Prevalence of Right Ventricle Strain Changes following Anthracycline Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
- (1)
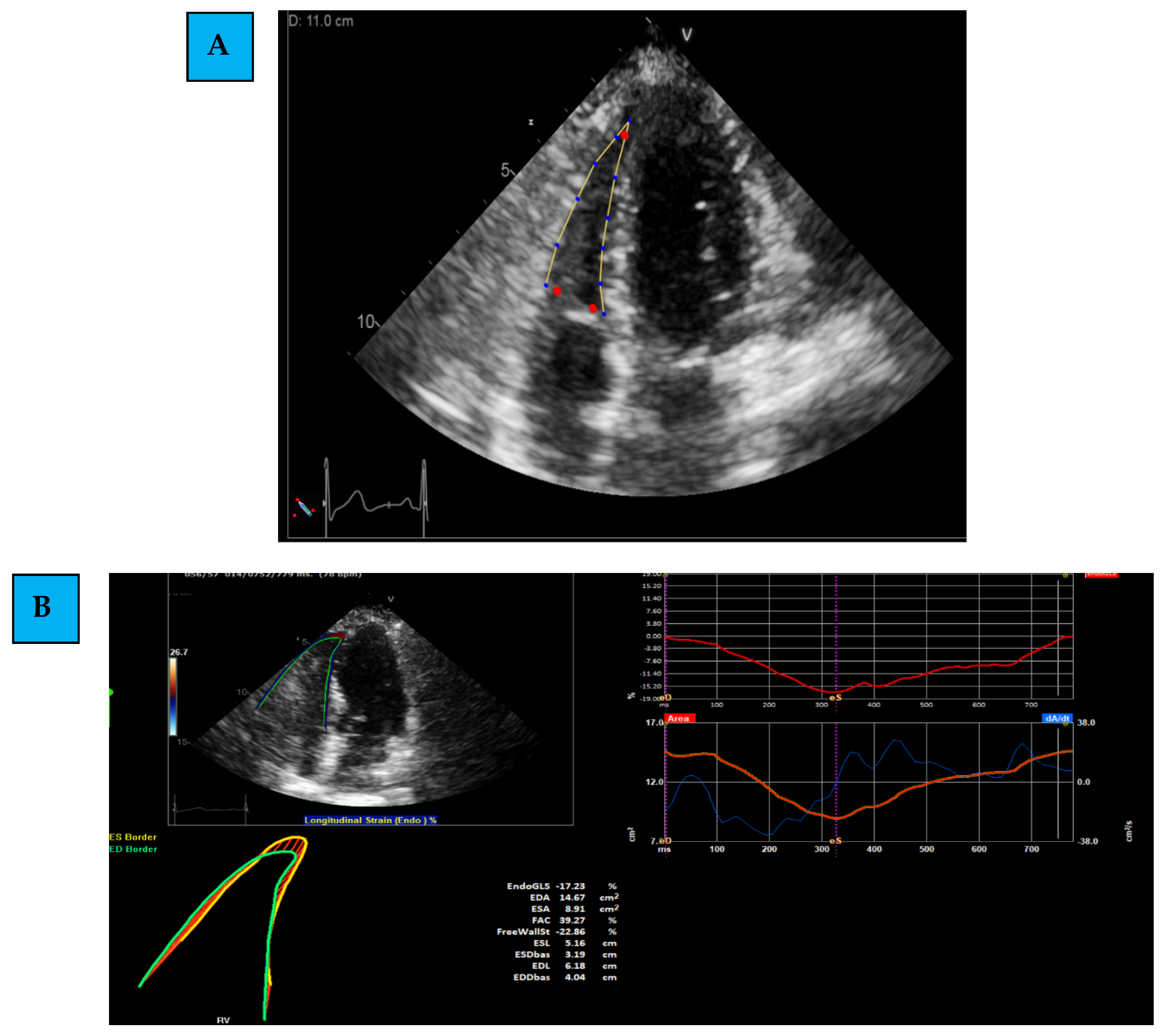
- Determining the region of interest (ROI): using the apical 4C RV-focused view, the margins of the RV are marked. This is first determined through automatic identification (by the software), and later, manual corrections and modifications are performed.
- (2)
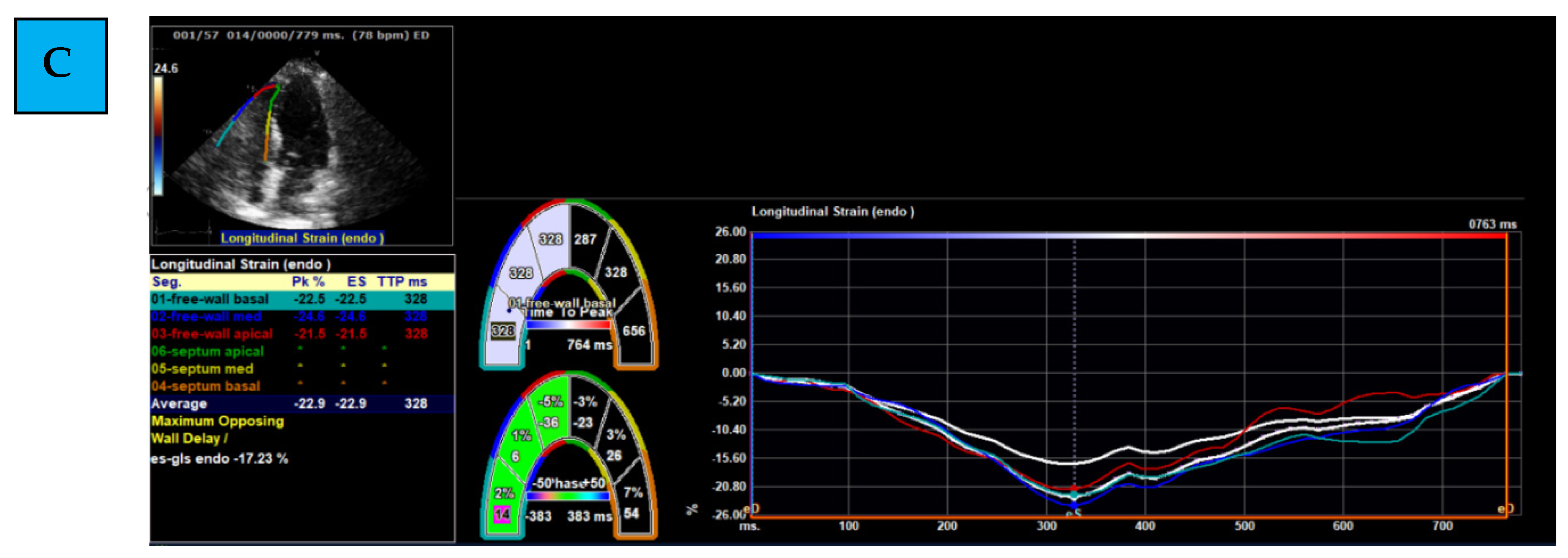
- Evaluating Global and Regional Longitudinal Strain: RV GLS is the average of 6 segments (3 of the free wall and 3 of the septum) (Figure 1B). Each wall of the RV is divided into 3 equal parts from the base to the apex. RV FWLS PK is, for example, the average measured strain of the basal, mid, and apical segments of the RV FWLS PK solely (Figure 1C).
- (3)
- Determining the timing of measurement: strain can be measured during several phases of the cardiac cycle: End Systolic (ES) strain (defined by pulmonary valve closure), Peak (PK) systolic strain (maximal ventricular contraction), or Peak strain (the highest value throughout the entire heart cycle). To date, PK systolic value is the recommended measurement to use [22].
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S.; et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, M.W.; Hamo, C.E.; Cardinale, D.; Ky, B.; Nohria, A.; Baer, L.; Skopicki, H.; Lenihan, D.J.; Gheorghiade, M.; Lyon, A.R.; et al. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure: Part 1: Definitions, Pathophysiology, Risk Factors, and Imaging. Circ. Heart Fail 2016, 9, e002661. [Google Scholar] [CrossRef] [Green Version]
- Vejpongsa, P.; Yeh, E.T. Prevention of anthracycline-induced cardiotoxicity: Challenges and opportunities. J. Am. Coll. Cardiol. 2014, 64, 938–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1063–1093. [Google Scholar] [CrossRef] [PubMed]
- Hardaway, B.W. Adriamycin-associated cardiomyopathy: Where are we now? updates in pathophysiology, dose recommendations, prognosis, and outcomes. Curr. Opin. Cardiol. 2019, 34, 289–295. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Gulati, G.; Heck, S.L.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.; Fagerland, M.W.; Gravdehaug, B.; von Knobelsdorff-Brenkenhoff, F.; Bratland, Å.; Storås, T.H.; et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): A 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016, 37, 1671–1680. [Google Scholar] [CrossRef] [Green Version]
- Eidem, B.W. Identification of anthracycline cardiotoxicity: Left ventricular ejection fraction is not enough. J. Am. Soc. Echocardiogr. 2008, 21, 1290–1292. [Google Scholar] [CrossRef]
- Anqi, Y.; Yu, Z.; Mingjun, X.; Xiaoli, K.; Mengmeng, L.; Fangfang, L.; Mei, Z. Use of echocardiography to monitor myocardial damage during anthracycline chemotherapy. Echocardiography 2019, 36, 495–502. [Google Scholar] [CrossRef]
- Santoro, C.; Arpino, G.; Esposito, R.; Lembo, M.; Paciolla, I.; Cardalesi, C.; de Simone, G.; Trimarco, B.; De Placido, S.; Galderisi, M. 2D and 3D strain for detection of subclinical anthracycline cardiotoxicity in breast cancer patients: A balance with feasibility. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Çetin, S.; Babaoğlu, K.; Başar, E.Z.; Deveci, M.; Çorapçıoğlu, F. Subclinical anthracycline-induced cardiotoxicity in long-term follow-up of asymptomatic childhood cancer survivors: Assessment by speckle tracking echocardiography. Echocardiography 2018, 35, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Poterucha, J.T.; Kutty, S.; Lindquist, R.K.; Li, L.; Eidem, B.W. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J. Am. Soc. Echocardiogr. 2012, 25, 733–740. [Google Scholar] [CrossRef] [PubMed]
- De Groote, P.; Millaire, A.; Foucher-Hossein, C.; Nugue, O.; Marchandise, X.; Ducloux, G.; Lablanche, J.M. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J. Am. Coll. Cardiol. 1998, 32, 948–954. [Google Scholar] [CrossRef] [Green Version]
- Boczar, K.E.; Aseyev, O.; Sulpher, J.; Johnson, C.; Burwash, I.G.; Turek, M.; Dent, S.; Dwivedi, G. Right heart function deteriorates in breast cancer patients undergoing anthracycline-based chemotherapy. Echo. Res. Pract. 2016, 3, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadic, M.; Baudisch, A.; Haßfeld, S.; Heinzel, F.; Cuspidi, C.; Burkhardt, F.; Escher, F.; Attanasio, P.; Pieske, B.; Genger, M. Right ventricular function and mechanics in chemotherapy- and radiotherapy-naive cancer patients. Int. J. Cardiovasc. Imaging 2018, 34, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.H. Strain Analysis of the Right Ventricle Using Two-dimensional Echocardiography. J. Cardiovasc. Imaging 2018, 26, 111–124. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Sarvari, S.I.; Haugaa, K.H.; Anfinsen, O.G.; Leren, T.P.; Smiseth, O.A.; Kongsgaard, E.; Amlie, J.P.; Edvardsen, T. Right ventricular mechanical dispersion is related to malignant arrhythmias: A study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur. Heart J. 2011, 32, 1089–1096. [Google Scholar] [CrossRef] [Green Version]
- Laufer-Perl, M.; Arias, O.; Dorfman, S.S.; Baruch, G.; Rothschild, E.; Beer, G.; Hasson, S.P.; Arbel, Y.; Rozenbaum, Z.; Topilsky, Y.; et al. Left Atrial Strain changes in patients with breast cancer during anthracycline therapy. Int. J. Cardiol. 2021, 330, 238–244. [Google Scholar] [CrossRef]
- Laufer-Perl, M.; Arnold, J.H.; Mor, L.; Amrami, N.; Derakhshesh, M.; Moshkovits, Y.; Sadeh, B.; Arbel, Y.; Topilsky, Y.; Rozenbaum, Z. The association of reduced global longitudinal strain with cancer therapy-related cardiac dysfunction among patients receiving cancer therapy. Clin. Res. Cardiol. 2020, 109, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Poulin, F.; Lim, K.D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J. Am. Coll. Cardiol. 2014, 63, 2751–2768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.; et al. Recommendations for chamber quantification. Eur. J. Echocardiogr. 2006, 7, 79–108. [Google Scholar] [CrossRef] [PubMed]
- Charbonnel, C.; Convers-Domart, R.; Rigaudeau, S.; Taksin, A.L.; Baron, N.; Lambert, J.; Ghez, S.; Georges, J.L.; Farhat, H.; Lambert, J.; et al. Assessment of global longitudinal strain at low-dose anthracycline-based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 392–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraru, D.; Onciul, S.; Peluso, D.; Soriani, N.; Cucchini, U.; Aruta, P.; Romeo, G.; Cavalli, G.; Iliceto, S.; Badano, L.P. Sex- and Method-Specific Reference Values for Right Ventricular Strain by 2-Dimensional Speckle-Tracking Echocardiography. Circ. Cardiovasc. Imaging 2016, 9, e003866. [Google Scholar] [CrossRef] [Green Version]
- Baruch, G.; Rothschild, E.; Kapusta, L.; Schwartz, L.A.; Biner, S.; Aviram, G.; Ingbir, M.; Nachmany, I.; Keren, G.; Topilsky, Y. Impact of right ventricular dysfunction and end-diastolic pulmonary artery pressure estimated from analysis of tricuspid regurgitant velocity spectrum in patients with preserved ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 446–454. [Google Scholar] [CrossRef]
- Ho, S.Y.; Nihoyannopoulos, P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart 2006, 92 (Suppl. 1), i2–i13. [Google Scholar] [CrossRef] [Green Version]
- Planek, M.I.C.; Manshad, A.; Hein, K.; Hemu, M.; Ballout, F.; Varandani, R.; Venugopal, P.; Okwuosa, T. Prediction of doxorubicin cardiotoxicity by early detection of subclinical right ventricular dysfunction. Cardio-Oncology 2020, 6, 10. [Google Scholar] [CrossRef]
- Arciniegas Calle, M.C.; Sandhu, N.P.; Xia, H.; Cha, S.S.; Pellikka, P.A.; Ye, Z.; Herrmann, J.; Villarraga, H.R. Two-dimensional speckle tracking echocardiography predicts early subclinical cardiotoxicity associated with anthracycline-trastuzumab chemotherapy in patients with breast cancer. BMC Cancer 2018, 18, 1037. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Shu, F.; Zhang, C.; Song, F.; Xu, Y.; Guo, Y.; Xue, K.; Lin, J.; Shu, X.; Hsi, D.H.; et al. Early Detection and Prediction of Anthracycline-Induced Right Ventricular Cardiotoxicity by 3-Dimensional Echocardiography. Cardio Oncol. 2020, 2, 13–22. [Google Scholar] [CrossRef]
- Ylänen, K.; Poutanen, T.; Savikurki-Heikkilä, P.; Rinta-Kiikka, I.; Eerola, A.; Vettenranta, K. Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among long-term survivors of childhood cancer. J. Am. Coll. Cardiol. 2013, 61, 1539–1547. [Google Scholar] [CrossRef] [Green Version]
- Calleja, A.; Poulin, F.; Khorolsky, C.; Shariat, M.; Bedard, P.L.; Amir, E.; Rakowski, H.; McDonald, M.; Delgado, D.; Thavendiranathan, P. Right Ventricular Dysfunction in Patients Experiencing Cardiotoxicity during Breast Cancer Therapy. J. Oncol. 2015, 2015, 609194. [Google Scholar] [CrossRef] [PubMed]
- Keramida, K.; Farmakis, D.; Bingcang, J.; Sulemane, S.; Sutherland, S.; Bingcang, R.A.; Ramachandran, K.; Tzavara, C.; Charalampopoulos, G.; Filippiadis, D. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur. J. Heart Fail. 2019, 21, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Monitillo, F.; Di Terlizzi, V.; Gioia, M.I.; Barone, R.; Grande, D.; Parisi, G.; Brunetti, N.D.; Iacoviello, M. Right Ventricular Function in Chronic Heart Failure: From the Diagnosis to the Therapeutic Approach. J. Cardiovasc. Dev. Dis. 2020, 7, 12. [Google Scholar]
- Bosch, L.; Lam, C.S.P.; Gong, L.; Chan, S.P.; Sim, D.; Yeo, D.; Jaufeerally, F.; Leong, K.T.G.; Ong, H.Y.; Ng, T.P.; et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 1664–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variables | All Patients (40) |
|---|---|
| Age (years) (mean, SD) | 50 (±13) |
| Hypertension (n, %) | 8 (20) |
| Ischemic heart disease (n, %) | 0 (0) |
| Diabetes mellitus (n, %) | 2 (5) |
| Chronic heart failure (n, %) | 0 (0) |
| Chronic kidney disease (n, %) | 0 (0) |
| Hyperlipidemia (n, %) | 5 (12.5) |
| Smoker (n, %) | |
| No | 25 (62.5) |
| Yes | 9 (22.5) |
| Past Smoker | 6 (15) |
| ACEi (n, %) | 2 (5) |
| ARB (n, %) | 4 (10) |
| BB (n, %) | 4 (10) |
| ACEi/ARB/BB (yes) | 6 (15.0) |
| Statins (n, %) | 5 (12.5) |
| Trastuzumab (n, %) | 10 (25) |
| Pertuzumab (n, %) | 9 (22.5) |
| Chest Radiation (n, %) | 18 (45) |
| Ejection fraction (%) (mean, SD) | 60 (±0) |
| Left Ventricle Global Longitudinal strain (%) (mean, SD) | −21.5 (±2) |
| RV GLS (mean, SD) | 26.8 (±4.7) |
| RV FWGLS PK (mean, SD) | 28.9 (±5.1) |
| RV GLS septum PK (Median (Q1, Q3)) | 23.6 (20.2, 28.0) |
| TAPSE (mean, SD) | 25 ± 3 |
| SPAP (15/40) (mean, SD) | 26 ± 6 |
| Variables | Number(%) |
|---|---|
| RV GLS 10% relative reduction (n, %) | 30 (75) |
| RV FWLS PK 10% relative reduction (n, %) | 23 (58) |
| RV GLS septum PK 10% relative reduction (n, %) | 31 (78) |
| RV GLS 15% relative reduction (n, %) | 28 (70) |
| RV FWLS PK 15% relative reduction (n, %) | 20 (50) |
| RV GLS septum PK 15% relative reduction (n, %) | 29 (73) |
| LV GLS (%) (mean, SD) | 19.7 (±1.8) |
| EF (%) (mean, SD) | 59 (±2) |
| LV GLS 10% relative reduction (n, %) | 14 (35) |
| LV GLS 15% relative reduction (n, %) | 8 (20) |
| CTRCD (n, %) | 2 (5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laufer-Perl, M.; Perelman-Gvili, M.; Sirota Dorfman, S.; Baruch, G.; Rothschild, E.; Beer, G.; Arbel, Y.; Arnold, J.H.; Rozenbaum, Z.; Banai, S.; et al. Prevalence of Right Ventricle Strain Changes following Anthracycline Therapy. Life 2022, 12, 291. https://doi.org/10.3390/life12020291
Laufer-Perl M, Perelman-Gvili M, Sirota Dorfman S, Baruch G, Rothschild E, Beer G, Arbel Y, Arnold JH, Rozenbaum Z, Banai S, et al. Prevalence of Right Ventricle Strain Changes following Anthracycline Therapy. Life. 2022; 12(2):291. https://doi.org/10.3390/life12020291
Chicago/Turabian StyleLaufer-Perl, Michal, Moran Perelman-Gvili, Svetlana Sirota Dorfman, Guy Baruch, Ehud Rothschild, Gil Beer, Yaron Arbel, Joshua H. Arnold, Zach Rozenbaum, Shmuel Banai, and et al. 2022. "Prevalence of Right Ventricle Strain Changes following Anthracycline Therapy" Life 12, no. 2: 291. https://doi.org/10.3390/life12020291






