The Anti-Inflammatory, Anti-Apoptotic, and Antioxidant Effects of a Pomegranate-Peel Extract against Acrylamide-Induced Hepatotoxicity in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experiments
2.2. Plant Samples and Extraction
2.3. HPLC Analysis for Phenol and Flavonoid Compounds
2.4. Blood Parameter Measurement
2.5. Measurement of Inflammatory and Anti-Inflammatory Cytokines
2.6. Quantification of Genes by Real-Time PCR (qRT-PCR)
2.7. Histopathological Examination
2.8. Immunohistochemical Study
2.9. Statistical Analysis
3. Results
3.1. Impacts of Pomegranate-Peel Extract on Acrylamide-Induced Liver Dysfunction in Rats
3.2. Impacts of Pomegranate-Peel Extract on Acrylamide-Induced Alterations of Serum MDA, Catalase, GSH and SOD Levels
3.3. Impacts of Pomegranate-Peel Extract on Acrylamide-Induced Alterations in Cytokine Levels
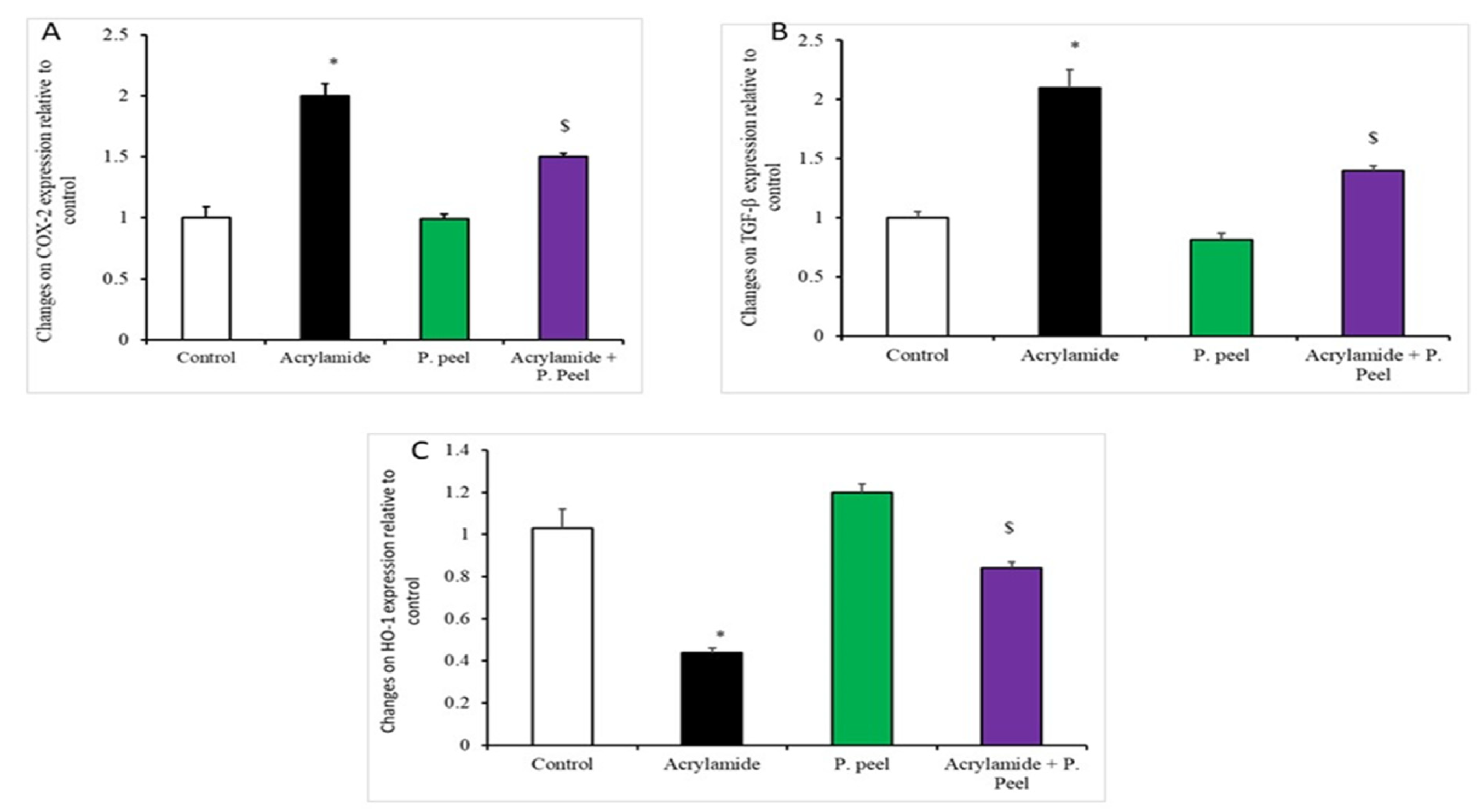
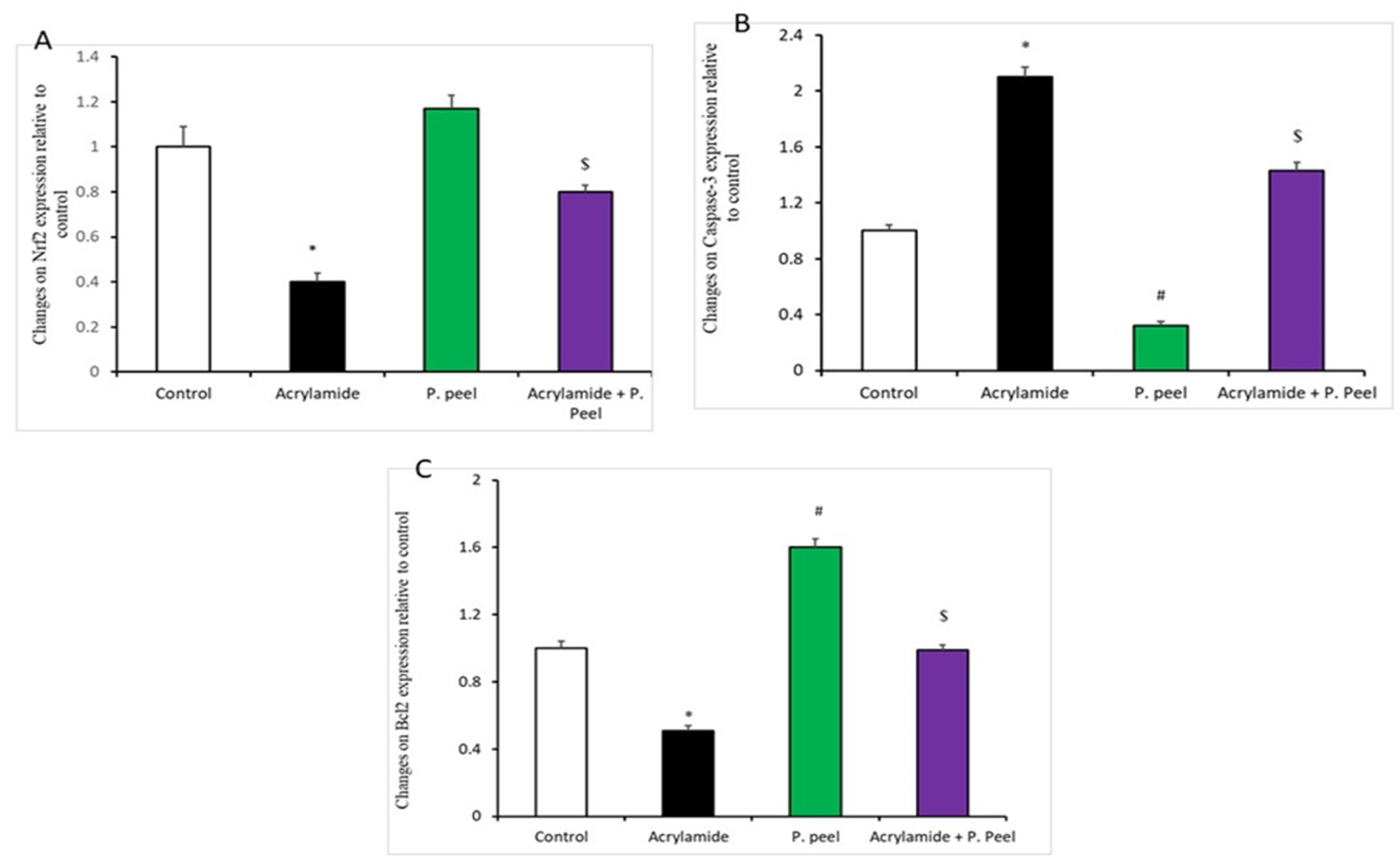
3.4. Impacts of Pomegranate-Peel Extract on the Expression of Liver Genes in Acrylamide-Induced Liver Dysfunction in Rats
3.5. Impacts of Pomegranate-Peel Extract on the Liver Architecture (Immunohistochemical and Histopathological Study)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Statement
References
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- Becalski, A.; Lau, B.; Lewis, D.; Seaman, S. Acrylamide in foods: Occurrence and sources. J. Agric. Food Chem. 2003, 51, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Moorman, W.J.; Reutman, S.S.; Shaw, P.B.; Blade, L.M.; Marlow, D.; Vesper, H.; Clark, J.C.; Schrader, S.M. Occupational exposure to acrylamide in closed system production plants: Air levels and biomonitoring. J. Toxicol. Environ. Health Part A 2012, 75, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Turkington, C.; Mitchell, D. The Encyclopedia of Poisons and Antidotes; Facts on File: New York, NY, USA, 2010. [Google Scholar]
- Fuhr, U.; Boettcher, M.I.; Kinzig-Schippers, M.; Weyer, A.; Jetter, A.; Lazar, A.; Taubert, D.; Tomalik-Scharte, D.; Pournara, P.; Jakob, V. Toxicokinetics of acrylamide in humans after ingestion of a defined dose in a test meal to improve risk assessment for acrylamide carcinogenicity. Cancer Epidemiol. Prev. Biomark. 2006, 15, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Adams, A.; Hamdani, S.; Van Lancker, F.; Méjri, S.; De Kimpe, N. Stability of acrylamide in model systems and its reactivity with selected nucleophiles. Food Res. Int. 2010, 43, 1517–1522. [Google Scholar] [CrossRef]
- Ghanayem, B.I.; Witt, K.L.; Kissling, G.E.; Tice, R.R.; Recio, L. Absence of acrylamide-induced genotoxicity in CYP2E1-null mice: Evidence consistent with a glycidamide-mediated effect. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 578, 284–297. [Google Scholar] [CrossRef]
- Alturfan, A.A.; Tozan-Beceren, A.; Şehirli, A.Ö.; Demiralp, E.; Şener, G.; Omurtag, G.Z. Resveratrol ameliorates oxidative DNA damage and protects against acrylamide-induced oxidative stress in rats. Mol. Biol. Rep. 2012, 39, 4589–4596. [Google Scholar] [CrossRef] [PubMed]
- Teodor, V.; Cuciureanu, M.; Filip, C.; Zamosteanu, N.; Cuciureanu, R. Protective effects of selenium on acrylamide toxicity in the liver of the rat. Effects on the oxidative stress. Rev. Med. Chir. Soc. Med. Nat. Iasi 2011, 115, 612–618. [Google Scholar]
- Pereira, P.H.; Oliveira, T.; Rosa, M.F.; Cavalcante, F.L.; Moates, G.K.; Wellner, N.; Waldron, K.W.; Azeredo, H.M. Pectin extraction from pomegranate peels with citric acid. Int. J. Biol. Macromol. 2016, 88, 373–379. [Google Scholar] [CrossRef]
- Joseph, M.M.; Aravind, S.R.; George, S.K.; Varghese, S.; Sreelekha, T.T. A galactomannan polysaccharide from Punica granatum imparts in vitro and in vivo anticancer activity. Carbohydr. Polym. 2013, 98, 1466–1475. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. An integrated green biorefinery approach towards simultaneous recovery of pectin and polyphenols coupled with bioethanol production from waste pomegranate peels. Bioresour. Technol. 2018, 266, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Bermudez, B.; de la Paz, S.M.; Jaramillo, S.; Abia, R.; Muriana, F.J. Virgin olive oil and hypertension. Curr. Vasc. Pharmacol. 2016, 14, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Hsu, Y.F.; Lin, T.C.; Hsu, H.Y. Antioxidant and hepatoprotective effects of punicalagin and punicalin on acetaminophen-induced liver damage in rats. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2001, 15, 206–212. [Google Scholar] [CrossRef]
- Alkathiri, B.; El-Khadragy, M.F.; Metwally, D.M.; Al-Olayan, E.M.; Bakhrebah, M.A.; Abdel Moneim, A.E. Pomegranate (Punica granatum) Juice Shows Antioxidant Activity against Cutaneous Leishmaniasis-Induced Oxidative Stress in Female BALB/c Mice. Int. J. Environ. Res. Public Health 2017, 14, 1592. [Google Scholar] [CrossRef] [Green Version]
- Amri, Z.; Ghorbel, A.; Turki, M.; Akrout, F.M.; Ayadi, F.; Elfeki, A.; Hammami, M. Effect of pomegranate extracts on brain antioxidant markers and cholinesterase activity in high fat-high fructose diet induced obesity in rat model. BMC Complement. Altern. Med. 2017, 17, 339. [Google Scholar] [CrossRef] [Green Version]
- Benchagra, L.; Berrougui, H.; Islam, M.O.; Ramchoun, M.; Boulbaroud, S.; Hajjaji, A.; Fulop, T.; Ferretti, G.; Khalil, A. Antioxidant Effect of Moroccan Pomegranate (Punica granatum L. Sefri Variety) Extracts Rich in Punicalagin against the Oxidative Stress Process. Foods 2021, 10, 2219. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Marhuenda-Egea, F.C.; Hernandez, F.; Rosas-Burgos, E.C.; Burgos-Hernandez, A.; Carbonell-Barrachina, A.A. Biological Activity of Conventional and Organic Pomegranate Juices: Antioxidant and Antimutagenic Potential. Plant Foods Hum. Nutr. 2016, 71, 375–380. [Google Scholar] [CrossRef]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 114, 108–111. [Google Scholar] [CrossRef]
- Di Stefano, V.; Pitonzo, R.; Novara, M.E.; Bongiorno, D.; Indelicato, S.; Gentile, C.; Avellone, G.; Bognanni, R.; Scandurra, S.; Melilli, M.G. Antioxidant activity and phenolic composition in pomegranate (Punica granatum L.) genotypes from south Italy by UHPLC-Orbitrap-MS approach. J. Sci. Food Agric. 2019, 99, 1038–1045. [Google Scholar] [CrossRef]
- Dzugan, M.; Wesolowska, M.; Zagula, G.; Puchalski, C. The comparison of the physicochemical parameters and antioxidant activity of homemade and commercial pomegranate juices. Acta Sci. Pol. Technol. Aliment. 2018, 17, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposto, S.; Veneziani, G.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Daidone, L.; Gironi, G.; Servili, M. Chemical Composition, Antioxidant Activity, and Sensory Characterization of Commercial Pomegranate Juices. Antioxidants 2021, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Pereira, J.A.; Lopez-Cortes, I.; Salazar, D.M.; Ramalhosa, E.C. Physicochemical Changes and Antioxidant Activity of Juice, Skin, Pellicle and Seed of Pomegranate (cv. Mollar de Elche) at Different Stages of Ripening. Food Technol. Biotechnol. 2015, 53, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Pontonio, E.; Montemurro, M.; Pinto, D.; Marzani, B.; Trani, A.; Ferrara, G.; Mazzeo, A.; Gobbetti, M.; Rizzello, C.G. Lactic Acid Fermentation of Pomegranate Juice as a Tool to Improve Antioxidant Activity. Front. Microbiol. 2019, 10, 1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, M.; Fanali, C.; Tripodo, G.; Dugo, P.; Muleo, R.; Dugo, L.; De Gara, L.; Mondello, L. Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: Application to different Italian varieties. Anal. Bioanal. Chem. 2018, 410, 3507–3520. [Google Scholar] [CrossRef]
- Tarantino, A.; Difonzo, G.; Disciglio, G.; Frabboni, L.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Fresh pomegranate juices from cultivars and local ecotypes grown in southeastern Italy: Comparison of physicochemical properties, antioxidant activity and bioactive compounds. J. Sci. Food Agric. 2021, 102, 1185–1192. [Google Scholar] [CrossRef]
- Salles, T.S.; Meneses, M.D.F.; Caldas, L.A.; Sa-Guimaraes, T.E.; de Oliveira, D.M.; Ventura, J.A.; Azevedo, R.C.; Kuster, R.M.; Soares, M.R.; Ferreira, D.F. Virucidal and antiviral activities of pomegranate (Punica granatum) extract against the mosquito-borne Mayaro virus. Parasites Vectors 2021, 14, 443. [Google Scholar] [CrossRef]
- Ahmed, M.M.; El-Shazly, A.S.; El-Shehawi, A.M.; Alkafafy, M.E. Anti-obesity effects of Taif and Egyptian pomegranates: Molecular study. Biosci. Biotechnol. Biochem. 2015, 79, 598–609. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Chu, Q.; Zheng, X. Pomegranate fruit pulp polyphenols reduce diet-induced obesity with modulation of gut microbiota in mice. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef]
- Aboelsoued, D.; Abo-Aziza, F.A.M.; Mahmoud, M.H.; Abdel Megeed, K.N.; Abu El Ezz, N.M.T.; Abu-Salem, F.M. Anticryptosporidial effect of pomegranate peels water extract in experimentally infected mice with special reference to some biochemical parameters and antioxidant activity. J. Parasit. Dis. 2019, 43, 215–228. [Google Scholar] [CrossRef]
- Kujawska, M.; Jourdes, M.; Witucki, L.; Karazniewicz-Lada, M.; Szulc, M.; Gorska, A.; Mikolajczak, P.L.; Teissedre, P.L.; Jodynis-Liebert, J. Pomegranate Juice Ameliorates Dopamine Release and Behavioral Deficits in a Rat Model of Parkinson’s Disease. Brain Sci. 2021, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Turgut, S.S.; Isikci, F.; Soyer, A. Antioxidant activity of pomegranate peel extract on lipid and protein oxidation in beef meatballs during frozen storage. Meat Sci. 2017, 129, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Zhu, C.; Li, Y.; Zhang, Y.; Duan, Z.; Yang, X. Optimization for pectinase-assisted extraction of polysaccharides from pomegranate peel with chemical composition and antioxidant activity. Int. J. Biol. Macromol. 2018, 109, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Malli Sureshbabu, N.; Ranjan, M.; Ranganath, A.; Siddique, R. The Antimicrobial Effect of Pomegranate Peel Extract versus Chlorhexidine in High Caries Risk Individuals Using Quantitative Real-Time Polymerase Chain Reaction: A Randomized Triple-Blind Controlled Clinical Trial. Int. J. Dent. 2021, 2021, 5563945. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Effect of Blanching Pomegranate Seeds on Physicochemical Attributes, Bioactive Compounds and Antioxidant Activity of Extracted Oil. Molecules 2020, 25, 2554. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Effects of Enzymatic Pretreatment of Seeds on the Physicochemical Properties, Bioactive Compounds, and Antioxidant Activity of Pomegranate Seed Oil. Molecules 2021, 26, 4575. [Google Scholar] [CrossRef]
- Al-Sodany, Y.M.; Bazaid, S.A.; Mosallam, H.A. Medicinal Plants in Saudi Arabia: I. Sarrwat Mountains at Taif, KSA. Acad. J. Plant Sci. 2013, 6, 134–145. [Google Scholar] [CrossRef]
- Dessoky, E.S.; Alqurashi, M.; Alotaibi, S.S.; Sadik, A.S. DNA Fingerprinting of in vitro Micropropagated Pomegranate Genotypes. Pak. J. Biol. Sci. 2020, 23, 619–627. [Google Scholar] [CrossRef]
- Elhelaly, A.E.; AlBasher, G.; Alfarraj, S.; Almeer, R.; Bahbah, E.I.; Fouda, M.M.A.; Bungău, S.G.; Aleya, L.; Abdel-Daim, M.M. Protective effects of hesperidin and diosmin against acrylamide-induced liver, kidney, and brain oxidative damage in rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 35151–35162. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abo El-Ela, F.I.; Alshahrani, F.K.; Bin-Jumah, M.; Al-Zharani, M.; Almutairi, B.; Alyousif, M.S.; Bungau, S.; Aleya, L.; Alkahtani, S. Protective effects of thymoquinone against acrylamide-induced liver, kidney and brain oxidative damage in rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 37709–37717. [Google Scholar] [CrossRef]
- Jiang, G.; Lei, A.; Chen, Y.; Yu, Q.; Xie, J.; Yang, Y.; Yuan, T.; Su, D. The protective effects of the Ganoderma atrum polysaccharide against acrylamide-induced inflammation and oxidative damage in rats. Food Funct. 2021, 12, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-L.; Fang, R.-T.; Yang, Y.-H.; Bi, X.-Y.; Ren, G.-X.; Luo, A.-L.; Zhao, M.; Zang, W.-J. Protective effects of extracts from Pomegranate peels and seeds on liver fibrosis induced by carbon tetrachloride in rats. BMC Complement. Altern. Med. 2015, 15, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Banchroft, J.; Stevens, A.; Turner, D. Theory and Practice of Histological Techniques; Churchil Livingstone: New York, NY, USA, 1996. [Google Scholar]
- Saber, S.; Khalil, R.M.; Abdo, W.S.; Nassif, D.; El-Ahwany, E. Olmesartan ameliorates chemically-induced ulcerative colitis in rats via modulating NFκB and Nrf-2/HO-1 signaling crosstalk. Toxicol. Appl. Pharmacol. 2019, 364, 120–132. [Google Scholar] [CrossRef]
- Devasagayam, T.; Tilak, J.; Boloor, K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India JAPI 2004, 52, 4. [Google Scholar]
- Al-Khalaf, M.; Ramadan, K.S. Oxidants and antioxidants status in bronchial asthma. Asian J. Appl. Sci. 2013, 1. [Google Scholar]
- Alkhalaf, M.I. Diosmin protects against acrylamide-induced toxicity in rats: Roles of oxidative stress and inflammation. J. King Saud Univ.-Sci. 2020, 32, 1510–1515. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, E.; Chen, F.; Yan, H.; Yuan, Y. Potential protective effects of oral administration of allicin on acrylamide-induced toxicity in male mice. Food Funct. 2013, 4, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, J.M.; Chiang, J.Y.L. Circadian rhythms in liver metabolism and disease. Acta Pharm. Sin. B 2015, 5, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Rivadeneyra-Domínguez, E.; Becerra-Contreras, Y.; Vázquez-Luna, A.; Díaz-Sobac, R.; Rodríguez-Landa, J.F. Alterations of blood chemistry, hepatic and renal function, and blood cytometry in acrylamide-treated rats. Toxicol. Rep. 2018, 5, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Benítez, C.; Lucas, A.; Aispur, G.; Álvarez, M.; Llanos, C.; Quintana, M.A.; Brandan, N. Efectos de un potencial hepatotóxico: La acrilamida monomérica, en un modelo murino. Comun. Cient. Tecnol. 2004. [Google Scholar]
- Gurung, R.B.; Purbe, B.; Gyawali, P.; Risal, P. The ratio of aspartate aminotransferase to alanine aminotransferase (AST/ALT): The correlation of value with underlying severity of alcoholic liver disease. Kathmandu Univ. Med. J. KUMJ 2013, 11, 233–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gedik, S.; Erdemli, M.E.; Gul, M.; Yigitcan, B.; Gozukara Bag, H.; Aksungur, Z.; Altinoz, E. Hepatoprotective effects of crocin on biochemical and histopathological alterations following acrylamide-induced liver injury in Wistar rats. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 95, 764–770. [Google Scholar] [CrossRef]
- Husain, H.; Latief, U.; Ahmad, R. Pomegranate action in curbing the incidence of liver injury triggered by Diethylnitrosamine by declining oxidative stress via Nrf2 and NFκB regulation. Sci. Rep. 2018, 8, 8606. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmed, M.M. Assessment the protective role of quercetin on acrylamide-induced oxidative stress in rats. J. Food Biochem. 2016, 40, 715–723. [Google Scholar] [CrossRef]
- Rosenblat, M.; Hayek, T.; Aviram, M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis 2006, 187, 363–371. [Google Scholar] [CrossRef]
- Mansouri, E.; Basgen, J.; Saremy, S. The effects of pomegranate extract on normal adult rat kidney: A stereological study. Vet. Res. Forum 2016, 7, 1–6. [Google Scholar] [PubMed]
- Zhang, L.; Zhang, H.; Miao, Y.; Wu, S.; Ye, H.; Yuan, Y. Protective effect of allicin against acrylamide-induced hepatocyte damage in vitro and in vivo. Food Chem. Toxicol. 2012, 50, 3306–3312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, P.; Zhu, Y.; Liu, X.; Hu, X.; Chen, F. The chemoprotection of a blueberry anthocyanin extract against the acrylamide-induced oxidative stress in mitochondria: Unequivocal evidence in mice liver. Food Funct. 2015, 6, 3006–3012. [Google Scholar] [CrossRef] [PubMed]
- Ghamarzad Shishavan, N.; Mesgari Abbasi, M.; Amini Afshar, R.; Zakeri Milani, P.; Yahyavi, F. The effects of pomegranate (Punica granatum L.) peel methanolic extract on methotrexate induced changes in hepatic antioxidant enzymes of rats. Jundishapur J. Nat. Pharm. Prod. 2017, 12, e57499. [Google Scholar] [CrossRef]
- Wei, X.; Li, S.; Li, T.; Liu, L.; Liu, Y.; Wang, H.; Zhou, Y.; Liang, F.; Yu, X.; Zang, W. Pomegranate peel extract ameliorates liver fibrosis induced by carbon tetrachloride in rats through suppressing p38MAPK/Nrf2 pathway. J. Funct. Foods 2020, 65, 103712. [Google Scholar] [CrossRef]
- Toklu, H.Z.; Sehirli, O.; Sener, G.; Dumlu, M.U.; Ercan, F.; Gedik, N.; Gökmen, V. Pomegranate peel extract prevents liver fibrosis in biliary-obstructed rats. J. Pharm. Pharmacol. 2007, 59, 1287–1295. [Google Scholar] [CrossRef]
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Singh, R.P. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J. Agric. Food Chem. 2002, 50, 4791–4795. [Google Scholar] [CrossRef]
- Toklu, H.Z.; Şehİrlİ, Ö.; Özyurt, H.; Ekşİoğlu-Demİralp, E.; Çetİnel, Ş.; Şahİn, H.; Yeğen, B.Ç.; Dumlu, M.U.; Gökmen, V.; Şener, G. Punica granatum peel extract protects against ionizing radiation-induced enteritis and leukocyte apoptosis in rats. J. Radiat. Res. 2009, 50, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Doostan, F.; Vafafar, R.; Zakeri-Milani, P.; Pouri, A.; Afshar, R.A.; Abbasi, M.M. Effects of pomegranate (Punica granatum L.) seed and peel methanolic extracts on oxidative stress and lipid profile changes induced by methotrexate in rats. Adv. Pharm. Bull. 2017, 7, 269. [Google Scholar] [CrossRef] [Green Version]
- Orgil, O.; Schwartz, E.; Baruch, L.; Matityahu, I.; Mahajna, J.; Amir, R. The antioxidative and anti-proliferative potential of non-edible organs of the pomegranate fruit and tree. LWT-Food Sci. Technol. 2014, 58, 571–577. [Google Scholar] [CrossRef]
- Zahin, M.; Aqil, F.; Ahmad, I. Broad spectrum antimutagenic activity of antioxidant active fraction of Punica granatum L. peel extracts. Mutat. Res. Genet. Toxicol. Environ. Mutagenes. 2010, 703, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. Int. J. Food Sci. Technol. 2010, 45, 216–222. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Aradhya, S.M.; Divakar, S. Isolation and identification of a radical scavenging antioxidant–punicalagin from pith and carpellary membrane of pomegranate fruit. Food Chem. 2004, 87, 551–557. [Google Scholar] [CrossRef]
- Ashoush, I.S.; El-Batawy, O.; El-Shourbagy, G.A. Antioxidant activity and hepatoprotective effect of pomegranate peel and whey powders in rats. Ann. Agric. Sci. 2013, 58, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.-Q.; Xin, T.; Men, X.-M.; Xu, Z.-W.; Tian, W. In vitro and in vivo antioxidant activities of three major polyphenolic compounds in pomegranate peel: Ellagic acid, punicalin, and punicalagin. J. Integr. Agric. 2017, 16, 1808–1818. [Google Scholar] [CrossRef] [Green Version]
- Qnais, E.; Elokda, A.; Abu Ghalyun, Y.; Abdulla, F. Antidiarrheal Activity of the Aqueous extract of Punica granatum (Pomegranate) peels. Pharm. Biol. 2007, 45, 715–720. [Google Scholar] [CrossRef]
- Lee, C.-J.; Chen, L.-G.; Liang, W.-L.; Wang, C.-C. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010, 118, 315–322. [Google Scholar] [CrossRef]
- Hollebeeck, S.; Winand, J.; Hérent, M.-F.; During, A.; Leclercq, J.; Larondelle, Y.; Schneider, Y.-J. Anti-inflammatory effects of pomegranate (Punica granatum L.) husk ellagitannins in Caco-2 cells, an in vitro model of human intestine. Food Funct. 2012, 3, 875–885. [Google Scholar] [CrossRef]
- Simpson, K.J.; Henderson, N.C.; Bone-Larson, C.L.; Lukacs, N.W.; Hogaboam, C.M.; Kunkel, S.L. Chemokines in the pathogenesis of liver disease: So many players with poorly defined roles. Clin. Sci. 2003, 104, 47–63. [Google Scholar] [CrossRef]
- Tilg, H.; Wilmer, A.; Vogel, W.; Herold, M.; Nölchen, B.; Judmaier, G.; Huber, C. Serum levels of cytokines in chronic liver diseases. Gastroenterology 1992, 103, 264–274. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abd Eldaim, M.A.; Hassan, A.G. Trigonella foenum-graecum ameliorates acrylamide-induced toxicity in rats: Roles of oxidative stress, proinflammatory cytokines, and DNA damage. Biochem. Cell Biol. 2015, 93, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cao, X.; Hu, X.; Li, S.; Wang, J. The anti-apoptotic, antioxidant and anti-inflammatory effects of curcumin on acrylamide-induced neurotoxicity in rats. BMC Pharmacol. Toxicol. 2020, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Wang, N.; Hafeez, B.B.; Cheruvu, V.K.; Haqqi, T.M. Punica granatum L. extract inhibits IL-1β–Induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-κB in human chondrocytes in vitro. J. Nutr. 2005, 135, 2096–2102. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, L.; Jiang, G.; Lei, A.; Yu, Q.; Xie, J.; Chen, Y. Evaluation of the protective effects of Ganoderma atrum polysaccharide on acrylamide-induced injury in small intestine tissue of rats. Food Funct. 2019, 10, 5863–5872. [Google Scholar] [CrossRef]
- Ahmed, M.A.; El Morsy, E.M.; Ahmed, A.A. Pomegranate extract protects against cerebral ischemia/reperfusion injury and preserves brain DNA integrity in rats. Life Sci. 2014, 110, 61–69. [Google Scholar] [CrossRef]
- Zhang, R.-L.; Chopp, M.; Chen, H.; Garcia, J.H. Temporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2H) middle cerebral artery occlusion in the rat. J. Neurol. Sci. 1994, 125, 3–10. [Google Scholar] [CrossRef]
- Ziebell, J.M.; Morganti-Kossmann, M.C. Involvement of pro-and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 2010, 7, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Ileriturk, M.; Benzer, F.; Aksu, E.H.; Yildirim, S.; Kandemir, F.M.; Dogan, T.; Dortbudak, M.B.; Genc, A. Chrysin protects against testicular toxicity caused by lead acetate in rats with its antioxidant, anti-inflammatory, and antiapoptotic properties. J. Food Biochem. 2021, 45, e13593. [Google Scholar] [CrossRef]
- Pan, X.; Wu, X.; Yan, D.; Peng, C.; Rao, C.; Yan, H. Acrylamide-induced oxidative stress and inflammatory response are alleviated by N-acetylcysteine in PC12 cells: Involvement of the crosstalk between Nrf2 and NF-κB pathways regulated by MAPKs. Toxicol. Lett. 2018, 288, 55–64. [Google Scholar] [CrossRef]
- Gur, C.; Kandemir, F.M.; Darendelioglu, E.; Caglayan, C.; Kucukler, S.; Kandemir, O.; Ileriturk, M. Morin protects against acrylamide-induced neurotoxicity in rats: An investigation into different signal pathways. Environ. Sci. Pollut. Res. 2021, 28, 49808–49819. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.E.; Dai, A.; Tiffee, J.C.; Li, H.H.; Mundy, G.R.; Boyce, B.F. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-β. Nat. Med. 1996, 2, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.I.-D.L.; Ruiz-Torres, P.; del Moral, R.G.; Rodríguez-Puyol, M.; Rodríguez-Puyol, D. Age-related progressive renal fibrosis in rats and its prevention with ACE inhibitors and taurine. Am. J. Physiol.-Ren. Physiol. 2000, 278, F122–F129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.-X.; Cui, N.; Zhang, C.-L.; Zhao, X.-L.; Yu, S.-F.; Xie, K.-Q. Effect of subchronic exposure to acrylamide induced on the expression of bcl-2, bax and caspase-3 in the rat nervous system. Toxicology 2006, 217, 46–53. [Google Scholar] [CrossRef]
- Oda, S.S. Metformin protects against experimental acrylamide neuropathy in rats. Drug Dev. Res. 2017, 78, 349–359. [Google Scholar] [CrossRef]
- Davuljigari, C.B.; Ekuban, F.A.; Zong, C.; Fergany, A.A.; Morikawa, K.; Ichihara, G. Nrf2 Activation Attenuates Acrylamide-Induced Neuropathy in Mice. Int. J. Mol. Sci. 2021, 22, 5995. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Mabrok, H.B. Protective Effect of Pomegranate Peel Powder against Gastric Ulcer in Rats. Biointerface Res. Appl. Chem. 2021, 12, 4888–4899. [Google Scholar]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Yuan, H.; He, Z. Melamine causes apoptosis of rat kidney epithelial cell line (NRK-52e cells) via excessive intracellular ROS (reactive oxygen species) and the activation of p38 MAPK pathway. Cell Biol. Int. 2012, 36, 383–389. [Google Scholar] [CrossRef]



| S.N. | Compounds | Retention Time (min) | Amount | Area (mAU*s) |
|---|---|---|---|---|
| 1 | Gallic acid | 4.028 | 0.96 | 28.84930 |
| 2 | 3-Hydroxytyrosol | 4.814 | 36.34 | 102.20006 |
| 3 | Catechol | 5.352 | 18.12 | 164.68491 |
| 4 | p- Hydroxy benzoic acid | 7.824 | 15.68 | 148.26949 |
| 5 | Catechin | 8.794 | 21.04 | 1268.24951 |
| 6 | Chlorogenic acid | 9.243 | 11.65 | 317.87186 |
| 7 | Vanillic acid | 9.740 | 8.63 | 138.28169 |
| 8 | Caffeic acid | 10.036 | 2.04 | 101.89981 |
| 9 | Syringic acid | 10.690 | 2.03 | 69.65744 |
| 10 | p- Coumaric acid | 13.069 | 0.71 | 48.37119 |
| 11 | Ferulic acid | 15.361 | 0.68 | 25.69361 |
| 12 | Ellagic acid | 16.914 | 1.50 | 38.31996 |
| 13 | o- Coumaric acid | 17.161 | 4.95 | 335.75812 |
| 14 | Cinnamic acid | 20.093 | 1.89 | 126.55923 |
| 15 | Quercitin | 21.599 | 2.35 | 4.63419 |
| 16 | Rosemarinic acid | 22.145 | 11.31 | 19.64437 |
| 17 | Myricetin | 23.016 | 5.60 | 9.34549 |
| 18 | Kampherol | 24.246 | 7.99 | 30.73397 |
| Total | 153.51 | |||
| Gene | Direction | Primer Sequence | Accession Number |
|---|---|---|---|
| TGF-β1 | Sense | GGACTACTACGCCAAAGAAG | NM_021578.2 |
| Antisense | TCAAAAGACAGCCACTCAGG | ||
| COX2 | Sense | TGATCTACCCTCCCCACGTC | NM 017232 |
| Antisense | ACACACTCTGTTGTGCTCCC | ||
| BAX | Sense | AGGACGCATCCACCAAGAAG | NM 017059 |
| Antisense | CAGTTGAAGTTGCCGTCTGC | ||
| Nrf2 | Sense | TTGTAGATGACCATGAGTCGC | NM_031789.2 |
| Antisense | TGTCCTGCTGTATGCTGCTT | ||
| HO-1 | Sense | GTAAATGCAGTGTTGGCCCC | NM_012580.2 |
| Antisense | ATGTGCCAGGCATCTCCTTC | ||
| Bcl2 | Sense | ACTCTTCAGGGATGGGGTGA | NM_016993 |
| Antisense | TGACATCTCCCTGTTGACGC | ||
| β-actin | Sense | AGGAGTACGATGAGTCCGGC | NM 031144 |
| Antisense | CGCAGCTCAGTAACAGTCCG |
| Control | Acrylamide | P.P. | P.P. + Acrylamide | |
|---|---|---|---|---|
| Urea (mg/dL) | 16.8 ± 1.1 d | 58.1 ± 1.9 a | 22.9 ± 2.9 c | 39.8 ± 2.2 b |
| Total proteins (g/dL) | 9.1 ± 0.4 a | 4.9 ± 0.1 c | 9.1 ± 0.5 a | 8.9 ± 0.3 b |
| AST (U/L) | 31.7 ± 2.6 c | 149.7 ± 10.8 a | 29.3 ± 1.58 c | 71.8 ± 1.6 b |
| ALT(U/L) | 32.1 ± 1.1 c | 135.1 ± 3.7 a | 30.7 ± 1.1 c | 52.1 ± 2.1 b |
| Control | Acrylamide | P.P. | P.P. + Acrylamide | |
|---|---|---|---|---|
| Catalase (U/mL) | 3.6 ± 0.2 a | 1.4 ± 0.04 b | 3.3 ± 0.3 a | 3.0 ± 0.05 a |
| SOD (U/mL) | 32.7 ± 0.6 b | 18.3 ± 0.8 c | 41.6 ± 0.9 a | 31.2 ± 1.5 b |
| GSH (nmol/L) | 33.4 ± 0.6 b | 16.7 ± 1.4 d | 36.6 ± 2.3 a | 29.2 ± 1.9 c |
| MDA (nmol/mL) | 24.4 ± 1.3 c | 72.5 ± 1.7 a | 23.7 ± 2.1 c | 34.5 ± 1.2 b |
| Control | Acrylamide | P.P. | P.P. + Acrylamide | |
|---|---|---|---|---|
| IL-1β (pg/mL) | 92.6 ± 5 c | 240.4 ± 8.7 a | 78.2 ± 9.6 d | 112.8 ± 7.3 b |
| IL-6 (pg/mL) | 77.0 ± 5.7 d | 274.4 ± 11.2 a | 100.8 ± 9.6 c | 119.5 ± 5.7 b |
| IL-10 (pg/mL) | 118.0 ± 6.9 b | 77.6 ± 3.9 d | 147.6 ± 7.6 a | 112.9 ± 6.2 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed, S.; Alotaibi, S.S.; El-Shehawi, A.M.; Hassan, M.M.; Shukry, M.; Alkafafy, M.; Soliman, M.M. The Anti-Inflammatory, Anti-Apoptotic, and Antioxidant Effects of a Pomegranate-Peel Extract against Acrylamide-Induced Hepatotoxicity in Rats. Life 2022, 12, 224. https://doi.org/10.3390/life12020224
Sayed S, Alotaibi SS, El-Shehawi AM, Hassan MM, Shukry M, Alkafafy M, Soliman MM. The Anti-Inflammatory, Anti-Apoptotic, and Antioxidant Effects of a Pomegranate-Peel Extract against Acrylamide-Induced Hepatotoxicity in Rats. Life. 2022; 12(2):224. https://doi.org/10.3390/life12020224
Chicago/Turabian StyleSayed, Samy, Saqer S. Alotaibi, Ahmed M. El-Shehawi, Mohamed M. Hassan, Mustafa Shukry, Mohamed Alkafafy, and Mohamed Mohamed Soliman. 2022. "The Anti-Inflammatory, Anti-Apoptotic, and Antioxidant Effects of a Pomegranate-Peel Extract against Acrylamide-Induced Hepatotoxicity in Rats" Life 12, no. 2: 224. https://doi.org/10.3390/life12020224
APA StyleSayed, S., Alotaibi, S. S., El-Shehawi, A. M., Hassan, M. M., Shukry, M., Alkafafy, M., & Soliman, M. M. (2022). The Anti-Inflammatory, Anti-Apoptotic, and Antioxidant Effects of a Pomegranate-Peel Extract against Acrylamide-Induced Hepatotoxicity in Rats. Life, 12(2), 224. https://doi.org/10.3390/life12020224











