Lavandula pedunculata (Mill.) Cav. Aqueous Extract Antibacterial Activity Improved by the Addition of Salvia rosmarinus Spenn., Salvia lavandulifolia Vahl and Origanum compactum Benth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Aqueous Extraction
2.4. Determination of Total Polyphenol Content
2.5. Determination of Total Tannin Content
2.6. UHPLC Analysis of Aqueous Extracts
2.7. Antibacterial Activity of Aqueous Extracts
2.7.1. Preparation of Bacterial Suspensions
2.7.2. Activity of Individual Extracts
2.7.3. Activity of Extracts in Mixtures
- FIC < 1: synergistic effect;
- FIC = 1: commutative effect;
- 1 < FIC ≤ 2: indifferent effect;
- 2 < FIC: antagonistic effect.
2.8. Statistical Analysis
3. Results
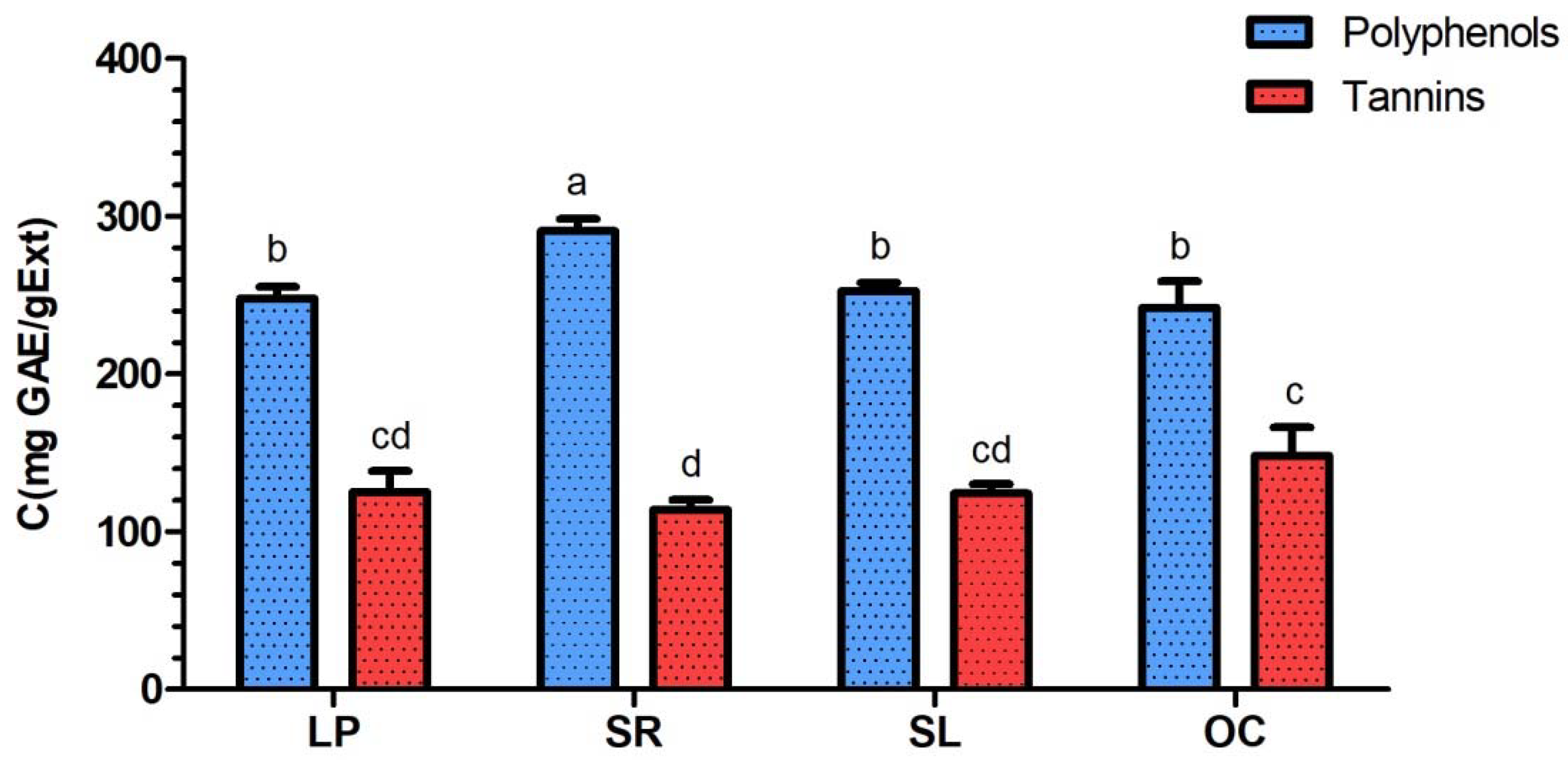
3.1. Determination of Total Polyphenol and Total Tannin Contents
3.2. UHPLC Analysis of Aqueous Extracts
3.3. Antibacterial Activity of Aqueous Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Alekshun, M.N.; Levy, S.B. Molecular Mechanisms of Antibacterial Multidrug Resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef] [Green Version]
- Qi, G.-B.; Zhang, D.; Liu, F.-H.; Qiao, Z.-Y.; Wang, H. An “On-Site Transformation” Strategy for Treatment of Bacterial Infection. Adv. Mater. 2017, 29, 1703461. [Google Scholar] [CrossRef]
- Levin, A.S.; Barone, A.A.; Penço, J.; Santos, M.V.; Marinho, I.S.; Arruda, E.A.; Manrique, E.I.; Costa, S.F. Intravenous Colistin as Therapy for Nosocomial Infections Caused by Multidrug-Resistant Pseudomonas Aeruginosa and Acinetobacter Baumannii. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1999, 28, 1008–1011. [Google Scholar] [CrossRef] [Green Version]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular Mechanisms of Antibiotic Resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Ghobadi, A.; Amini-Behbahani, F.; Yousefi, A.; Shirazi, M.T.; Behnoud, N. Medicinal and Nutritional Properties of Ziziphus Jujuba Mill. in Traditional Persian Medicine and Modern Phytotherapy. Crescent J. Med. Biol. Sci. 2019, 6, 146–150. [Google Scholar]
- Mickymaray, S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Baba, D. Stratégies Nationale de Développement Des Plantes Aromatiques et Médicinales Spontannées; Royaume du Maroc, Haut Commissariat aux Eaux et Forêts et à la Lutte Contre la Désertification, Direction du Développenment Forestier: Marrakesh, Morocco, 2015.
- Erland, L.A.E.; Mahmoud, S.S. Chapter 57—Lavender (Lavandula Angustifolia) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 501–508. ISBN 978-0-12-416641-7. [Google Scholar]
- Nafis, A.; Ouedrhiri, W.; Iriti, M.; Mezrioui, N.; Marraiki, N.; Elgorban, A.M.; Syed, A.; Hassani, L. Chemical Composition and Synergistic Effect of Three Moroccan Lavender EOs with Ciprofloxacin against Foodborne Bacteria: A Promising Approach to Modulate Antimicrobial Resistance. Lett. Appl. Microbiol. 2021, 72, 698–705. [Google Scholar] [CrossRef]
- Ouhaddou, H.; Boubaker, H.; Msanda, F.; El Mousadik, A. An Ethnobotanical Study of Medicinal Plants of the Agadir Ida Ou Tanane Province (Southwest Morocco). J. Appl. Biosci. 2015, 84, 7707–7722. [Google Scholar] [CrossRef] [Green Version]
- Teixidor-Toneu, I.; Martin, G.J.; Ouhammou, A.; Puri, R.K.; Hawkins, J.A. An Ethnomedicinal Survey of a Tashelhit-Speaking Community in the High Atlas, Morocco. J. Ethnopharmacol. 2016, 188, 96–110. [Google Scholar] [CrossRef]
- Almohawes, Z.N.; Alruhaimi, H.S. Effect of Lavandula Dentata Extract on Ovalbumin-Induced Asthma in Male Guinea Pigs. Braz. J. Biol. 2019, 80, 87–96. [Google Scholar] [CrossRef]
- Baptista, R.; Madureira, A.M.; Jorge, R.; Adão, R.; Duarte, A.; Duarte, N.; Lopes, M.M.; Teixeira, G. Antioxidant and Antimycotic Activities of Two Native Lavandula Species from Portugal. Evid. Based Complement. Alternat. Med. 2015, 2015, 570521. [Google Scholar] [CrossRef] [Green Version]
- Kulabas, S.S.; Ipek, H.; Tufekci, A.R.; Arslan, S.; Demirtas, I.; Ekren, R.; Sezerman, U.; Tumer, T.B. Ameliorative Potential of Lavandula Stoechas in Metabolic Syndrome via Multitarget Interactions. J. Ethnopharmacol. 2018, 223, 88–98. [Google Scholar] [CrossRef]
- Bint Mustafa, S.; Akram, M.; Muhammad Asif, H.; Qayyum, I.; Mehmood Hashmi, A.; Munir, N.; Said Khan, F.; Riaz, M.; Ahmad, S. Antihyperglycemic Activity of Hydroalcoholic Extracts of Selective Medicinal Plants Curcuma Longa, Lavandula Stoechas, Aegle Marmelos, and Glycyrrhiza Glabra and Their Polyherbal Preparation in Alloxan-Induced Diabetic Mice. Dose-Response Int. J. 2019, 17, 1559325819852503. [Google Scholar] [CrossRef] [Green Version]
- Rahmati, B.; Kiasalari, Z.; Roghani, M.; Khalili, M.; Ansari, F. Antidepressant and Anxiolytic Activity of Lavandula Officinalis Aerial Parts Hydroalcoholic Extract in Scopolamine-Treated Rats. Pharm. Biol. 2017, 55, 958–965. [Google Scholar] [CrossRef] [Green Version]
- Sadraei, H.; Asghari, G.; Rahmati, M. Study of Antispasmodic Action of Lavandula Angustifolia Mill Hydroalcoholic Extract on Rat Ileum. J. Herbmed Pharmacol. 2019, 8, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Shahdadi, H.; Bahador, R.S.; Eteghadi, A.; Boraiinejad, S. Lavender a Plant for Medical Uses: A Literature Review. Indian J. Public Health Res. Dev. 2017, 8, 328–332. [Google Scholar] [CrossRef]
- Boutahiri, S.; Bouhrim, M.; Abidi, C.; Mechchate, H.; Alqahtani, A.S.; Noman, O.M.; Kouoh Elombo, F.; Gressier, B.; Sahpaz, S.; Bnouham, M.; et al. Antihyperglycemic Effect of Lavandula Pedunculata: In Vivo, In Vitro and Ex Vivo Approaches. Pharmaceutics 2021, 13, 2019. [Google Scholar] [CrossRef]
- Chaachouay, N.; Benkhnigue, O.; Fadli, M.; El Ibaoui, H.; El Ayadi, R.; Zidane, L. Ethnobotanical and Ethnopharmacological Study of Medicinal and Aromatic Plants Used in the Treatment of Respiratory System Disorders in the Moroccan Rif. Ethnobot. Res. Appl. 2019, 18, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Zougagh, S.; Belghiti, A.; Rochd, T.; Zerdani, I.; Mouslim, J. Medicinal and Aromatic Plants Used in Traditional Treatment of the Oral Pathology: The Ethnobotanical Survey in the Economic Capital Casablanca, Morocco (North Africa). Nat. Prod. Bioprospecting 2019, 9, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Zeroual, A.; Eloutassi, N.; Chaouch, M.; Chaqroune, A. Antimicrobial, Antioxidant Activity, and Chemical Composition of Origanum Compactum Benth from Taounate Province, North Morocco. Asian J. Pharm. Clin. Res. 2020, 13, 126–131. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; El-Ansary, D.O.; Al-Mana, F.A.; Mahmoud, E.A. Saudi Rosmarinus Officinalis and Ocimum Basilicum L. Polyphenols and Biological Activities. Processes 2020, 8, 446. [Google Scholar] [CrossRef] [Green Version]
- Pop (Cuceu), A.V.; Tofană, M.; Socaci, S.A.; Pop, C.; Rotar, A.M.; Nagy, M.; Salanţă, L. Determination of Antioxidant Capacity and Antimicrobial Activity of Selected Salvia Species. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2016, 73, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Lopes, C.L.; Pereira, E.; Soković, M.; Carvalho, A.M.; Barata, A.M.; Lopes, V.; Rocha, F.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Phenolic Composition and Bioactivity of Lavandula Pedunculata (Mill.) Cav. Samples from Different Geographical Origin. Molecules 2018, 23, 1037. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A Simple 96-Well Microplate Method for Estimation of Total Polyphenol Content in Seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Olounlade, P.A.; Hounzangbe-Adote, M.S.; Azando, E.V.B.; Ha, T.T.; Brunet, S.; Moulis, C.; Fabre, N.; Fouraste, I.; Hoste, H.; Valentin, A. Etude in vitro de l’effet Des Tanins de Newbouldia Laevis et de Zanthoxylum Zanthoxyloïdes Sur La Migration Des Larves Infestantes de Haemonchus Contortus. Int. J. Biol. Chem. Sci. 2011, 5, 1414–1422. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; CLSI document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; Volume 40. [Google Scholar]
- Fratini, F.; Mancini, S.; Turchi, B.; Friscia, E.; Pistelli, L.; Giusti, G.; Cerri, D. A Novel Interpretation of the Fractional Inhibitory Concentration Index: The Case Origanum Vulgare L. and Leptospermum Scoparium J. R. et G. Forst Essential Oils against Staphylococcus Aureus Strains. Microbiol. Res. 2017, 195, 11–17. [Google Scholar] [CrossRef]
- Blažeković, B.; Yang, W.; Wang, Y.; Li, C.; Kindl, M.; Pepeljnjak, S.; Vladimir-Knežević, S. Chemical Composition, Antimicrobial and Antioxidant Activities of Essential Oils of Lavandula × Intermedia ‘Budrovka’ and L. Angustifolia Cultivated in Croatia. Ind. Crops Prod. 2018, 123, 173–182. [Google Scholar] [CrossRef]
- Moussi Imane, M.; Houda, F.; Said Amal, A.H.; Kaotar, N.; Mohammed, T.; Imane, R.; Farid, H. Phytochemical Composition and Antibacterial Activity of Moroccan Lavandula Angustifolia Mill. J. Essent. Oil Bear. Plants 2017, 20, 1074–1082. [Google Scholar] [CrossRef]
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical Survey of Medicinal Plants Used in the Traditional Treatment of Diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350. [Google Scholar] [CrossRef]
- Orch, H.; Douira, A.; Zidane, L. Étude Ethnobotanique Des Plantes Médicinales Utilisées Dans Le Traitement Du Diabète, et Des Maladies Cardiaques Dans La Région d’Izarène (Nord Du Maroc). J. Appl. Biosci. 2015, 86, 7940–7956. [Google Scholar] [CrossRef]
- Zaher, A.; Boufellous, M.; Jaber, H.; Hartiti, H.E.; Barrahi, M.; Ouhssine, M.; Bourkhiss, B. Ethnobotanical Study of Medicinal Plants Used in the Province of Sidi Slimane (Morocco). J. Biosci. Med. 2018, 6, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Horky, P.; Skalickova, S.; Smerkova, K.; Skladanka, J. Essential Oils as a Feed Additives: Pharmacokinetics and Potential Toxicity in Monogastric Animals. Animals 2019, 9, 352. [Google Scholar] [CrossRef] [Green Version]
- Ramdan, B.; El Malki, F.; Eddarraji, K.; Greche, H.; Nhiri, M. Composition and Antibacterial Activity of Hydro-Alcohol and Aqueous Extracts Obtained from the Lamiaceae Family. Pharmacogn. J. 2018, 10, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Giner, M.J.; Vegara, S.; Funes, L.; Martí, N.; Saura, D.; Micol, V.; Valero, M. Antimicrobial Activity of Food-Compatible Plant Extracts and Chitosan against Naturally Occurring Micro-Organisms in Tomato Juice. J. Sci. Food Agric. 2012, 92, 1917–1923. [Google Scholar] [CrossRef]
- Casciaro, B.; Calcaterra, A.; Cappiello, F.; Mori, M.; Loffredo, M.R.; Ghirga, F.; Mangoni, M.L.; Botta, B.; Quaglio, D. Nigritanine as a New Potential Antimicrobial Alkaloid for the Treatment of Staphylococcus Aureus-Induced Infections. Toxins 2019, 11, 511. [Google Scholar] [CrossRef] [Green Version]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus Aureus Bloodstream Infections—United States. Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Z.; Fu, S.-G.; Wang, S.-Y.; Yang, D.-J.; Wu, Y.-H.S.; Chen, Y.-C. Effects of a Natural Antioxidant, Polyphenol-Rich Rosemary (Rosmarinus Officinalis L.) Extract, on Lipid Stability of Plant-Derived Omega-3 Fatty-Acid Rich Oil. LWT Food Sci. Technol. 2018, 89, 210–216. [Google Scholar] [CrossRef]
- Bourhia, M.; Laasri, F.E.; Aourik, H.; Boukhris, A.; Ullah, R.; Bari, A.; Ali, S.S.; El Mzibri, M.; Benbacer, L.; Gmouh, S. Antioxidant and Antiproliferative Activities of Bioactive Compounds Contained in Rosmarinus Officinalis Used in the Mediterranean Diet. Evid. Based Complement. Alternat. Med. 2019, 2019, 7623830. [Google Scholar] [CrossRef] [Green Version]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. Phytochemical Composition and Bioactive Effects of Salvia Africana, Salvia Officinalis ‘Icterina’ and Salvia Mexicana Aqueous Extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef] [Green Version]
- Bouyahya, A.; Abrini, J.; Bakri, Y.; Dakka, N. Screening phytochimique et évaluation de l’activité antioxydante et antibactérienne des extraits d’Origanum compactum. Phytothérapie 2017, 15, 379–383. [Google Scholar] [CrossRef]
- Dong, G.; Liu, H.; Yu, X.; Zhang, X.; Lu, H.; Zhou, T.; Cao, J. Antimicrobial and Anti-Biofilm Activity of Tannic Acid against Staphylococcus Aureus. Nat. Prod. Res. 2018, 32, 2225–2228. [Google Scholar] [CrossRef] [PubMed]
- Štumpf, S.; Hostnik, G.; Primožič, M.; Leitgeb, M.; Salminen, J.-P.; Bren, U. The Effect of Growth Medium Strength on Minimum Inhibitory Concentrations of Tannins and Tannin Extracts against E. coli. Molecules 2020, 25, 2947. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef] [Green Version]
- Matejczyk, M.; Świsłocka, R.; Golonko, A.; Lewandowski, W.; Hawrylik, E. Cytotoxic, Genotoxic and Antimicrobial Activity of Caffeic and Rosmarinic Acids and Their Lithium, Sodium and Potassium Salts as Potential Anticancer Compounds. Adv. Med. Sci. 2018, 63, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, E.T.; Mustafa, Y.F. Coumarins from Red Delicious Apple Seeds: Extraction, Phytochemical Analysis, and Evaluation as Antimicrobial Agents. Syst. Rev. Pharm. 2020, 11, 64–70. [Google Scholar]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. BioMed Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Almeida, C.; Nogueira, J.M.F.; Romano, A. Metabolic Profile and Biological Activities of Lavandula Pedunculata Subsp. Lusitanica (Chaytor) Franco: Studies on the Essential Oil and Polar Extracts. Food Chem. 2013, 141, 2501–2506. [Google Scholar] [CrossRef]
- Barbieri, J.B.; Goltz, C.; Batistão Cavalheiro, F.; Theodoro Toci, A.; Igarashi-Mafra, L.; Mafra, M.R. Deep Eutectic Solvents Applied in the Extraction and Stabilization of Rosemary (Rosmarinus Officinalis L.) Phenolic Compounds. Ind. Crops Prod. 2020, 144, 112049. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Polyphenolics of Salvia—A Review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]




| Plant Species | Family | Voucher Number | Used Organ | Harvest Region | Geographic Coordinates | Harvest Date |
|---|---|---|---|---|---|---|
| Lavandula pedunculata (Mill.) Cav. | Lamiaceae | RAB111854 | Flowering tops | Taza | 34°04′01.2″ N 4°07′42.5″ W | May 2019 |
| Salvia rosmarinus Spenn. | Lamiaceae | RAB111855 | Leaves | Oulad Ali | 33°27′46.6″ N 3°58′34.4″ W | May 2017 |
| Salvia lavandulifolia Vahl | Lamiaceae | RAB111857 | Leaves | Oulad Ali | 33°27′45.2″ N 3°58′39.8″ W | May 2017 |
| Origanum compactum Benth. | Lamiaceae | RAB111858 | Leaves and flowers | Bouyablane | 33°39′02.4″ N 4°09′49.6″ W | June 2018 |
| tR (min) | λmax (nm) | [M − H]− (m/z) | [M + H]+ (m/z) | Compounds | LP | SR | SL | OC |
|---|---|---|---|---|---|---|---|---|
| 6.869 | 278.1 | WD | 147 |  | + | T | T | T |
| 8.259 | 255.5, 297.2 | 147 | 149 |  | T | + | T | − |
| 2.697 | 259.1, 293.6 | 153 | 155 |  | + | + | + | + |
| 3.923 | 260.3, 292.4 | 167 | 169 |  | + | + | T | + |
| 1.518 | 371.0 | 169 | 171 |  | + | T | T | T |
| 7.761 | 307.9 | WD | 177 |  | + | − | + | T |
| 3.976 | 324.7 | 179 | 181 |  | + | + | + | + |
| 6.536 | 322.3 | 193 | 195 |  | + | + | − | − |
| 8.606 | 338.8 | 269 | 271 |  | + | + | + | + |
| 8.368 | 254.3, 350.4 | 285 | 287 |  | + | + | + | + |
| 7.792 | 253.2, 372.1 | 317 | 319 |  | + | − | + | T |
| 3.680 | 240.1, 325.8 | 353 | 355 |  | − | T | T | T |
| 7.831 | 329.4 | 359 | WD |  | ++ | ++ | ++ | ++ |
| Microorganisms | Reference | Antibiotics (MIC Values in mg/L) | |||
|---|---|---|---|---|---|
| Gentamicin | Vancomycin | Amoxicillin | |||
| Gram + | Enterococcus faecalis | C159-6 | 2 | 0.5 | 64 |
| Enterococcus sp. | 8153 | 2 | 4 | 2 | |
| Mycobacterium smegmatis | 5003 | 0.03 | 0.5 | 1 | |
| Staphylococcus aureus | 8146 | 0.5 | 1 | 4 | |
| Staphylococcus aureus | 8241 | 0.5 | 1 | 16 | |
| Staphylococcus aureus | ATCC 6538 | 0.25 | 1 | 0.125 | |
| Staphylococcus aureus | T28-1 | 0.5 | 1 | 2 | |
| Staphylococcus aureus | T17-4 | 0.5 | 1 | 1 | |
| Staphylococcus epidermidis | T46A1 | 0.06 | 2 | 1 | |
| Staphylococcus epidermidis | T19A1 | 32 | 2 | 16 | |
| Staphylococcus epidermidis | T21A5 | 0.06 | 2 | 16 | |
| Staphylococcus warneri | T12A12 | 0.06 | 4 | 1 | |
| Staphylococcus warneri | T26A1 | 0.06 | 2 | 0.25 | |
| Staphylococcus pettenkoferi | T47.A6 | 0.06 | 2 | 0.25 | |
| Streptococcus agalactiae | T38.2 | ND | ND | ND | |
| Streptococcus agalactiae | T53C9 | 0.5 | 0.25 | 0.03 | |
| Streptococcus pyogenes | 16138 | 0.125 | 0.25 | 0.03 | |
| Streptococcus pyogenes | 16135 | 0.125 | 0.25 | 0.03 | |
| Corynebacterium striatum | T40A3 | 0.06 | 0.5 | 1 | |
| Gram − | Citrobacter freundii | 11041 | 0.25 | NA | 2 |
| Citrobacter freundii | 10268 | ND | ND | ND | |
| Escherichia coli | ATCC 25922 | 0.5 | NA | 16 | |
| Escherichia coli | T20A1 | 0.25 | NA | NA | |
| Escherichia coli | 8138 | 0.5 | NA | NA | |
| Escherichia coli | 8157 | 0.5 | NA | NA | |
| Enterobacter aerogenes | 9004 | 0.5 | NA | NA | |
| Klebsiella pneumoniae | 10270 | 8 | NA | NA | |
| Klebsiella pneumoniae | 11016 | 0.25 | NA | NA | |
| Proteus mirabilis | 11060 | 0.5 | NA | 2 | |
| Proteus mirabilis | T28-3 | 0.25 | NA | 1 | |
| Pseudomonas aeruginosa | 8131 | 1 | NA | NA | |
| Pseudomonas aeruginosa | ATCC 27583 | 2 | NA | NA | |
| Pseudomonas aeruginosa | 8129 | 0.03 | NA | NA | |
| Salmonella sp. | 11033 | 0.25 | NA | 2 | |
| MIC ± SD (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Microorganisms | Reference | LP | SR | Combination | FIC | ||
| LP | SR | ||||||
| Gram + | Enterococcus faecalis | C159-6 | NA | NA | 1.00 ± 0.28 | 0.70 ± 0.37 | - |
| Enterococcus sp. | 8153 | NA | NA | NA | NA | - | |
| Mycobacterium smegmatis | 5003 | 0.43 ± 0.25 | 0.70 ± 0.37 | 0.13 ± 0.04 | 0.40 ± 0.14 | 0.87 | |
| Staphylococcus aureus | 8146 | 0.60 ± 0.00 | 0.60 ± 0.00 | 0.18 ± 0.09 | 0.30 ± 0.00 | 0.79 | |
| Staphylococcus aureus | 8241 | 0.50 ± 0.14 | 0.60 ± 0.00 | 0.15 ± 0.11 | 0.25 ± 0.07 | 0.72 | |
| Staphylococcus aureus | ATCC 6538 | 0.60 ± 0.00 | 0.50 ± 0.14 | 0.18 ± 0.09 | 0.25 ± 0.07 | 0.79 | |
| Staphylococcus aureus | T28-1 | 0.40 ± 0.14 | 0.50 ± 0.14 | 0.20 ± 0.07 | 0.13 ± 0.04 | 0.75 | |
| Staphylococcus aureus | T17-4 | 0.60 ± 0.00 | 0.80 ± 0.28 | 0.08 ± 0.00 | 0.40 ± 0.14 | 0.63 | |
| Staphylococcus epidermidis | T46A1 | 0.20 ± 0.07 | 0.20 ± 0.07 | 0.10 ± 0.04 | 0.10 ± 0.04 | 1.00 | |
| Staphylococcus epidermidis | T19A1 | 0.25 ± 0.07 | 0.25 ± 0.07 | 0.18 ± 0.09 | 0.08 ± 0.00 | 1.00 | |
| Staphylococcus epidermidis | T21A5 | 0.30 ± 0.00 | 0.25 ± 0.07 | 0.13 ± 0.04 | 0.10 ± 0.04 | 0.82 | |
| Staphylococcus warneri | T12A12 | 0.30 ± 0.00 | 0.25 ± 0.07 | 0.13 ± 0.04 | 0.13 ± 0.04 | 0.92 | |
| Staphylococcus warneri | T26A1 | 0.30 ± 0.00 | 0.25 ± 0.07 | 0.13 ± 0.04 | 0.13 ± 0.04 | 0.92 | |
| Staphylococcus pettenkoferi | T47.A6 | 0.30 ± 0.00 | 0.25 ± 0.07 | 0.10 ± 0.04 | 0.13 ± 0.04 | 0.83 | |
| Streptococcus agalactiae | T38.2 | 0.75 ± 0.45 | 0.75 ± 0.45 | 0.38 ± 0.23 | 0.60 ± 0.00 | 1.30 | |
| Streptococcus agalactiae | T53C9 | NA | 1.20 ± 0.00 | 0.65 ± 0.43 | 0.50 ± 0.14 | - | |
| Streptococcus pyogenes | 16138 | 1.20 ± 0.00 | 1.20 ± 0.00 | 0.11 ± 0.04 | 0.38 ± 0.23 | 0.41 | |
| Streptococcus pyogenes | 16135 | 0.90 ± 0.30 | 0.60 ± 0.00 | 0.15 ± 0.00 | 0.45 ± 0.15 | 0.92 | |
| Corynebacterium striatum | T40A3 | NA | NA | 0.80 ± 0.28 | 0.35 ± 0.19 | - | |
| Gram − | Citrobacter freundii | 11041 | NA | NA | 0.80 ± 0.28 | 0.60 ± 0.00 | - |
| Citrobacter freundii | 10268 | 1.20 ± 0.00 | NA | 0.80 ± 0.28 | 0.45 ± 0.21 | - | |
| Escherichia coli | ATCC 25922 | NA | NA | NA | NA | - | |
| Escherichia coli | T20A1 | NA | NA | 1.20 ± 0.00 | 1.20 ± 0.00 | - | |
| Escherichia coli | 8138 | NA | NA | 1.20 ± 0.00 | 1.20 ± 0.00 | - | |
| Escherichia coli | 8157 | NA | NA | NA | NA | - | |
| Enterobacter aerogenes | 9004 | NA | NA | 1.00 ± 0.28 | 0.70 ± 0.37 | - | |
| Klebsiella pneumoniae | 10270 | NA | NA | 1.20 ± 0.00 | 1.20 ± 0.00 | - | |
| Klebsiella pneumoniae | 11016 | NA | NA | NA | NA | - | |
| Proteus mirabilis | 11060 | 1.20 ± 0.00 | 1.20 ± 0.00 | 0.35 ± 0.19 | 0.33 ± 0.22 | 0.56 | |
| Proteus mirabilis | T28-3 | 1.00 ± 0.28 | 0.60 ± 0.00 | 0.35 ± 0.19 | 0.33 ± 0.22 | 0.89 | |
| Pseudomonas aeruginosa | 8131 | NA | NA | 0.65 ± 0.43 | 0.80 ± 0.28 | - | |
| Pseudomonas aeruginosa | ATCC 27583 | 1.20 ± 0.00 | 1.20 ± 0.00 | 0.70 ± 0.37 | 0.28 ± 0.23 | 0.81 | |
| Pseudomonas aeruginosa | 8129 | 0.50 ± 0.14 | 0.60 ± 0.42 | 0.20 ± 0.07 | 0.15 ± 0.11 | 0.65 | |
| Salmonella sp. | 11033 | NA | NA | 1.20 ± 0.00 | 0.80 ± 0.28 | - | |
| MIC ± SD (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Microorganisms | Reference | LP | SL | Combination | FIC | ||
| LP | SL | ||||||
| Gram + | Enterococcus faecalis | C159-6 | NA | NA | 0.10 ± 0.04 | 0.83 ± 0.53 | - |
| Enterococcus sp. | 8153 | NA | NA | 0.85 ± 0.49 | 1.20 ± 0.00 | - | |
| Mycobacterium smegmatis | 5003 | 0.43 ± 0.25 | 0.50 ± 0.14 | 0.10 ± 0.04 | 0.33 ± 0.22 | 0.89 | |
| Staphylococcus aureus | 8146 | 0.60 ± 0.00 | 0.60 ± 0.00 | 0.08 ± 0.00 | 0.30 ± 0.00 | 0.63 | |
| Staphylococcus aureus | 8241 | 0.50 ± 0.14 | 0.60 ± 0.00 | 0.08 ± 0.00 | 0.30 ± 0.00 | 0.65 | |
| Staphylococcus aureus | ATCC 6538 | 0.60 ± 0.00 | 0.50 ± 0.14 | 0.15 ± 0.11 | 0.23 ± 0.11 | 0.70 | |
| Staphylococcus aureus | T28-1 | 0.40 ± 0.14 | 0.50 ± 0.14 | 0.15 ± 0.11 | 0.23 ± 0.11 | 0.83 | |
| Staphylococcus aureus | T17-4 | 0.60 ± 0.00 | 0.60 ± 0.00 | 0.13 ± 0.04 | 0.30 ± 0.00 | 0.71 | |
| Staphylococcus epidermidis | T46A1 | 0.20 ± 0.07 | 0.25 ± 0.07 | 0.10 ± 0.04 | 0.13 ± 0.04 | 1.00 | |
| Staphylococcus epidermidis | T19A1 | 0.25 ± 0.07 | 0.25 ± 0.07 | 0.13 ± 0.04 | 0.10 ± 0.04 | 0.90 | |
| Staphylococcus epidermidis | T21A5 | 0.30 ± 0.00 | 0.25 ± 0.07 | 0.10 ± 0.04 | 0.10 ± 0.04 | 0.73 | |
| Staphylococcus warneri | T12A12 | 0.30 ± 0.00 | 0.25 ± 0.07 | 0.13 ± 0.04 | 0.10 ± 0.04 | 0.82 | |
| Staphylococcus warneri | T26A1 | 0.30 ± 0.00 | 0.15 ± 0.00 | 0.13 ± 0.04 | 0.08 ± 0.00 | 0.92 | |
| Staphylococcus pettenkoferi | T47.A6 | 0.30 ± 0.00 | 0.20 ± 0.07 | 0.13 ± 0.04 | 0.10 ± 0.04 | 0.92 | |
| Streptococcus agalactiae | T38.2 | 0.75 ± 0.45 | ND | ND | ND | - | |
| Streptococcus agalactiae | T53C9 | NA | 1.20 ± 0.00 | 0.18 ± 0.09 | 0.60 ± 0.00 | - | |
| Streptococcus pyogenes | 16138 | 1.20 ± 0.00 | 1.20 ± 0.00 | 0.20 ± 0.07 | 0.50 ± 0.14 | 0.58 | |
| Streptococcus pyogenes | 16135 | 0.90 ± 0.30 | 1.00 ± 0.28 | 0.18 ± 0.09 | 0.30 ± 0.21 | 0.49 | |
| Corynebacterium striatum | T40A3 | NA | NA | 0.25 ± 0.25 | 0.90 ± 0.42 | - | |
| Gram − | Citrobacter freundii | 11041 | NA | NA | 1.00 ± 0.28 | 0.25 ± 0.25 | - |
| Citrobacter freundii | 10268 | 1.20 ± 0.00 | NA | 0.90 ± 0.42 | 0.28 ± 0.23 | - | |
| Escherichia coli | ATCC 25922 | NA | NA | 1.20 ± 0.00 | 1.20 ± 0.00 | - | |
| Escherichia coli | T20A1 | NA | NA | 1.00 ± 0.28 | 1.20 ± 0.00 | - | |
| Escherichia coli | 8138 | NA | NA | 1.20 ± 0.00 | 0.80 ± 0.28 | - | |
| Escherichia coli | 8157 | NA | NA | NA | NA | - | |
| Enterobacter aerogenes | 9004 | NA | NA | 1.20 ± 0.00 | 0.10 ± 0.04 | - | |
| Klebsiella pneumoniae | 10270 | NA | NA | 1.20 ± 0.00 | 1.20 ± 0.00 | - | |
| Klebsiella pneumoniae | 11016 | NA | NA | 1.20 ± 0.00 | 1.20 ± 0.00 | - | |
| Proteus mirabilis | 11060 | 1.20 ± 0.00 | 1.00 ± 0.28 | 0.20 ± 0.07 | 0.50 ± 0.14 | 0.67 | |
| Proteus mirabilis | T28-3 | 1.00 ± 0.28 | 0.90 ± 0.30 | 0.30 ± 0.00 | 0.30 ± 0.00 | 0.63 | |
| Pseudomonas aeruginosa | 8131 | NA | NA | 0.55 ± 0.46 | 0.85 ± 0.49 | - | |
| Pseudomonas aeruginosa | ATCC 27583 | 1.20 ± 0.00 | 1.20 ± 0.00 | 0.50 ± 0.14 | 0.60 ± 0.00 | 0.92 | |
| Pseudomonas aeruginosa | 8129 | 0.50 ± 0.14 | 0.50 ± 0.14 | 0.15 ± 0.00 | 0.15 ± 0.00 | 0.60 | |
| Salmonella sp. | 11033 | NA | NA | 1.20 ± 0.00 | 0.60 ± 0.00 | - | |
| MIC ± SD (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Microorganisms | Reference | LP | OC | Combination | FIC | ||
| LP | OC | ||||||
| Gram + | Enterococcus faecalis | C159-6 | NA | NA | 1.20 ± 0.00 | 0.90 ± 0.42 | - |
| Enterococcus sp. | 8153 | NA | NA | NA | NA | - | |
| Mycobacterium smegmatis | 5003 | 0.43 ± 0.25 | 1.00 ± 0.28 | 0.15 ± 0.11 | 0.45 ± 0.21 | 0.80 | |
| Staphylococcus aureus | 8146 | 0.60 ± 0.00 | 1.00 ± 0.28 | 0.15 ± 0.11 | 0.50 ± 0.14 | 0.75 | |
| Staphylococcus aureus | 8241 | 0.50 ± 0.14 | 0.80 ± 0.28 | 0.18 ± 0.09 | 0.23 ± 0.11 | 0.63 | |
| Staphylococcus aureus | ATCC 6538 | 0.60 ± 0.00 | 1.00 ± 0.28 | 0.30 ± 0.00 | 0.15 ± 0.11 | 0.65 | |
| Staphylococcus aureus | T28-1 | 0.40 ± 0.14 | 0.80 ± 0.28 | 0.13 ± 0.04 | 0.30 ± 0.00 | 0.69 | |
| Staphylococcus aureus | T17-4 | 0.60 ± 0.00 | 0.80 ± 0.28 | 0.23 ± 0.11 | 0.25 ± 0.25 | 0.69 | |
| Staphylococcus epidermidis | T46A1 | 0.20 ± 0.07 | 0.35 ± 0.19 | 0.10 ± 0.04 | 0.10 ± 0.04 | 0.79 | |
| Staphylococcus epidermidis | T19A1 | 0.25 ± 0.07 | 0.40 ± 0.14 | 0.08 ± 0.00 | 0.20 ± 0.07 | 0.80 | |
| Staphylococcus epidermidis | T21A5 | 0.30 ± 0.00 | 0.40 ± 0.14 | 0.08 ± 0.00 | 0.20 ± 0.07 | 0.75 | |
| Staphylococcus warneri | T12A12 | 0.30 ± 0.00 | 0.50 ± 0.14 | 0.10 ± 0.04 | 0.20 ± 0.07 | 0.73 | |
| Staphylococcus warneri | T26A1 | 0.30 ± 0.00 | 0.60 ± 0.00 | 0.13 ± 0.04 | 0.20 ± 0.07 | 0.75 | |
| Staphylococcus pettenkoferi | T47.A6 | 0.30 ± 0.00 | 0.50 ± 0.14 | 0.13 ± 0.04 | 0.15 ± 0.00 | 0.72 | |
| Streptococcus agalactiae | T38.2 | 0.75 ± 0.45 | NA | 0.64 ± 0.56 | 0.19 ± 0.11 | - | |
| Streptococcus agalactiae | T53C9 | NA | NA | 1.00 ± 0.28 | 0.33 ± 0.22 | - | |
| Streptococcus pyogenes | 16138 | 1.20 ± 0.00 | 1.20 ± 0.00 | 0.45 ± 0.15 | 0.60 ± 0.00 | 0.88 | |
| Streptococcus pyogenes | 16135 | 0.90 ± 0.30 | 1.20 ± 0.00 | 0.08 ± 0.00 | 0.90 ± 0.30 | 0.83 | |
| Corynebacterium striatum | T40A3 | NA | NA | 0.80 ± 0.28 | 0.45 ± 0.21 | - | |
| Gram − | Citrobacter freundii | 11041 | NA | NA | 1.20 ± 0.00 | 0.33 ± 0.22 | - |
| Citrobacter freundii | 10268 | 1.20 ± 0.00 | NA | 1.20 ± 0.00 | 0.23 ± 0.11 | - | |
| Escherichia coli | ATCC 25922 | NA | NA | NA | NA | - | |
| Escherichia coli | T20A1 | NA | NA | NA | NA | - | |
| Escherichia coli | 8138 | NA | NA | NA | NA | - | |
| Escherichia coli | 8157 | NA | NA | NA | NA | - | |
| Enterobacter aerogenes | 9004 | NA | NA | 1.20 ± 0.00 | 0.38 ± 0.23 | - | |
| Klebsiella pneumoniae | 10270 | NA | NA | NA | NA | - | |
| Klebsiella pneumoniae | 11016 | NA | NA | NA | NA | - | |
| Proteus mirabilis | 11060 | 1.20 ± 0.00 | 1.20 ± 0.00 | 0.60 ± 0.00 | 0.25 ± 0.07 | 0.71 | |
| Proteus mirabilis | T28-3 | 1.00 ± 0.28 | 1.20 ± 0.00 | 0.43 ± 0.25 | 0.35 ± 0.19 | 0.72 | |
| Pseudomonas aeruginosa | 8131 | NA | NA | NA | NA | - | |
| Pseudomonas aeruginosa | ATCC 27583 | 1.20 ± 0.00 | NA | 0.65 ± 0.43 | 0.70 ± 0.37 | - | |
| Pseudomonas aeruginosa | 8129 | 0.50 ± 0.14 | 0.80 ± 0.28 | 0.18 ± 0.09 | 0.33 ± 0.22 | 0.76 | |
| Salmonella sp. | 11033 | NA | NA | NA | NA | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutahiri, S.; Eto, B.; Bouhrim, M.; Mechchate, H.; Saleh, A.; Al kamaly, O.; Drioiche, A.; Remok, F.; Samaillie, J.; Neut, C.; et al. Lavandula pedunculata (Mill.) Cav. Aqueous Extract Antibacterial Activity Improved by the Addition of Salvia rosmarinus Spenn., Salvia lavandulifolia Vahl and Origanum compactum Benth. Life 2022, 12, 328. https://doi.org/10.3390/life12030328
Boutahiri S, Eto B, Bouhrim M, Mechchate H, Saleh A, Al kamaly O, Drioiche A, Remok F, Samaillie J, Neut C, et al. Lavandula pedunculata (Mill.) Cav. Aqueous Extract Antibacterial Activity Improved by the Addition of Salvia rosmarinus Spenn., Salvia lavandulifolia Vahl and Origanum compactum Benth. Life. 2022; 12(3):328. https://doi.org/10.3390/life12030328
Chicago/Turabian StyleBoutahiri, Salima, Bruno Eto, Mohamed Bouhrim, Hamza Mechchate, Asmaa Saleh, Omkulthom Al kamaly, Aziz Drioiche, Firdaous Remok, Jennifer Samaillie, Christel Neut, and et al. 2022. "Lavandula pedunculata (Mill.) Cav. Aqueous Extract Antibacterial Activity Improved by the Addition of Salvia rosmarinus Spenn., Salvia lavandulifolia Vahl and Origanum compactum Benth" Life 12, no. 3: 328. https://doi.org/10.3390/life12030328
APA StyleBoutahiri, S., Eto, B., Bouhrim, M., Mechchate, H., Saleh, A., Al kamaly, O., Drioiche, A., Remok, F., Samaillie, J., Neut, C., Gressier, B., Kouoh Elombo, F., Nassiri, L., Zair, T., & Sahpaz, S. (2022). Lavandula pedunculata (Mill.) Cav. Aqueous Extract Antibacterial Activity Improved by the Addition of Salvia rosmarinus Spenn., Salvia lavandulifolia Vahl and Origanum compactum Benth. Life, 12(3), 328. https://doi.org/10.3390/life12030328






