Whole Body MRI in the Detection of Lymph Node Metastases in Patients with Testicular Germ Cell Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Imaging Protocols
2.2.1. Computed Tomography (CT)
2.2.2. Whole-Body Magnetic Resonance Imaging (WB-MRI)
2.3. Image Analysis
2.4. Reference Standard
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Inter-Reader Agreement
3.3. Performance of CT and WB-MRI
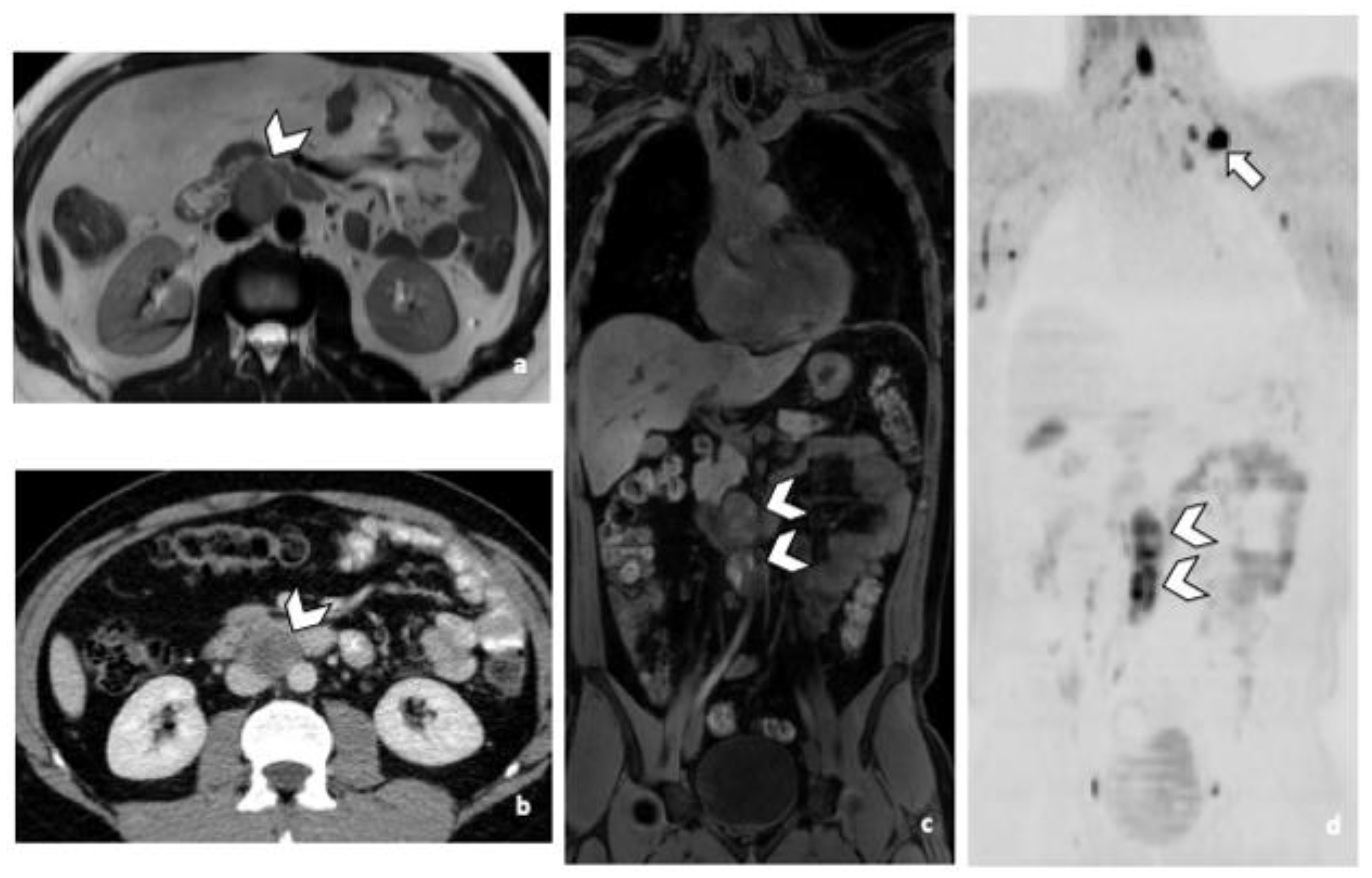
3.3.1. RPLN Metastases
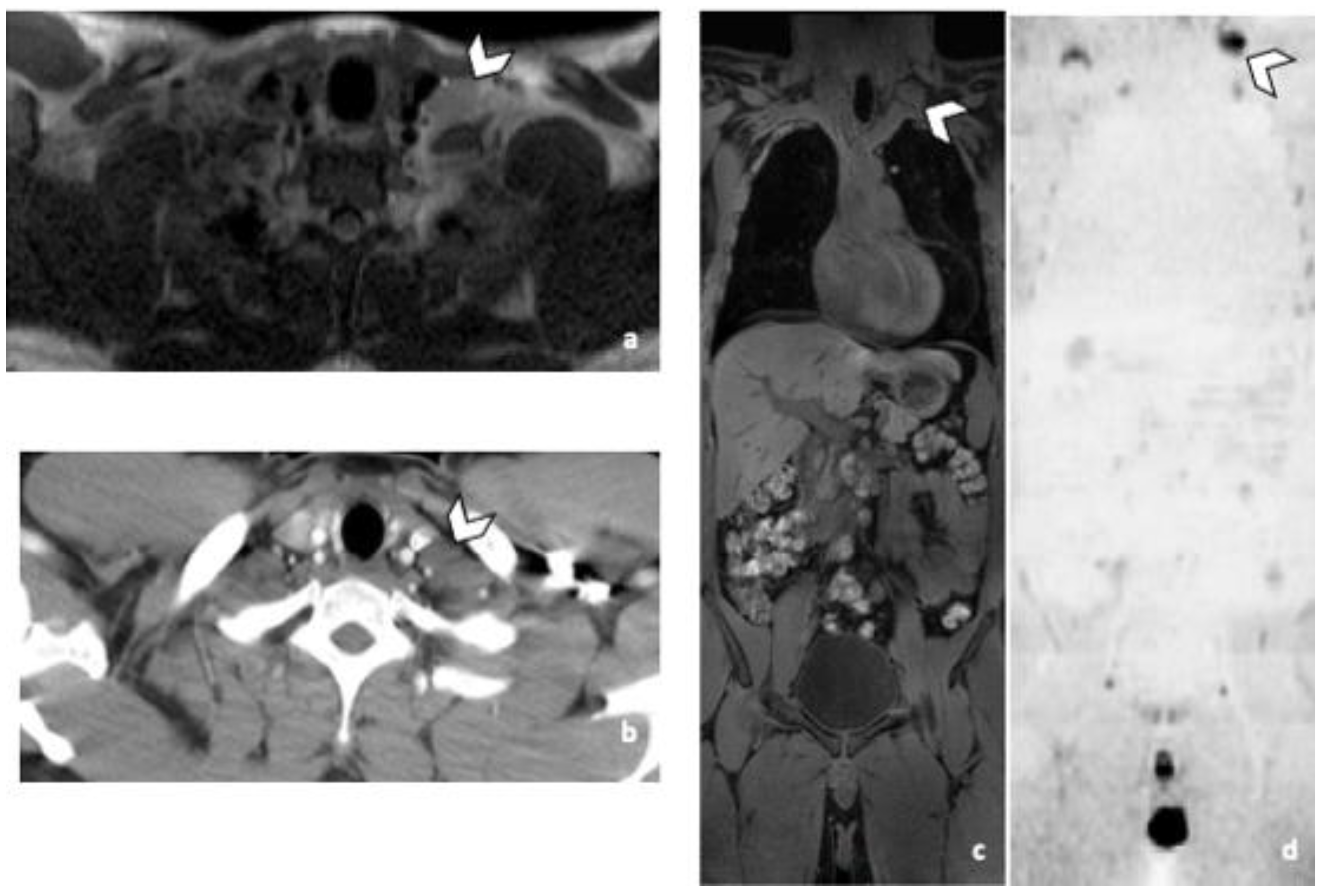
3.3.2. DLN Metastases
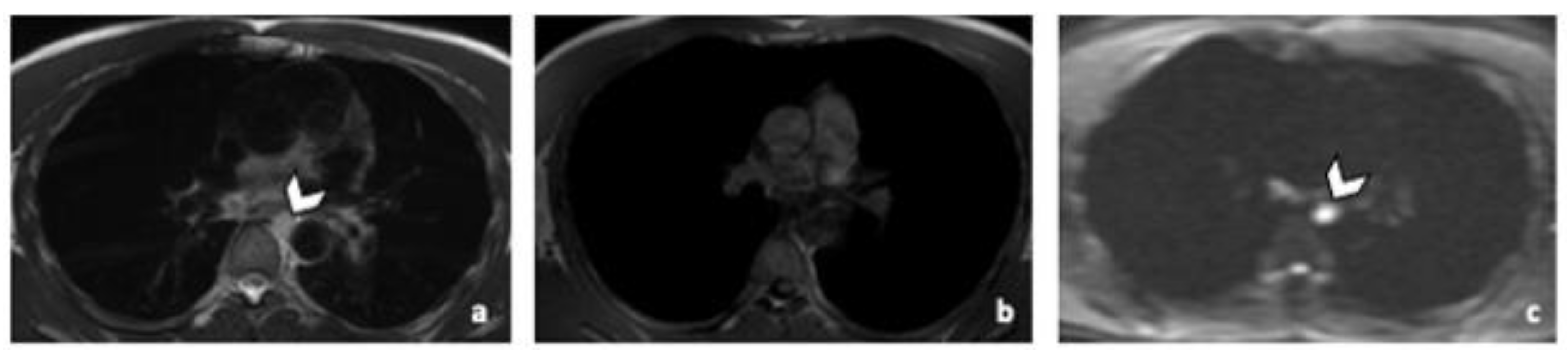
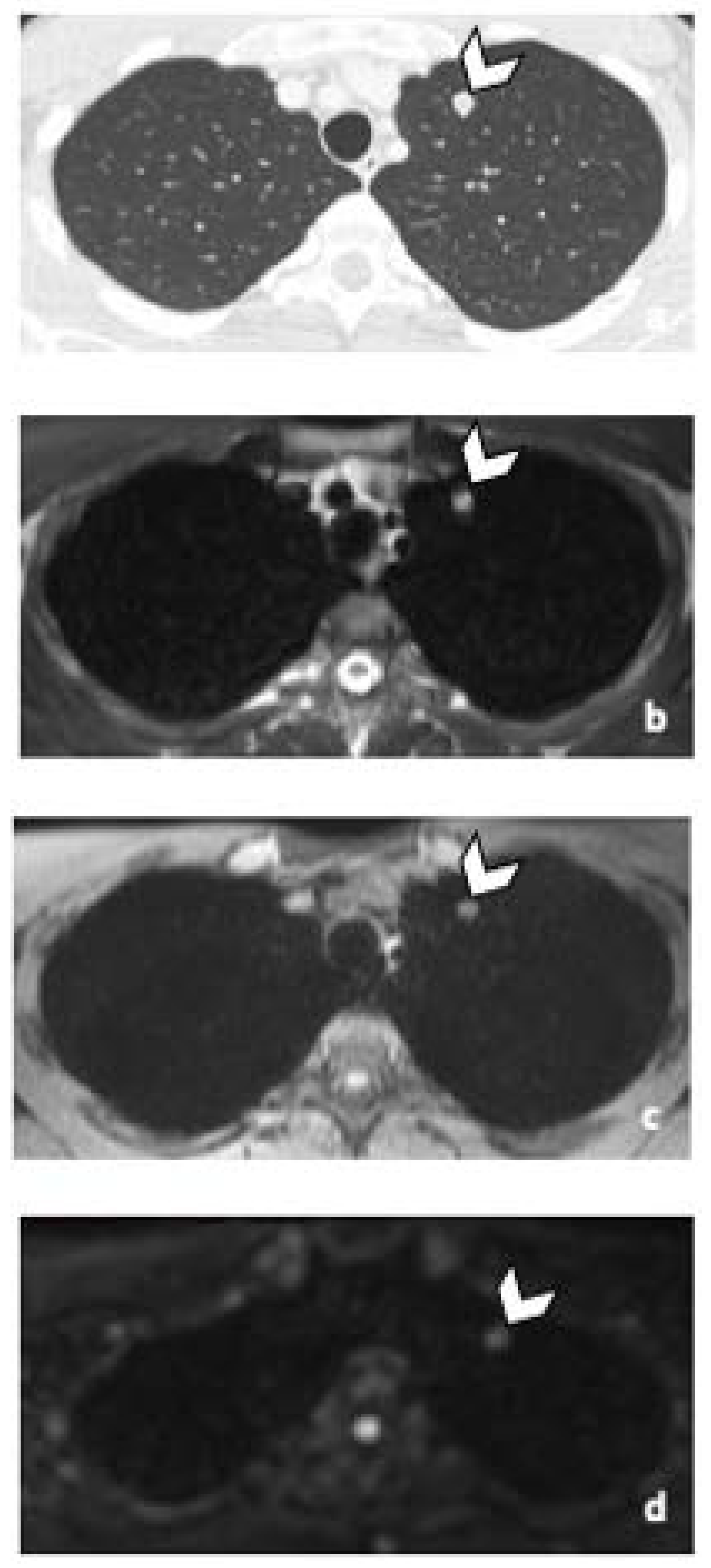
3.3.3. Other Metastatic Lesions
3.3.4. Comparison of Acc
3.4. Image Quality Assessment
3.4.1. Likert Scale
3.4.2. SNR & CNR
3.4.3. Radiation Dose Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cancer Institute. The Surveillance, Epidemiology, and End Results Program: Cancer Stat Facts: Testicular Cancer. Available online: https://seer.cancer.gov/statfacts/html/testis.html (accessed on 27 December 2012).
- Bosl, G.J.; Motzer, R.J. Testicular germ-cell cancer. N. Engl. J. Med. 1997, 337, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Reydin, E.I.; Barrisford, G.W.; Feldman, A.S.; Preston, M.A. Testicular cancer: What the radiologist needs to know. AJR Am. J. Roentgenol. 2013, 200, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.D.J.; Morse, M.J.; Grando, R.; Kleinert, E.L.; Whitmore, W.F. Lymphoscintigraphic studies of lymphatic drainage from the testes. Clin. Nucl. Med. 1986, 11, 823–827. [Google Scholar] [CrossRef]
- Groll, R.J.; Warde, P.; Jewett, M.A. A comprehensive systematic review of testicular germ cell tumor surveillance. Crit Rev. Oncol. Hematol. 2007, 64, 182–197. [Google Scholar] [CrossRef]
- Sternberg, C.N. The management of stage I testis cancer. Urol. Clin. N. Am. 1998, 25, 435–449. [Google Scholar] [CrossRef]
- Honecker, F.; Aparicio, J.; Berney, D.; Beyer, J.; Bokemeyer, C.; Cathomas, R.; Clarke, N.; Cohn-Cedermark, G.; Daugaard, G.; Dieckmann, K.-P.; et al. ESMO Consensus Conference on testicular germ cell cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, 1658–1686. [Google Scholar] [CrossRef]
- Munro, L. Basics of Radiation Protection How to Achieve ALARA: Working Tips and Guidelines; WHO Library: Valletta, Malta, 2004. [Google Scholar]
- Lecouvet, F.E. Whole-Body MR Imaging: Musculoskeletal Applications. Radiology 2016, 279, 345–365. [Google Scholar] [CrossRef]
- Schmidt, G.; Dinter, D.; Reiser, M.F.; Schoenberg, S.O. The uses and limitations of whole-body magnetic resonance imaging. Dtsch Arztebl. Int. 2010, 107, 383–389. [Google Scholar] [CrossRef]
- Pasoglou, V.; Michoux, N.; Larbi, A.; Van Nieuwenhove, S.; Lecouvet, F. Whole Body MRI and oncology: Recent major advances. Br. J. Radiol. 2018, 91, 20170664. [Google Scholar] [CrossRef]
- The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007, 37, 1–332.
- Koh, D.M.; Hughes, M.; Husband, J.E. Cross-sectional imaging of nodal metastases in the abdomen and pelvis. Abdom. Imaging 2006, 31, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Lengele, B.; Nyssen-Behets, C.; Scalliet, P. Anatomical bases for the radiological delineation of lymph node areas. Upper limbs, chest and abdomen. Radiother. Oncol. 2007, 84, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Gwet, K.L. Computing inter-rater reliability and its variance in the presence of high agreement. Br. J. Math. Stat. Psychol. 2008, 61 Pt 1, 29–48. [Google Scholar] [CrossRef] [Green Version]
- Sprawls, P. AAPM tutorial. CT image detail and noise. Radiographics 1992, 12, 1041–1046. [Google Scholar] [PubMed] [Green Version]
- Albers, P.; Albrecht, W.; Algaba, F.; Bokemeyer, C.; Cohn-Cedermark, G.; Fizazi, K.; Horwich, A.; Laguna, M. EAU guidelines on testicular cancer: 2011 update. European Association of Urology. Actas Urol. Esp. 2012, 36, 127–145. [Google Scholar] [CrossRef]
- Cardis, E.; Vrijheid, M.; Blettner, M.; Gilbert, E.; Hakama, M.; Hill, C.; Howe, G.; Kaldor, J.; Muirhead, C.; Schubauer-Berigan, M.; et al. Risk of cancer after low doses of ionising radiation: Retrospective cohort study in 15 countries. BMJ 2005, 331, 77. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, A.M.; Jones, A.E.; Tumlin, J.A.; Kline, J.A. Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient setting. Clin. J. Am. Soc. Nephrol. 2010, 5, 4–9. [Google Scholar] [CrossRef]
- Laukka, M.; Mannisto, S.; Beule, A.; Kouri, M.; Blomqvist, C. Comparison between CT and MRI in detection of metastasis of the retroperitoneum in testicular germ cell tumors: A prospective trial. Acta Oncol. 2020, 59, 660–665. [Google Scholar] [CrossRef]
- Sohaib, S.; Koh, D.; Barbachano, Y.; Parikh, J.; Husband, J.; Dearnaley, D.; Horwich, A.; Huddart, R. Prospective assessment of MRI for imaging retroperitoneal metastases from testicular germ cell tumours. Clin. Radiol. 2009, 64, 362–367. [Google Scholar] [CrossRef]
- Mosavi, F.; Laurell, A.; Ahlstrom, H. Whole body MRI, including diffusion-weighted imaging in follow-up of patients with testicular cancer. Acta Oncol. 2015, 54, 1763–1769. [Google Scholar] [CrossRef] [Green Version]
- Narine, R.; Osman, H.; Wongwaisayawan, S.; Morgan, S.; Lavallee, L.T.; Schieda, N. Unenhanced MRI of the abdomen and pelvis for surveillance of patients with stage 1 testicular cancer post-radical orchiectomy. Abdom. Radiol. 2021, 46, 1157–1162. [Google Scholar] [CrossRef]
- Pierorazio, P.M.; Cheaib, J.G.; Tema, G.; Patel, H.D.; Gupta, M.; Sharma, R.; Zhang, A.; Bass, E.B. Performance Characteristics of Clinical Staging Modalities for Early Stage Testicular Germ Cell Tumors: A Systematic Review. J. Urol. 2020, 203, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Baumann, T.; Ludwig, U.; Pache, G.; Gall, C.; Saueressig, U.; Fisch, D.; Stankovic, Z.; Bartholomae, J.-P.; Honal, M.; Bley, T.A.; et al. Detection of pulmonary nodules with move-during-scan magnetic resonance imaging using a free-breathing turbo inversion recovery magnitude sequence. Investig. Radiol. 2008, 43, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Vogt, F.M.; Herborn, C.U.; Hunold, P.; Lauenstein, T.C.; Schröder, T.; Debatin, J.F.; Barkhausen, J. HASTE MRI versus chest radiography in the detection of pulmonary nodules: Comparison with MDCT. AJR Am. J. Roentgenol. 2004, 183, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.M.; Fain, S.; Schiebler, M.; Nagle, S. Optimized 3D ultrashort echo time pulmonary MRI. Magn. Reson. Med. 2013, 70, 1241–1250. [Google Scholar] [CrossRef] [Green Version]
- Tombal, B.; Stordeur, J.V.S.; de Meerleer, G.; Gil, T.; Renard, L.; Rorive, S.; Rottey, S.; Salmon, I.; Schrijvers, D.; Villeirs, G. Soutien Scientifique au Collège d’Oncologie: Mise à Jour Des Recommandations de Bonne Pratique Pour la Prise en Charge du Cancer du Testicule. KCE Reports 142B. 2010. Available online: http://www.kce.fgov.be (accessed on 2 February 2020).
- Tsili, A.C.; Bertolotto, M.; Turgut, A.T.; Dogra, V.; Freeman, S.; Rocher, L.; Belfield, J.; Studniarek, M.; Ntorkou, A.; Derchi, L.E.; et al. MRI of the scrotum: Recommendations of the ESUR Scrotal and Penile Imaging Working Group. Eur. Radiol. 2018, 28, 31–43. [Google Scholar] [CrossRef]
- Motzer, R.J.; Agarwal, N.; Beard, C.; Bolger, G.B.; Boston, B.; Carducci, M.A.; Choueiri, T.K.; Figlin, R.A.; Fishman, M.; Hancock, S.L.; et al. NCCN Clinical Practice Guidelines in Oncology: Testicular Cancer. J. Nat. Compr. Cancer Netw. JNCCN 2009, 7, 672–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Target | Thorax-Abdomen-Pelvis | Thorax-Abdomen-Pelvis | Thorax-Abdomen-Pelvis |
|---|---|---|---|
| Acquisition | T2W | T1W DIXON | Diffusion |
| Time (min:s) | 2:45 (55 s × 3 stacks) | 0:47 (19 s × 3 stacks) | 11:40 (228 s × 3 stacks) |
| Sequence type | TSE | MDIXON | Diffusion |
| Plane | axial | coronal | axial |
| Slice × thickness/gap | 70 × 4 mm/0 | 153 × 3 mm/−1.5 (overlap) | 50 × 6 mm/0.1 |
| FOV, matrix | 400 × 300, 308 × 188 | 300 × 450, 200 × 300 | 440 × 348, 100 × 73 |
| TR, TE, NSA, TF (TI for Diffusion) | 788, 80, 1.74 | 3.6, 1.32/2.3, 1,- | 6329, 66, 1, - (250) |
| Specific parameters | b-values: 0, 50, 150, 1000 |
| Patients’ Characteristics (N = 43) | |
|---|---|
| Age (years) at diagnosis mean (SD) | 35.16 (9.92) |
| Primary Histology n (%) | |
| Seminoma | 19 (44.2) |
| Non-seminoma components | 24 (55.8) |
| Mixed Germ Cell Tumor | 9 (37.5) |
| Embryonal Carcinoma | 16 (66.7) |
| Yolk Sac Tumor | 6 (25) |
| Teratoma | 10 (41.7) |
| Trophoblastic Tumor | 7 (29.2) |
| Clinical Staging n (%) | |
| Stage I | 21 (48.8) |
| Stage II | 5 (11.6) |
| Stage III | 17 (39.6) |
| Stage IV | 0 |
| IGCCCG n (%) | |
| Good | 15 (68.2) |
| Intermediate | 6 (27.2) |
| Poor | 1 (4.6) |
| Treatment after orchidectomy n (%) | |
| Chemotherapy | 21 (48.8) |
| Radiotherapy | 1 (2.4) |
| Active surveillance | 21 (48.8) |
| RPLN | ||||||||
|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | Se | Sp | Acc | AC1 | |
| CT | 22 | 0 | 0 | 21 | 100 [85; 100] | 100 [84; 100] | 100 [92; 100] | 1.00 [1.00; 1.00] |
| totalWB-MRI | 22 | 0 | 0 | 21 | 100 [85; 100] | 100 [84; 100] | 100 [92; 100] 1 | 1.00 [1.00; 1.00] |
| T1W + DWI | 22 | 0 | 0 | 21 | 100 [85; 100] | 100 [84; 100] | 100 [92; 100] | 1.00 [1.00; 1.00] |
| T2W + DWI | 22 | 0 | 0 | 21 | 100 [85; 100] | 100 [84; 100] | 100 [92; 100] | 1.00 [1.00; 1.00] |
| CT | 22 | 0 | 0 | 21 | 100 [85; 100] | 100 [84; 100] | 100 [92; 100] | 1.00 [1.00; 1.00] |
| totalWB-MRI | 22 | 0 | 0 | 21 | 100 [85; 100] | 100 [84; 100] | 100 [92; 100] 2 | 1.00 [1.00; 1.00] |
| T1W + DWI | 22 | 0 | 0 | 21 | 100 [85; 100] | 100 [84; 100] | 100 [92; 100] | 1.00 [1.00; 1.00] |
| T2W + DWI | 22 | 0 | 0 | 21 | 100 [85; 100] | 100 [84; 100] | 100 [92; 100] | 1.00 [1.00; 1.00] |
| DLN | ||||||||
| TP | FP | FN | TN | Se | Sp | Acc | AC1 | |
| CT | 8 | 0 | 3 | 32 | 73 [39; 94] | 100 [89; 100] | 93 [81; 99] | 0.89 [0.77; 1.02] |
| totalWB-MRI | 11 | 0 | 0 | 32 | 100 [72; 100] | 100 [89; 100] | 100 [92; 100] 3 | 1.00 [1.00; 1.00] |
| T1W + DWI | 11 | 0 | 0 | 32 | 100 [72; 100] | 100 [89; 100] | 100 [92; 100] | 1.00 [1.00; 1.00] |
| T2W + DWI | 11 | 0 | 0 | 32 | 100 [72; 100] | 100 [89; 100] | 100 [92; 100] | 1.00 [1.00; 1.00] |
| CT | 9 | 1 | 2 | 31 | 82 [48; 98] | 97 [84; 100] | 93 [81; 99] | 0.89 [0.76; 1.02] |
| totalWB-MRI | 11 | 1 | 0 | 31 | 100 [72; 100] | 97 [84; 100] | 98 [88; 100] 4 | 0.96 [0.89; 1.04] |
| T1W + DWI | 11 | 1 | 0 | 31 | 100 [72; 100] | 97 [84; 100] | 98 [88; 100] | 0.96 [0.89; 1.04] |
| T2W + DWI | 11 | 1 | 0 | 31 | 100 [72; 100] | 97 [84; 100] | 98 [88; 100] | 0.96 [0.89; 1.04] |
| Other | ||||||||
| TP | FP | FN | TN | Se | Sp | Acc | AC1 | |
| CT | 8 | 0 | 1 | 34 | 89 [52; 100] | 100 [90; 100] | 98 [88; 100] | 0.97 [0.89; 1.04] |
| totalWB-MRI | 7 | 0 | 2 | 34 | 78 [40; 97] | 100 [90; 100] | 95 [84; 99] 5 | 0.93 [0.84; 1.03] |
| T1W + DWI | 7 | 0 | 2 | 34 | 78 [40; 97] | 100 [90; 100] | 95 [84; 99] | 0.93 [0.84; 1.03] |
| T2W + DWI | 7 | 0 | 2 | 34 | 78 [40; 97] | 100 [90; 100] | 95 [84; 99] | 0.93 [0.84; 1.03] |
| CT | 8 | 0 | 1 | 34 | 89 [52, 100] | 100 [90; 100] | 98 [88; 100] | 0.97 [0.90; 1.03] |
| totalWB-MRI | 7 | 0 | 2 | 34 | 78 [40; 97] | 100 [90; 100] | 95 [84; 99] 6 | 0.93 [0.84; 1.03] |
| T1W + DWI | 7 | 0 | 2 | 34 | 78 [40; 97] | 100 [90; 100] | 95 [84; 99] | 0.93 [0.84; 1.03] |
| T2W + DWI | 7 | 0 | 2 | 34 | 78 [40; 97] | 100 [90; 100] | 95 [84; 99] | 0.93 [0.84; 1.03] |
| RPLN | DLN | Other | |
|---|---|---|---|
| CT | 1.00 [1.00; 1.00] | 0.93 [0.83; 1.03] | 0.93 [0.84; 1.03] |
| totalWB-MRI | 1.00 [1.00; 1.00] | 0.96 [0.89; 1.04] | 1.00 [1.00; 1.00] |
| T1W + DWI | 1.00 [1.00; 1.00] | 0.96 [0.89; 1.04] | 1.00 [1.00; 1.00] |
| T2W + DWI | 1.00 [1.00; 1.00] | 0.96 [0.89; 1.04] | 1.00 [1.00; 1.00] |
| RPLN (Total Number = 22) | |||||||
|---|---|---|---|---|---|---|---|
| Likert Score | |||||||
| 0 | 1 | 2 | 3 | 4 | 5 | Total Score | |
| Very Poor | Poor | Fair | Good | Very Good | Excellent | ||
| Reader 1 | |||||||
| DWI | 0 | 0 | 0 | 0 | 5 | 17 | 105 |
| 0% | 0% | 0% | 0% | 22.7% | 77.3% | ||
| T2W | 0 | 0 | 0 | 4 | 13 | 5 | 89 |
| 0% | 0% | 0% | 18.2% | 59.1% | 22.7% | ||
| T1W DIXON | 0 | 0 | 0 | 12 | 8 | 2 | 78 |
| 0% | 0% | 0% | 54.5% | 36.4% | 9.1% | ||
| Reader 2 | |||||||
| DWI | 0 | 0 | 0 | 0 | 7 | 15 | 103 |
| 0% | 0% | 0% | 0% | 31.8% | 68.2% | ||
| T2 | 0 | 0 | 0 | 5 | 11 | 6 | 89 |
| 0% | 0% | 0% | 22.7% | 50%% | 22.3%% | ||
| T1W DIXON | 0 | 0 | 0 | 11 | 11 | 0 | 77 |
| 0% | 0% | 0% | 50.0% | 50.0% | 0.0% | ||
| DLN (Total Number = 11) | |||||||
| Likert Score | |||||||
| 0 | 1 | 2 | 3 | 4 | 5 | Total score | |
| very poor | poor | fair | good | very good | excellent | ||
| Reader 1 | |||||||
| DWI | 0 | 0 | 0 | 0 | 3 | 8 | 52 |
| 0% | 0% | 0% | 0.0% | 27.3% | 72.7% | ||
| T2 | 0 | 0 | 0 | 4 | 5 | 2 | 42 |
| 0% | 0% | 0% | 36.4% | 45.4% | 18.2% | ||
| T1W DIXON | 0 | 0 | 0 | 7 | 4 | 0 | 37 |
| 0% | 0% | 0% | 63.6% | 36.4% | 0.0% | ||
| Reader 2 | |||||||
| DWI | 0 | 0 | 0 | 0 | 3 | 8 | 52 |
| 0% | 0% | 0% | 0.0% | 27.3% | 72.7% | ||
| T2W | 0 | 0 | 0 | 6 | 3 | 2 | 40 |
| 0% | 0% | 0% | 54.5% | 27.3% | 18.2% | ||
| T1W DIXON | 0 | 0 | 0 | 6 | 4 | 1 | 39 |
| 0% | 0% | 0% | 54.5% | 36.4% | 9.1% | ||
| SNR | CNR | |
|---|---|---|
| DWI | 392 [214; 607] | 335 [173; 584] |
| T1W | 43.5 [36.1; 53.0] | 61.2 [50.0; 80.2] |
| T2W | 97.1 [61.3; 112] | 84.9 [56.4; 122] |
| Significance level after Bonferroni correction: p = 0.0167 | ||
| SNRDWI vs. SNRT1W: p < 0.0001 | ||
| SNRDWI vs. SNRT2W: p < 0.0001 | ||
| SNRT2W vs. SNRT1W: p < 0.0002 | ||
| CNRDWI vs. CNRT1W: p < 0.0001 | ||
| CNRDWI vs. CNRT2W: p < 0.0001 | ||
| CNRT2W vs. CNRT1W: p = 0.0982 | ||
| Recommended Minimum Follow up for Group 1 | Year 1 | Year 2 | Year 3 | Year 4 and 5 |
|---|---|---|---|---|
| Abdominal CT | 2 times | 2 times | at 36 months | 1 at 60 months |
| Mean accumulated Effective dose per year (mSv) * | 10.2 | 10.2 | 5.1 | 5.1 |
| Recommended minimum follow up for Group 2 | Year 1 | Year 2 | Year 3 | Year 4 and 5 |
| Chest X-ray | 2 times | 2 times | 1 if LVI + | At 60 months if LVI+ |
| Abdominal CT | 2 times | at 24 months | at 36 months | at 60 months (optional) |
| Mean accumulated Effective dose per year (mSv) ** | 10.201 | 5.101 | 5.1005 | 5.1005 |
| Recommended minimum follow up for Group 3 | Year 1 | Year 2 | Year 3 | Year 4 and 5 |
| Chest X-ray | 1–2 | 1 | 1 | 1 |
| Abdominal CT | 1–2 | at 24 months | at 36 months | at 60 months (optional) |
| Mean accumulated Effective dose per year (mSv) | 5.1005–10.201 | 5.1005 | 5.1005 | 5.1005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasoglou, V.; Van Nieuwenhove, S.; Van Damme, J.; Michoux, N.; Van Maanen, A.; Annet, L.; Machiels, J.-P.; Tombal, B.; Lecouvet, F.E. Whole Body MRI in the Detection of Lymph Node Metastases in Patients with Testicular Germ Cell Cancer. Life 2022, 12, 212. https://doi.org/10.3390/life12020212
Pasoglou V, Van Nieuwenhove S, Van Damme J, Michoux N, Van Maanen A, Annet L, Machiels J-P, Tombal B, Lecouvet FE. Whole Body MRI in the Detection of Lymph Node Metastases in Patients with Testicular Germ Cell Cancer. Life. 2022; 12(2):212. https://doi.org/10.3390/life12020212
Chicago/Turabian StylePasoglou, Vassiliki, Sandy Van Nieuwenhove, Julien Van Damme, Nicolas Michoux, Aline Van Maanen, Laurence Annet, Jean-Pascal Machiels, Bertrand Tombal, and Frederic E. Lecouvet. 2022. "Whole Body MRI in the Detection of Lymph Node Metastases in Patients with Testicular Germ Cell Cancer" Life 12, no. 2: 212. https://doi.org/10.3390/life12020212
APA StylePasoglou, V., Van Nieuwenhove, S., Van Damme, J., Michoux, N., Van Maanen, A., Annet, L., Machiels, J.-P., Tombal, B., & Lecouvet, F. E. (2022). Whole Body MRI in the Detection of Lymph Node Metastases in Patients with Testicular Germ Cell Cancer. Life, 12(2), 212. https://doi.org/10.3390/life12020212






