP-VMAT | DP-VMAT-CO-VMAT | MP-VMAT-CO-VMAT | ||
|---|---|---|---|---|---|---|---|
| Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | Diff | p | Diff | p | |
| 30 Gy (%) | 21.55 ± 9.08 | 19.67 ± 8.49 | 19.52 ± 8.39 | −1.89 * | <0.001 | −2.03 * | <0.001 |
| 15 Gy (%) | 50.45 ± 18.30 | 45.23 ± 16.12 | 46.05 ± 18.01 | −5.22 * | <0.001 | −4.40 * | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheung, E.Y.W.; Ng, S.S.H.; Yung, S.H.Y.; Cheng, D.Y.T.; Chan, F.Y.C.; Cheng, J.K.Y. Multi-Planar VMAT Plans for High-Grade Glioma and Glioblastoma Targeting the Hypothalamic-Pituitary Axis Sparing. Life 2022, 12, 195. https://doi.org/10.3390/life12020195
Cheung EYW, Ng SSH, Yung SHY, Cheng DYT, Chan FYC, Cheng JKY. Multi-Planar VMAT Plans for High-Grade Glioma and Glioblastoma Targeting the Hypothalamic-Pituitary Axis Sparing. Life. 2022; 12(2):195. https://doi.org/10.3390/life12020195
Chicago/Turabian StyleCheung, Eva Y. W., Shirley S. H. Ng, Sapphire H. Y. Yung, Dominic Y. T. Cheng, Fandy Y. C. Chan, and Janice K. Y. Cheng. 2022. "Multi-Planar VMAT Plans for High-Grade Glioma and Glioblastoma Targeting the Hypothalamic-Pituitary Axis Sparing" Life 12, no. 2: 195. https://doi.org/10.3390/life12020195
APA StyleCheung, E. Y. W., Ng, S. S. H., Yung, S. H. Y., Cheng, D. Y. T., Chan, F. Y. C., & Cheng, J. K. Y. (2022). Multi-Planar VMAT Plans for High-Grade Glioma and Glioblastoma Targeting the Hypothalamic-Pituitary Axis Sparing. Life, 12(2), 195. https://doi.org/10.3390/life12020195





