Marked to Die-Cell Death Mechanisms for Keratinocyte Acantholysis in Pemphigus Diseases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Signaling Pathways: TNF, FAS, and Other Proteins
2.2. Cell Death Controversy
2.2.1. TUNEL Assays–Are the Cells Dead or Alive?
2.2.2. Caspase Activity—Are the Cells Apoptotic or Not?
2.3. Morphology
2.4. Apoptolysis and Mitochondria
2.5. p38 MAPK, and Other Signaling Molecules
3. Conclusions and Perspectives
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, E.; Kasperkiewicz, M.; Joly, P. Pemphigus. Lancet 2019, 394, 882–894. [Google Scholar] [CrossRef]
- Waschke, J.; Spindler, V. Desmosomes and Extradesmosomal Adhesive Signaling Contacts in Pemphigus: DESMOSOMES IN HEALTH AND DISEASE. Med. Res. Rev. 2014, 34, 1127–1145. [Google Scholar] [CrossRef]
- Amagai, M.; Stanley, J.R. Desmoglein as a Target in Skin Disease and Beyond. J. Investig. Dermatol. 2012, 132, 776–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spindler, V.; Drenckhahn, D.; Zillikens, D.; Waschke, J. Pemphigus IgG Causes Skin Splitting in the Presence of Both Desmoglein 1 and Desmoglein 3. Am. J. Pathol. 2007, 171, 906–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahoney, M.G.; Wang, Z.; Rothenberger, K.; Koch, P.J.; Amagai, M.; Stanley, J.R. Explanations for the Clinical and Microscopic Localization of Lesions in Pemphigus Foliaceus and Vulgaris. J. Clin. Invest. 1999, 103, 461–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grando, S.A. Pemphigus Autoimmunity: Hypotheses and Realities. Autoimmunity 2012, 45, 7–35. [Google Scholar] [CrossRef] [Green Version]
- Schiltz, J.R.; Michel, B. Production Of Epidermal Acantholysis In Normal Human Skin In Vitro By The Igg Fraction From Pemphigus Serum. J. Investig. Dermatol. 1976, 67, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roscoe, J.T.; Diaz, L.; Sampaio, S.A.P.; Castro, R.M.; Labib, R.S.; Takahashi, Y.; Patel, H.; Anhalt, G.J. Brazilian Pemphigus Foliaceus Autoantibodies Are Pathogenic to BALB/c Mice by Passive Transfer. J. Investig. Dermatol. 1985, 85, 538–541. [Google Scholar] [CrossRef] [Green Version]
- Anhalt, G.J.; Labib, R.S.; Voorhees, J.J.; Beals, T.F.; Diaz, L.A. Induction of Pemphigus in Neonatal Mice by Passive Transfer of IgG from Patients with the Disease. N. Engl. J. Med. 1982, 306, 1189–1196. [Google Scholar] [CrossRef]
- Hashimoto, T. Recent Advances in the Study of the Pathophysiology of Pemphigus. Arch. Dermatol. Res. 2003, 295, S2–S11. [Google Scholar] [CrossRef]
- Sajda, T.; Sinha, A.A. Autoantibody Signaling in Pemphigus Vulgaris: Development of an Integrated Model. Front. Immunol. 2018, 9, 692. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, A.; Ishiko, A.; Ota, T.; Tsunoda, K.; Amagai, M.; Nishikawa, T. IgG Binds to Desmoglein 3 in Desmosomes and Causes a Desmosomal Split Without Keratin Retraction in a Pemphigus Mouse Model. J. Investig. Dermatol. 2004, 122, 1145–1153. [Google Scholar] [CrossRef] [Green Version]
- Heupel, W.-M.; Zillikens, D.; Drenckhahn, D.; Waschke, J. Pemphigus Vulgaris IgG Directly Inhibit Desmoglein 3-Mediated Transinteraction. J. Immunol. 2008, 181, 1825–1834. [Google Scholar] [CrossRef] [Green Version]
- Waschke, J.; Spindler, V.; Bruggeman, P.; Zillikens, D.; Schmidt, G.; Drenckhahn, D. Inhibition of Rho A Activity Causes Pemphigus Skin Blistering. J. Cell Biol. 2006, 175, 721–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esaki, C.; Seishima, M.; Yamada, T.; Osada, K.; Kitajima, Y. Pharmacologic Evidence for Involvement of Phospholiphase C in Pemphigus IgG-Induced Inositol 1,4,5-Trisphosphate Generation, Intracellular Calcium Increase, and Plasminogen Activator Secretion in DJM-1 Cells, a Squamous Cell Carcinoma Line. J. Investig. Dermatol. 1995, 105, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Berkowitz, P.; Hu, P.; Liu, Z.; Diaz, L.A.; Enghild, J.J.; Chua, M.P.; Rubenstein, D.S. Desmosome Signaling. J. Biol. Chem. 2005, 280, 23778–23784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.E.; Berkowitz, P.; Jolly, P.S.; Diaz, L.A.; Chua, M.P.; Rubenstein, D.S. Biphasic Activation of P38MAPK Suggests That Apoptosis Is a Downstream Event in Pemphigus Acantholysis. J. Biol. Chem. 2009, 284, 12524–12532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, L.; Raess, N.A.; Caldelari, R.; Zakher, A.; de Bruin, A.; Posthaus, H.; Bolli, R.; Hunziker, T.; Suter, M.M.; Müller, E.J. Pemphigus Vulgaris Identifies Plakoglobin as Key Suppressor of C-Myc in the Skin. EMBO J. 2006, 25, 3298–3309. [Google Scholar] [CrossRef]
- Frusić-Zlotkin, M.; Raichenberg, D.; Wang, X.; David, M.; Michel, B.; Milner, Y. Apoptotic Mechanism in Pemphigus Autoimmunoglobulins-Induced Acantholysis--Possible Involvement of the EGF Receptor. Autoimmunity 2006, 39, 563–575. [Google Scholar] [CrossRef]
- Aoyama, Y.; Owada, M.K.; Kitajima, Y. A Pathogenic Autoantibody, Pemphigus Vulgaris-IgG, Induces Phosphorylation of Desmoglein 3, and Its Dissociation from Plakoglobin in Cultured Keratinocytes. Eur. J. Immunol. 1999, 29, 2233–2240. [Google Scholar] [CrossRef]
- Sharma, P.; Mao, X.; Payne, A.S. Beyond Steric Hindrance: The Role of Adhesion Signaling Pathways in the Pathogenesis of Pemphigus. J. Dermatol. Sci. 2007, 48, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kalantari-Dehaghi, M.; Chen, Y.; Deng, W.; Chernyavsky, A.; Marchenko, S.; Wang, P.H.; Grando, S.A. Mechanisms of Mitochondrial Damage in Keratinocytes by Pemphigus Vulgaris Antibodies. J. Biol. Chem. 2013, 288, 16916–16925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasperkiewicz, M.; Ellebrecht, C.T.; Takahashi, H.; Yamagami, J.; Zillikens, D.; Payne, A.S.; Amagai, M. Pemphigus. Nat. Rev. Dis. Primers 2017, 3, 17026. [Google Scholar] [CrossRef] [Green Version]
- Arredondo, J.; Chernyavsky, A.I.; Karaouni, A.; Grando, S.A. Novel Mechanisms of Target Cell Death and Survival and of Therapeutic Action of IVIg in Pemphigus. Am. J. Pathol. 2005, 167, 1531–1544. [Google Scholar] [CrossRef] [Green Version]
- Joly, P.; Maho-Vaillant, M.; Prost-Squarcioni, C.; Hebert, V.; Houivet, E.; Calbo, S.; Caillot, F.; Golinski, M.L.; Labeille, B.; Picard-Dahan, C.; et al. First-Line Rituximab Combined with Short-Term Prednisone versus Prednisone Alone for the Treatment of Pemphigus (Ritux 3): A Prospective, Multicentre, Parallel-Group, Open-Label Randomised Trial. Lancet 2017, 389, 2031–2040. [Google Scholar] [CrossRef]
- Bystryn, J.-C.; Rudolph, J.L. Pemphigus. Lancet 2005, 366, 61–73. [Google Scholar] [CrossRef]
- Bastuji-Garin, S.; Souissi, R.; Blum, L.; Turki, H.; Nouira, R.; Jomaa, B.; Zahaf, A.; Osman, A.B.; Mokhtar, I.; Fazaa, B.; et al. Comparative Epidemiology of Pemphigus in Tunisia and France: Unusual Incidence of Pemphigus Foliaceus in Young Tunisian Women. J. Investig. Dermatol. 1995, 104, 302–305. [Google Scholar] [CrossRef] [Green Version]
- Meyer, N.; Misery, L. Geoepidemiologic Considerations of Auto-Immune Pemphigus. Autoimmun. Rev. 2010, 9, A379–A382. [Google Scholar] [CrossRef]
- Alpsoy, E.; Akman-Karakas, A.; Uzun, S. Geographic Variations in Epidemiology of Two Autoimmune Bullous Diseases: Pemphigus and Bullous Pemphigoid. Arch. Dermatol. Res. 2015, 307, 291–298. [Google Scholar] [CrossRef]
- Castro, R.M.; Roscoe, J.T.; Sampaio, S.A. Brazilian Pemphigus Foliaceus. Clin. Dermatol. 1983, 1, 22–41. [Google Scholar] [CrossRef]
- Abréu-Vélez, A.M.; de Messias Reason, I.J.; Howard, M.S.; Roselino, A.M. Endemic Pemphigus Foliaceus over a Century: Part I. N. Am. J. Med. Sci. 2010, 2, 51–59. [Google Scholar]
- Robledo, M.A. Chronic Methyl Mercury Poisoning May Trigger Endemic Pemphigus Foliaceus “Fogo Selvagem. Med. Hypotheses 2012, 78, 60–66. [Google Scholar] [CrossRef]
- Hans-Filho, G.; dos Santos, V.; Katayama, J.H.; Aoki, V.; Rivitti, E.A.; Sampaio, S.A.; Friedman, H.; Moraes, J.R.; Moraes, M.E.; Eaton, D.P.; et al. An Active Focus of High Prevalence of Fogo Selvagem on an Amerindian Reservation in Brazil. Cooperative Group on Fogo Selvagem Research. J. Investig. Dermatol. 1996, 107, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Ruocco, V.; Ruocco, E.; Lo Schiavo, A.; Brunetti, G.; Guerrera, L.P.; Wolf, R. Pemphigus: Etiology, Pathogenesis, and Inducing or Triggering Factors: Facts and Controversies. Clin. Dermatol. 2013, 31, 374–381. [Google Scholar] [CrossRef]
- Diaz, L.A.; Sampaio, S.A.; Rivitti, E.A.; Martins, C.R.; Cunha, P.R.; Lombardi, C.; Almeida, F.A.; Castro, R.M.; Macca, M.L.; Lavrado, C. Endemic Pemphigus Foliaceus (Fogo Selvagem). I. Clinical Features and Immunopathology. J. Am. Acad. Dermatol. 1989, 20, 657–669. [Google Scholar] [CrossRef]
- Petzl-Erler, M.L. Beyond the HLA Polymorphism: A Complex Pattern of Genetic Susceptibility to Pemphigus. Genet. Mol. Biol. 2020, 43, e20190369. [Google Scholar] [CrossRef] [PubMed]
- Bumiller-Bini, V.; Cipolla, G.A.; Spadoni, M.B.; Augusto, D.G.; Petzl-Erler, M.L.; Beltrame, M.H.; Boldt, A.B.W. Condemned or Not to Die? Gene Polymorphisms Associated With Cell Death in Pemphigus Foliaceus. Front. Immunol. 2019, 10, 2416. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, C.; Borges, P.C.; Chaul, A.; Sampaio, S.A.; Rivitti, E.A.; Friedman, H.; Martins, C.R.; Sanches Júnior, J.A.; Cunha, P.R.; Hoffmann, R.G. Environmental Risk Factors in Endemic Pemphigus Foliaceus (Fogo Selvagem). “The Cooperative Group on Fogo Selvagem Research”. J. Investig. Dermatol. 1992, 98, 847–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernal, S.; Pepinelli, M.; Casanova, C.; Goulart, T.M.; Kim, O.; De Paula, N.A.; Pinto, M.C.; Sá-Nunes, A.; Roselino, A.M. Insights into the Epidemiological Link between Biting Flies and Pemphigus Foliaceus in Southeastern Brazil. Acta Trop. 2017, 176, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Jeong, J.S.; Maldonado, M.; Valenzuela, J.G.; Gomes, R.; Teixeira, C.; Evangelista, F.; Qaqish, B.; Aoki, V.; Hans, G.; et al. Cutting Edge: Brazilian Pemphigus Foliaceus Anti-Desmoglein 1 Autoantibodies Cross-React with Sand Fly Salivary LJM11 Antigen. J. Immunol. 2012, 189, 1535–1539. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, F.G.; Franco, A.M.R. Pênfigo Foliáceo Endêmico (Fogo Selvagem) Em Indígena Yanomami No Município de São Gabriel Da Cachoeira, Estado Do Amazonas, Brasil. Rev. Pan-Amaz. Saúde 2014, 5, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Reis, V.M.; Toledo, R.P.; Lopez, A.; Diaz, L.A.; Martins, J.E. UVB-Induced Acantholysis in Endemic Pemphigus Foliaceus (Fogo Selvagem) and Pemphigus Vulgaris. J. Am. Acad. Dermatol. 2000, 42, 571–576. [Google Scholar] [CrossRef]
- Stanley, J.R.; Klaus-Kovtun, V.; Sampaio, S.A. Antigenic Specificity of Fogo Selvagem Autoantibodies Is Similar to North American Pemphigus Foliaceus and Distinct from Pemphigus Vulgaris Autoantibodies. J. Investig. Dermatol. 1986, 87, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Diaz, L.A.; Prisayanh, P.S.; Dasher, D.A.; Li, N.; Evangelista, F.; Aoki, V.; Hans-Filho, G.; dos Santos, V.; Qaqish, B.F.; Rivitti, E.A.; et al. The IgM Anti-Desmoglein 1 Response Distinguishes Brazilian Pemphigus Foliaceus (Fogo Selvagem) from Other Forms of Pemphigus. J. Investig. Dermatol. 2008, 128, 667–675. [Google Scholar] [CrossRef]
- Qian, Y.; Prisayanh, P.; Andraca, E.; Qaqish, B.F.; Aoki, V.; Hans-Filhio, G.; Rivitti, E.A.; Diaz, L.A. Cooperative Group on Fogo Selvagem Research IgE, IgM, and IgG4 Anti-Desmoglein 1 Autoantibody Profile in Endemic Pemphigus Foliaceus (Fogo Selvagem). J. Investig. Dermatol. 2011, 131, 985–987. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Jeong, J.S.; Abdeladhim, M.; Valenzuela, J.G.; Aoki, V.; Hans-Filhio, G.; Rivitti, E.A.; Diaz, L.A. IgE Anti-LJM11 Sand Fly Salivary Antigen May Herald the Onset of Fogo Selvagem in Endemic Brazilian Regions. J. Investig. Dermatol. 2015, 135, 913–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha-Alvarez, R.; Friedman, H.; Campbell, I.T.; Souza-Aguiar, L.; Martins-Castro, R.; Diaz, L.A. Pregnant Women with Endemic Pemphigus Foliaceus (Fogo Selvagem) Give Birth to Disease-Free Babies. J. Investig. Dermatol. 1992, 99, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiko, A.; Hashimoto, T.; Shimizu, H.; Masunaga, T.; Nishibori, Y.; Watanabe, K.; Nishikawa, T. Combined Features of Pemphigus Foliaceus and Bullous Pemphigoid: Immunoblot and Immunoelectron Microscopic Studies. Arch. Dermatol. 1995, 131, 732–734. [Google Scholar] [CrossRef]
- Huh, W.K.; Tada, J.; Fujimoto, W.; Toi, Y.; Arakawa, K.; Arata, J.; Morita, H.; Hamada, H. Thyroid Gland Tumour, Pemphigus Foliaceus and Myasthenia Gravis in the Daughter of a Woman with Myasthenia Gravis. Clin. Exp. Dermatol. 2001, 26, 504–506. [Google Scholar] [CrossRef]
- Ameri, P.; Cinotti, E.; Mussap, M.; Murialdo, G.; Parodi, A.; Cozzani, E. Association of Pemphigus and Bullous Pemphigoid with Thyroid Autoimmunity in Caucasian Patients. J. Am. Acad. Dermatol. 2013, 68, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, A.; Attwood, K.; Sato, R.; Seiffert-Sinha, K.; Sinha, A.A. Identification of a New Disease Cluster of Pemphigus Vulgaris with Autoimmune Thyroid Disease, Rheumatoid Arthritis and Type I Diabetes. Br. J. Dermatol. 2015, 172, 729–738. [Google Scholar] [CrossRef]
- Nisihara, R.M.; de Bem, R.S.; Hausberger, R.; Roxo, V.S.; Pavoni, D.P.; Petzl-Erler, M.L.; de Messias-Reason, I.J. Prevalence of Autoantibodies in Patients with Endemic Pemphigus Foliaceus (Fogo Selvagem). Arch. Dermatol. Res. 2003, 295, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, D.J.B.; Christopher, M.; Lian, F.; Sligh, J.E. A Blistering Response: Concurrent Psoriasis and Pemphigus Foliaceus. Am. J. Med. 2015, 128, 24–26. [Google Scholar] [CrossRef]
- Liebman, T.N.; Lieberman, M.R.; Burris, K. Pemphigus Foliaceus Exacerbated by Radiation, in Association with Myasthenia Gravis. Dermatol. Online J. 2016, 22. [Google Scholar] [CrossRef]
- EL-Komy, M.H.M.; Samir, N.; Shaker, O.G. Estimation of Vitamin D Levels in Patients with Pemphigus Vulgaris. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Waschke, J. Apoptosis in Pemphigus. Autoimmun. Rev. 2009, 8, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Bektas, M.; Jolly, P.; Rubenstein, D.S. Apoptotic Pathways in Pemphigus. Dermatol. Res. Pract. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lotti, R.; Marconi, A.; Pincelli, C. Apoptotic Pathways in the Pathogenesis of Pemphigus: Targets for New Therapies. CPB 2012, 13, 1877–1881. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Lotti, R.; Shu, E.; Petrachi, T.; Marconi, A.; Palazzo, E.; Quadri, M.; Lin, A.; O’Reilly, L.A.; Pincelli, C. Soluble Fas Ligand Is Essential for Blister Formation in Pemphigus. Front. Immunol. 2018, 9, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gniadecki, R.; Jemec, G.B.; Thomsen, B.M.; Hansen, M. Relationship between Keratinocyte Adhesion and Death: Anoikis in Acantholytic Diseases. Arch. Dermatol. Res. 1998, 290, 528–532. [Google Scholar] [CrossRef]
- Janse, I.C.; van der Wier, G.; Jonkman, M.F.; Pas, H.H.; Diercks, G.F.H. No Evidence of Apoptotic Cells in Pemphigus Acantholysis. J. Investig. Dermatol. 2014, 134, 2039–2041. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, E.; Gutberlet, J.; Siegmund, D.; Berg, D.; Wajant, H.; Waschke, J. Apoptosis Is Not Required for Acantholysis in Pemphigus Vulgaris. Am. J. Physiol.-Cell Physiol. 2009, 296, C162–C172. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.B.R.; Pereira, S.A.L.; dos Reis, M.A.; Adad, S.J.; Caixeta, J.E.; Chiba, A.M.; Sousa, R.; Rodrigues, V., Jr. In Situ Detection of Inflammatory Cytokines and Apoptosis in Pemphigus Foliaceus Patients. Am. Pathol. Lab. Med. 2009, 133, 97–100. [Google Scholar] [CrossRef]
- Sokol, E.; Kramer, D.; Diercks, G.F.H.; Kuipers, J.; Jonkman, M.F.; Pas, H.H.; Giepmans, B.N.G. Large-Scale Electron Microscopy Maps of Patient Skin and Mucosa Provide Insight into Pathogenesis of Blistering Diseases. J. Investig. Dermatol. 2015, 135, 1763–1770. [Google Scholar] [CrossRef] [Green Version]
- Renehan, A.G. What Is Apoptosis, and Why Is It Important? BMJ 2001, 322, 1536–1538. [Google Scholar] [CrossRef] [Green Version]
- Luyet, C.; Schulze, K.; Sayar, B.S.; Howald, D.; Müller, E.J.; Galichet, A. Preclinical Studies Identify Non-Apoptotic Low-Level Caspase-3 as Therapeutic Target in Pemphigus Vulgaris. PLoS ONE 2015, 10, e0119809. [Google Scholar] [CrossRef] [Green Version]
- Dusek, R.L.; Getsios, S.; Chen, F.; Park, J.K.; Amargo, E.V.; Cryns, V.L.; Green, K.J. The Differentiation-Dependent Desmosomal Cadherin Desmoglein 1 Is a Novel Caspase-3 Target That Regulates Apoptosis in Keratinocytes. J. Biol. Chem. 2006, 281, 3614–3624. [Google Scholar] [CrossRef] [Green Version]
- Weiske, J.; Schöneberg, T.; Schröder, W.; Hatzfeld, M.; Tauber, R.; Huber, O. The Fate of Desmosomal Proteins in Apoptotic Cells. J. Biol. Chem. 2001, 276, 41175–41181. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; El-Deiry, W.S. Overview of Cell Death Signaling Pathways. Cancer Biol. Ther. 2005, 4, 147–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, A.J.; Konstantinou, M.; Goode, E.F.; Meier, P. The Diversification of Cell Death and Immunity: Memento Mori. Mol. Cell 2019, 76, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Abida, O.; Mahfoudh, N.; Kammoun, A.; Gaddour, L.; Hakim, F.; Toumi, A.; Masmoudi, A.; Ben Ayed, M.; Turki, H.; Masmoudi, H.; et al. Polymorphisms of HLA Microsatellite Marker in Tunisian Pemphigus Foliaceus. Hum. Immunol. 2013, 74, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Ameglio, F.; D’Auria, L.; Cordiali-Fei, P.; Trento, E.; D’Agosto, G.; Mastroianni, A.; Giannetti, A.; Giacalone, B. Anti-Intercellular Substance Antibody Log Titres Are Correlated with Serum Concentrations of Interleukin-6, Interleukin-15 and Tumor Necrosis Factor-Alpha in Patients with Pemphigus Vulgaris Relationships with Peripheral Blood Neutrophil Counts, Disease Severity and Duration and Patients’ Age. J. Biol. Regul. Homeost. Agents 1999, 13, 220–224. [Google Scholar]
- Asahina, A.; Uno, K.; Fujita, H. Sequential Occurrence of Pemphigus Vulgaris and Palmoplantar Pustulosis: Possible Role of Cytokine Profile. Acta Derm. Venerol. 2012, 92, 89–90. [Google Scholar] [CrossRef] [Green Version]
- Baroni, A.; Buommino, E.; Paoletti, I.; Orlando, M.; Ruocco, E.; Ruocco, V. Pemphigus Serum and Captopril Induce Heat Shock Protein 70 and Inducible Nitric Oxide Synthase Overexpression, Triggering Apoptosis in Human Keratinocytes. Br. J. Dermatol. 2004, 150, 1070–1080. [Google Scholar] [CrossRef]
- Chen, Y.; Chernyavsky, A.; Webber, R.J.; Grando, S.A.; Wang, P.H. Critical Role of the Neonatal Fc Receptor (FcRn) in the Pathogenic Action of Antimitochondrial Autoantibodies Synergizing with Anti-Desmoglein Autoantibodies in Pemphigus Vulgaris. J. Biol. Chem. 2015, 290, 23826–23837. [Google Scholar] [CrossRef] [Green Version]
- Chernyavsky, A.; Chen, Y.; Wang, P.H.; Grando, S.A. Pemphigus Vulgaris Antibodies Target the Mitochondrial Nicotinic Acetylcholine Receptors That Protect Keratinocytes from Apoptolysis. Int. Immunopharmacol. 2015, 29, 76–80. [Google Scholar] [CrossRef]
- Chernyavsky, A.; Amber, K.T.; Agnoletti, A.F.; Wang, C.; Grando, S.A. Synergy among Non-Desmoglein Antibodies Contributes to the Immunopathology of Desmoglein Antibody–Negative Pemphigus Vulgaris. J. Biol. Chem. 2019, 294, 4520–4528. [Google Scholar] [CrossRef] [Green Version]
- Chiapa-Labastida, M.; Zentella-Dehesa, A.; León-Dorantes, G.; Becker, I. Pemphigus Vulgaris: Accumulation of Apoptotic Cells in Dermis and Epidermis Possibly Relates to Pathophysiology through TNF-Alpha Production by Phagocytes. Eur. J. Dermatol. 2011, 21, 874–888. [Google Scholar] [CrossRef]
- Chriguer, R.S.; Roselino, A.M.; de Castro, M. Glucocorticoid Sensitivity and Proinflammatory Cytokines Pattern in Pemphigus. J. Clin. Immunol. 2012, 32, 786–793. [Google Scholar] [CrossRef]
- Cuevas-Gonzalez, J.C.; Vega-Memíje, M.E.; García-Vázquez, F.J.; Aguilar-Urbano, M.A. Detection of Apoptosis in Pemphigus Vulgaris by TUNEL Technique. An. Bras. Dermatol. 2016, 91, 296–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Auria, L.; Bonifati, C.; Mussi, A.; D’Agosto, G.; De Simone, C.; Giacalone, B.; Ferraro, C.; Ameglio, F. Cytokines in the Sera of Patients with Pemphigus Vulgaris: Interleukin-6 and Tumour Necrosis Factor-Alpha Levels Are Significantly Increased as Compared to Healthy Subjects and Correlate with Disease Activity. Eur. Cytokine Netw. 1997, 8, 383–387. [Google Scholar]
- Deyhimi, P.; Tavakoli, P. Study of Apoptosis in Oral Pemphigus Vulgaris Using Immunohistochemical Marker Bax and TUNEL Technique. J. Oral. Pathol. Med. 2013, 42, 409–414. [Google Scholar] [CrossRef]
- Dey-Rao, R.; Seiffert-Sinha, K.; Sinha, A.A. Genome-Wide Expression Analysis Suggests Unique Disease-Promoting and Disease-Preventing Signatures in Pemphigus Vulgaris. Genes Immun. 2013, 14, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, Y.; Burgos, E.; Gagliardi, J.; Vullo, C.M.; Borosky, A.; Pesoa, S.; Serra, H.M. Cytokine Polymorphisms in Patients with Pemphigus. Arch. Dermatol. Res. 2005, 296, 309–313. [Google Scholar] [CrossRef]
- Feliciani, C.; Toto, P.; Amerio, P. In Vitro C3 MRNA Expression in Pemphigus Vulgaris: Complement Activation Is Increased by IL-1alpha and TNF-Alpha. J. Cutan. Med. Surg. 1999, 3, 140–144. [Google Scholar] [CrossRef]
- Feliciani, C.; Toto, P.; Amerio, P.; Pour, S.M.; Coscione, G.; Shivji, G.; Wang, B.; Sauder, D.N. In Vitro and in Vivo Expression of Interleukin-1alpha and Tumor Necrosis Factor-Alpha MRNA in Pemphigus Vulgaris: Interleukin-1alpha and Tumor Necrosis Factor-Alpha Are Involved in Acantholysis. J. Investig. Dermatol. 2000, 114, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Frusic-Zlotkin, M.; Pergamentz, R.; Michel, B.; David, M.; Mimouni, D.; Brégégère, F.; Milner, Y. The Interaction of Pemphigus Autoimmunoglobulins with Epidermal Cells: Activation of the Fas Apoptotic Pathway and the Use of Caspase Activity for Pathogenicity Tests of Pemphigus Patients. Ann. N. Y. Acad. Sci. 2005, 1050, 371–379. [Google Scholar] [CrossRef]
- Gil, M.P.; Modol, T.; España, A.; López-Zabalza, M.J. Inhibition of FAK Prevents Blister Formation in the Neonatal Mouse Model of Pemphigus Vulgaris: FAK in Pemphigus Vulgaris. Exp. Dermatol. 2012, 21, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Javor, J.; Chmurova, N.; Parnicka, Z.; Ferencik, S.; Grosse-Wilde, H.; Buc, M.; Svecova, D. TNF-α and IL-10 Gene Polymorphisms Show a Weak Association with Pemphigus Vulgaris in the Slovak Population: TNF-α and IL-10 Gene Polymorphisms in Pemphigus Vulgaris. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 65–68. [Google Scholar] [CrossRef]
- Khozeimeh, F.; Savabi, O.; Esnaashari, M. Evaluation of Interleukin-1α, Interleukin-10, Tumor Necrosis Factor-α and Transforming Growth Factor-β in the Serum of Patients with Pemphigus Vulgaris. J. Contemp. Dent. Pract. 2014, 15, 746–749. [Google Scholar] [CrossRef]
- Kohler, K.F.; Petzl-Erler, M.L. No Evidence for Association of the TP53 12139 and the BAX-248 Polymorphisms with Endemic Pemphigus Foliaceus (Fogo Selvagem). Int. J. Immunogenet. 2006, 33, 141–144. [Google Scholar] [CrossRef]
- Lanza, A.; Lanza, M.; Santoro, R.; Soro, V.; Prime, S.S.; Cirillo, N. Deregulation of PERK in the Autoimmune Disease Pemphigus Vulgaris Occurs via IgG-Independent Mechanisms: Deregulation of PERK in PV. Br. J. Dermatol. 2011, 164, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, M.; Wang, J.; Liu, Z.; Diaz, L.A. Involvement of the Apoptotic Mechanisms in Pemphigus Foliaceus Autoimmune Injury of the Skin. J. Immunol. 2009, 182, 711–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Halipu, Y.; Hu, F.; Yakeya, B.; Chen, W.; Zhang, H.; Kang, X. Naringenin Protects Keratinocytes from Oxidative Stress Injury via Inhibition of the NOD2-Mediated NF-ΚB Pathway in Pemphigus Vulgaris. Biomed. Pharmacother. 2017, 92, 796–801. [Google Scholar] [CrossRef]
- Lopez-Robles, E.; Avalos-Diaz, E.; Vega-Memije, E.; Hojyo-Tomoka, T.; Villalobos, R.; Fraire, S.; Domiguez-Soto, L.; Herrera-Esparza, R. TNFalpha and IL-6 Are Mediators in the Blistering Process of Pemphigus. Int. J. Dermatol. 2001, 40, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Malheiros, D.; Panepucci, R.A.; Roselino, A.M.; Araújo, A.G.; Zago, M.A.; Petzl-Erler, M.L. Genome-Wide Gene Expression Profiling Reveals Unsuspected Molecular Alterations in Pemphigus Foliaceus. Immunology 2014, 143, 381–395. [Google Scholar] [CrossRef] [Green Version]
- Marchenko, S.; Chernyavsky, A.I.; Arredondo, J.; Gindi, V.; Grando, S.A. Antimitochondrial Autoantibodies in Pemphigus Vulgaris. J. Biol. Chem. 2010, 285, 3695–3704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moravvej, H.; Yousefi, M.; Farrokhi, B.; Mosaffa, N. Soluble Fas in Pemphigus Vulgaris. Arch. Iran. Med. 2011, 14, 200–201. [Google Scholar]
- Mosaad, Y.M.; Fathy, H.; Fawzy, Z.; El-Saied, M.A. Tumor Necrosis Factor-α -308 G>A and Interleukin-6 -174 G>C Promoter Polymorphisms and Pemphigus. Hum. Immunol. 2012, 73, 560–565. [Google Scholar] [CrossRef]
- Narbutt, J.; Lukamowicz, J.; Bogaczewicz, J.; Sysa-Jedrzejowska, A.; Torzecka, J.D.; Lesiak, A. Serum Concentration of Interleukin-6 Is Increased Both in Active and Remission Stages of Pemphigus Vulgaris. Med. Inflamm. 2008, 2008, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.T.; Arredondo, J.; Chernyavsky, A.I.; Kitajima, Y.; Pittelkow, M.; Grando, S.A. Pemphigus Vulgaris IgG and Methylprednisolone Exhibit Reciprocal Effects on Keratinocytes. J. Biol. Chem. 2004, 279, 2135–2146. [Google Scholar] [CrossRef] [Green Version]
- Orlov, M.D.; Chernyavsky, A.I.; Arredondo, J.; Grando, S.A. Synergistic Actions of Pemphigus Vulgaris IgG, Fas-Ligand and Tumor Necrosis Factor-α during Induction of Basal Cell Shrinkage and Acantholysis. Autoimmunity 2006, 39, 557–562. [Google Scholar] [CrossRef]
- Pacheco-Tovar, M.; Avalos-Díaz, E.; Vega-Memije, E.; Bollain-y-Goytia, J.; López-Robles, E.; Hojyo-Tomoka, M.; Domínguez-Soto, L.; Herrera-Esparza, R. The Final Destiny of Acantholytic Cells in Pemphigus Is Fas Mediated. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Pretel, M.; España, A.; Marquina, M.; Pelacho, B.; López-Picazo, J.M.; López-Zabalza, M.J. An Imbalance in Akt/MTOR Is Involved in the Apoptotic and Acantholytic Processes in a Mouse Model of Pemphigus Vulgaris. Exp. Dermatol. 2009, 18, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Puviani, M.; Marconi, A.; Pincelli, C.; Cozzani, E. Fas Ligand in Pemphigus Sera Induces Keratinocyte Apoptosis through the Activation of Caspase-8. J. Investig. Dermatol. 2003, 120, 164–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragab, N.; Abdallah, M.; El-Gohary, E.; Elewa, R. Stress and Serum TNF-Alpha Levels May Predict Disease Outcome in Patients with Pemphigus: A Preliminary Study. Cutis 2011, 87, 189–194. [Google Scholar]
- Rehman, A.; Cai, Y.; Hünefeld, C.; Jedličková, H.; Huang, Y.; Teck Teh, M.; Sharif Ahmad, U.; Uttagomol, J.; Wang, Y.; Kang, A.; et al. The Desmosomal Cadherin Desmoglein-3 Acts as a Keratinocyte Anti-Stress Protein via Suppression of P53. Cell Death Dis. 2019, 10, 750. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Rodrigues, D.B.; Paschoini, G.; Pereira, S.A.L.; dos Reis, M.A.; Vicente de Paula, A.T.; Rodrigues, V. High Levels of Interleukin-1 in Patients with Endemic Pemphigus Foliaceus. Clin. Vaccine Immunol. 2003, 10, 741–743. [Google Scholar] [CrossRef]
- Roxo, V.M.M.S.; Pereira, N.F.; Pavoni, D.P.; Lin, M.-T.; Hansen, J.A.; Poersch, O.D.C.; Filho, A.M.; Petzl-Erler, M.L. Polymorphisms within the Tumor Necrosis Factor and Lymphotoxin-Alpha Genes and Endemic Pemphigus Foliaceus - Are There Any Associations?: Roxo et al: Endemic Pemphigus Foliaceus and TNF and LTA Gene Polymorphisms. Tissue Antigens 2003, 62, 394–400. [Google Scholar] [CrossRef]
- Sarig, O.; Bercovici, S.; Zoller, L.; Goldberg, I.; Indelman, M.; Nahum, S.; Israeli, S.; Sagiv, N.; Martinez de Morentin, H.; Katz, O.; et al. Population-Specific Association between a Polymorphic Variant in ST18, Encoding a Pro-Apoptotic Molecule, and Pemphigus Vulgaris. J. Investig. Dermatol. 2012, 132, 1798–1805. [Google Scholar] [CrossRef] [Green Version]
- Sayama, K.; Yonehara, S.; Watanabe, Y.; Miki, Y. Expression of Fas Antigen on Keratinocytes In Vivo and Induction of Apoptosis in Cultured Keratinocytes. J. Investig. Dermatol. 1994, 103, 330–334. [Google Scholar] [CrossRef] [Green Version]
- Seiffert-Sinha, K.; Yang, R.; Fung, C.K.; Lai, K.W.; Patterson, K.C.; Payne, A.S.; Xi, N.; Sinha, A.A. Nanorobotic Investigation Identifies Novel Visual, Structural and Functional Correlates of Autoimmune Pathology in a Blistering Skin Disease Model. PLoS ONE 2014, 9, e106895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamsabadi, R.M.; Basafa, S.; Yarahmadi, R.; Goorani, S.; Khani, M.; Kamarehei, M.; Hossein Kiani, A. Elevated Expression of NLRP1 and IPAF Are Related to Oral Pemphigus Vulgaris Pathogenesis. Inflammation 2015, 38, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.L.; Dahl, M.V. Methylprednisolone Inhibits Pemphigus Acantholysis in Skin Cultures. J. Investig. Dermatol. 1983, 81, 258–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timoteo, R.P.; da Silva, M.V.; Miguel, C.B.; Silva, D.A.A.; Catarino, J.D.S.; Rodrigues Junior, V.; Sales-Campos, H.; Freire Oliveira, C.J. Th1/Th17-Related Cytokines and Chemokines and Their Implications in the Pathogenesis of Pemphigus Vulgaris. Mediat. Inflamm. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Toosi, S.; Habib, N.; Torres, G.; Reynolds, S.R.; Bystryn, J.-C. Serum Levels of Inhibitors of Apoptotic Proteins (IAPs) Change with IVIg Therapy in Pemphigus. J. Investig. Dermatol. 2011, 131, 2327–2329. [Google Scholar] [CrossRef] [Green Version]
- Torzecka, J.D.; Narbutt, J.; Sysa-jedrzejowska, A.; Borowiec, M.; Ptasinska, A.; Woszczek, G.; Kowalski, M.L. Tumour Necrosis Factor-α Polymorphism as One of the Complex Inherited Factors in Pemphigus. Mediat. Inflamm. 2003, 12, 303–307. [Google Scholar] [CrossRef]
- Wang, X.; Brégégère, F.; Frušić-Zlotkin, M.; Feinmesser, M.; Michel, B.; Milner, Y. Possible Apoptotic Mechanism in Epidermal Cell Acantholysis Induced by Pemphigus Vulgaris Autoimmunoglobulins. Apoptosis 2004, 9, 131–143. [Google Scholar] [CrossRef]
- Wang, X.; Brégégère, F.; Soroka, Y.; Frusic-Zlotkin, M.; Milner, Y. Replicative Senescence Enhances Apoptosis Induced by Pemphigus Autoimmune Antibodies in Human Keratinocytes. FEBS Lett. 2004, 567, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuccolotto, I.; Roselino, A.M.; Ramalho, L.N.Z.; Zucoloto, S. Apoptosis and P63 Expression in the Pathogenesis of Bullous Lesions of Endemic Pemphigus Foliaceus. Arch. Dermatol. Res. 2003, 295, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Hajeer, A.H.; Hutchinson, I.V. TNF-Alpha Gene Polymorphism: Clinical and Biological Implications. Microsc. Res. Tech. 2000, 50, 216–228. [Google Scholar] [CrossRef]
- Tavakolpour, S.; Mahmoudi, H.; Mirzazadeh, A.; Balighi, K.; Darabi-Monadi, S.; Hatami, S.; GhasemiAdl, M.; Daneshpazhooh, M. Pathogenic and Protective Roles of Cytokines in Pemphigus: A Systematic Review. Cytokine 2020, 129, 155026. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Liu, X.-Z.; Sun, J.; Yin, Y.-W.; Sun, Q.-Q. Association between TNF α Gene Polymorphisms and the Risk of Duodenal Ulcer: A Meta-Analysis. PLoS ONE 2013, 8, e57167. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Piras, V.; Tabata, S.; Tomita, M.; Selvarajoo, K. A Systems Biology Approach to Suppress TNF-Induced Proinflammatory Gene Expressions. Cell Commun. Signal. 2013, 11, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoog, T.; van’t Hooft, F.M.; Kallin, B.; Jovinge, S.; Boquist, S.; Nilsson, J.; Eriksson, P.; Hamsten, A. A Common Functional Polymorphism (C-->A Substitution at Position -863) in the Promoter Region of the Tumour Necrosis Factor-Alpha (TNF-Alpha) Gene Associated with Reduced Circulating Levels of TNF-Alpha. Hum. Mol. Genet. 1999, 8, 1443–1449. [Google Scholar] [CrossRef] [Green Version]
- Kiss-Toth, E.; Harlock, E.; Lath, D.; Quertermous, T.; Wilkinson, J.M. A TNF Variant That Associates with Susceptibility to Musculoskeletal Disease Modulates Thyroid Hormone Receptor Binding to Control Promoter Activation. PLoS ONE 2013, 8, e76034. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Kong, Z.; Zhao, H. Relationship Between Tumor Necrosis Factor-α Rs361525 Polymorphism and Gastric Cancer Risk: A Meta-Analysis. Front. Physiol. 2018, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Krammer, P.H. CD95’s Deadly Mission in the Immune System. Nature 2000, 407, 789–795. [Google Scholar] [CrossRef]
- Jackson, N.M.; Ceresa, B.P. EGFR-Mediated Apoptosis via STAT3. Exp. Cell Res. 2017, 356, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M. BCL2A1: The Underdog in the BCL2 Family. Cell Death Differ. 2012, 19, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizushima, T.; Arakawa, S.; Sanada, Y.; Yoshino, I.; Miyazaki, D.; Urushima, H.; Tsujimoto, Y.; Ito, T.; Shimizu, S. Inhibition of Epithelial Cell Death by Bcl-2 Improved Chronic Colitis in IL-10 KO Mice. Am. J. Pathol. 2013, 183, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Verfaillie, T.; Rubio, N.; Garg, A.D.; Bultynck, G.; Rizzuto, R.; Decuypere, J.-P.; Piette, J.; Linehan, C.; Gupta, S.; Samali, A.; et al. PERK Is Required at the ER-Mitochondrial Contact Sites to Convey Apoptosis after ROS-Based ER Stress. Cell Death Differ. 2012, 19, 1880–1891. [Google Scholar] [CrossRef] [Green Version]
- Shao, B.-Z.; Xu, Z.-Q.; Han, B.-Z.; Su, D.-F.; Liu, C. NLRP3 Inflammasome and Its Inhibitors: A Review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef] [Green Version]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef]
- Moore, C.L.; Savenka, A.V.; Basnakian, A.G. TUNEL Assay: A Powerful Tool for Kidney Injury Evaluation. Int. J. Mol. Sci. 2021, 22, 412. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Alexander, J.S.; Babu, P.P. PARP-1 Cleavage Fragments: Signatures of Cell-Death Proteases in Neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Taatjes, D.J.; Sobel, B.E.; Budd, R.C. Morphological and Cytochemical Determination of Cell Death by Apoptosis. Histochem. Cell Biol. 2008, 129, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Grando, S.A.; Bystryn, J.-C.; Chernyavsky, A.I.; Frušić-Zlotkin, M.; Gniadecki, R.; Lotti, R.; Milner, Y.; Pittelkow, M.R.; Pincelli, C. Apoptolysis: A Novel Mechanism of Skin Blistering in Pemphigus Vulgaris Linking the Apoptotic Pathways to Basal Cell Shrinkage and Suprabasal Acantholysis. Exp. Dermatol. 2009, 18, 764–770. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
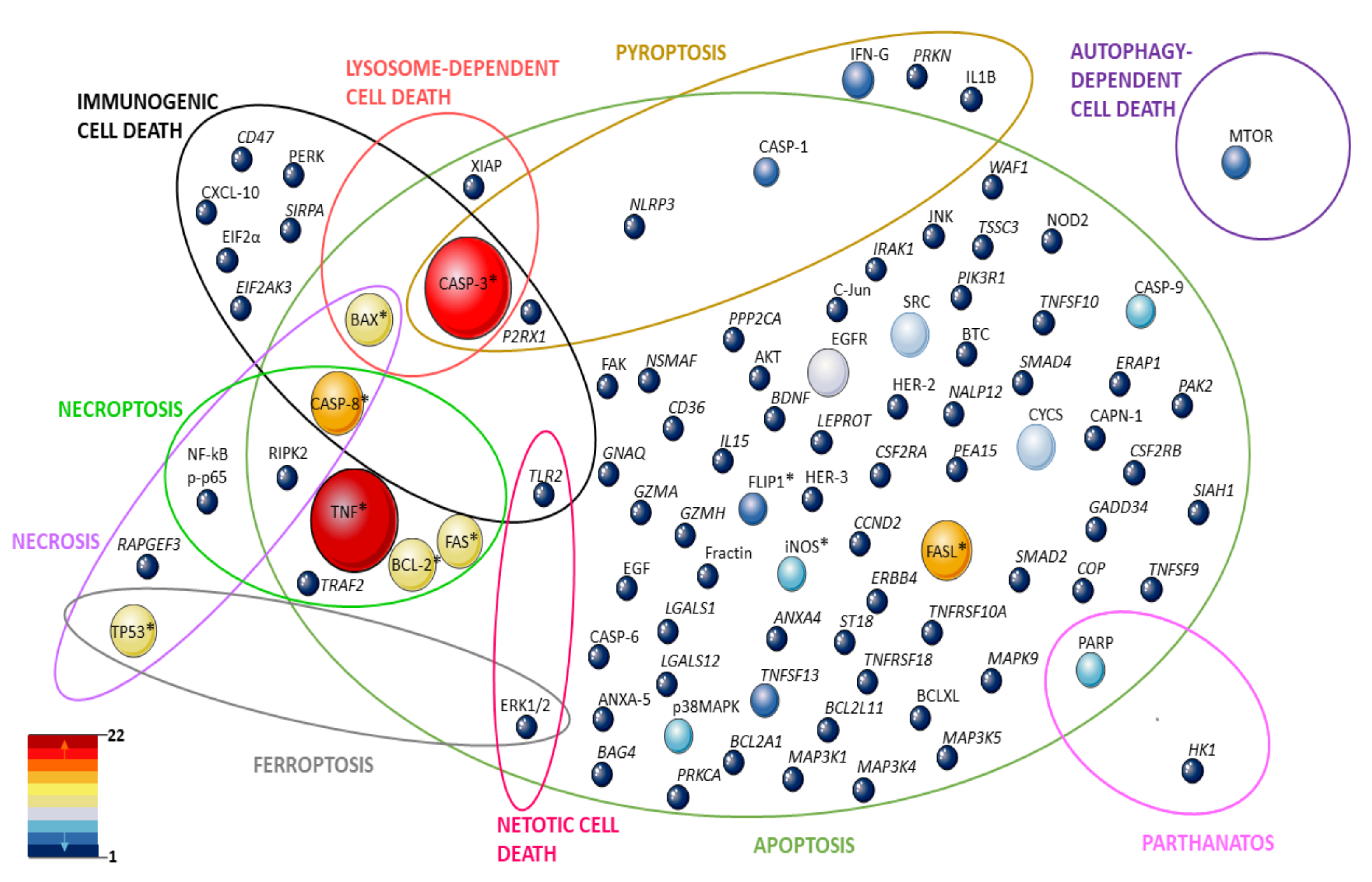
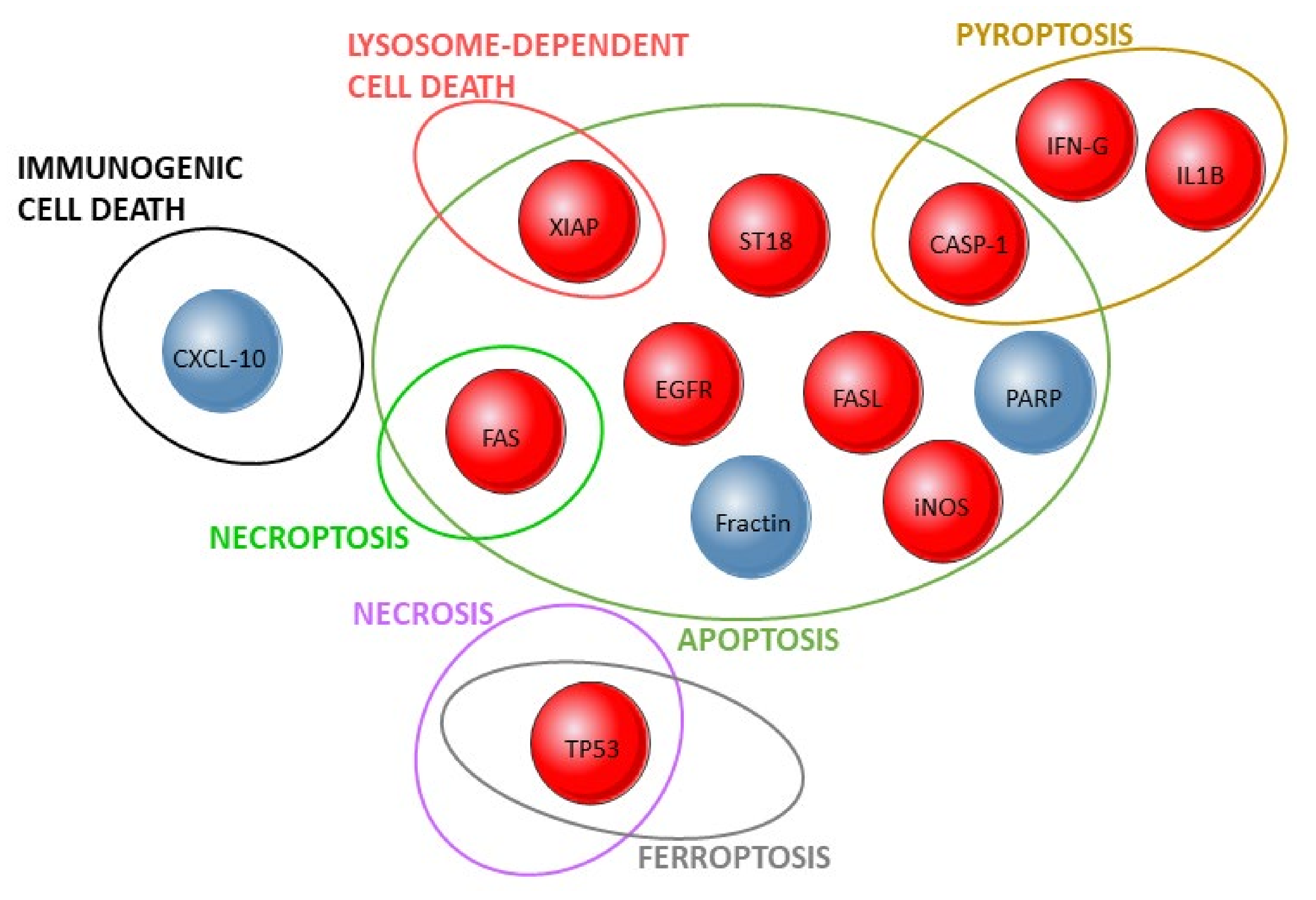
| References | Target(s) Investigated | Study Design | |||
|---|---|---|---|---|---|
| Patients | Human Skin Organ Culture | Cell Lines | Mouse Model | ||
| [73] | TNF | ✓ | |||
| [74] | TNF | ✓ | |||
| [24] | CASP3 *, CASP8 *, FASL *, FLIP1 *, CAPN1, DNA fragmentation, membrane cell permeability | ✓ | ✓ | ||
| [75] | TNF | ✓ | |||
| [76] | iNOS *, TP53 *, BCL2, DNA fragmentation | ✓ | |||
| [37] | TNF, TRAF2, CD36, SIRPA, CD47, HK1, PAK2, EIF2AK3, RAPGEF3, PRKN | ✓ | |||
| [77] | CYCS | ✓ | |||
| [78] | CYCS | ✓ | |||
| [79] | CASP9, CYCS, SRC, p38 MAPK | ✓ | |||
| [80] | CASP3, TNF, DNA fragmentation | ✓ | |||
| [81] | TNF | ✓ | |||
| [82] | DNA fragmentation | ✓ | |||
| [83] | TNF | ✓ | |||
| [84] | BAX, DNA fragmentation | ✓ | |||
| [85] | BAG4, BCL2L11, CSF2RA, CSF2RB, GNAQ, IL15, LEPROT, MAP3K1, MAP3K4, MAP3K5, MAPK9, NSMAF, PPP2CA, SMAD2, SMAD4, ERAP1, ERBB4, BDNF, IRAK1, TNFSF13 | ✓ | |||
| [86] | TNF | ✓ | |||
| [87] | TNF | ✓ | |||
| [88] | TNF * | ✓ | ✓ | ✓ | |
| [89] | CASP3, CASP8, membrane cell permeability, degraded cells and condensed chromatin | ✓ | ✓ | ✓ | |
| [19] | CASP3, CASP8, EGFR, ERK1/2, FASL, JUN, membrane cell permeability | ✓ | ✓ | ✓ | |
| [90] | BAX, BCL2, CASP3, CASP9, EGFR, MTOR, SRC, FAK | ✓ | |||
| [62] | DNA fragmentation | ✓ | |||
| [63] | CASP3, CASP8, Fractin, PARP, DNA fragmentation | ✓ | ✓ | ||
| [91] | TNF | ✓ | |||
| [22] | mitochondrial damage | ✓ | |||
| [92] | TNF | ✓ | |||
| [93] | TP53, BAX | ✓ | |||
| [94] | EIF2α, PERK | ✓ | |||
| [17] | CASP3, p38 MAPK, PARP, DNA fragmentation | ✓ | ✓ | ||
| [95] | BAX, BCLXL, CASP3, CASP6, DNA fragmentation | ✓ | |||
| [96] | BAX, BCL2, CASP3, NOD2, RIPK2, NF-kB p-p65, ROS, membrane cell permeability | ✓ | |||
| [97] | TNF | ✓ | |||
| [61] | CASP8, FAS, FASL, DNA fragmentation | ✓ | ✓ | ✓ | |
| [68] | CASP3, PARP, DNA fragmentation | ✓ | ✓ | ✓ | |
| [98] | ANXA4, BCL2A1, COP, GZMA, GZMH, LGALS1, LGALS12, NALP12, P2RX1, PIK3R1, PRKCA, SIAH1, TLR2, TNFRSF10A, TNFRSF18, TNFSF9, TNFSF10, TNFSF13 | ✓ | |||
| [99] | CASP3, CASP8, CASP9, CYCS, EGFR, FASL, JNK, p38 MAPK, SRC | ✓ | |||
| [100] | FASL | ✓ | |||
| [101] | TNF | ✓ | |||
| [102] | TNF | ✓ | |||
| [103] | BAX, CCND2, GADD34, PEA15, TSSC3, WAF1 | ✓ | |||
| [104] | TNF, FASL, membrane cell permeability | ✓ | |||
| [105] | CASP3 *, FAZ *, FASL *, DNA fragmentation | ✓ | |||
| [106] | AKT, BTC, EGF, EGFR, HER2, HER3, P-SCR, TNF, MTOR, DNA fragmentation | ✓ | |||
| [107] | FASL, CASP8, DNA fragmentation | ✓ | ✓ | ||
| [108] | TNF | ✓ | |||
| [109] | BAX, CASP3, TP53 | ✓ | ✓ | ||
| [110] | IL1B | ✓ | |||
| [65] | BCL2, FAS, IFNG, IL1-B, iNOS, TNF, DNA fragmentation | ✓ | |||
| [111] | TNF | ✓ | |||
| [112] | ST18 | ✓ | |||
| [113] | FAS | ✓ | |||
| [64] | CASP3, FLIP, DNA fragmentation | ✓ | ✓ | ||
| [114] | membrane cell permeability | ✓ | |||
| [115] | NLRP3 | ✓ | |||
| [116] | membrane cell permeability | ✓ | |||
| [117] | CXCL10, IFNG, TNF | ✓ | |||
| [118] | LIVIN, XIAP | ✓ | |||
| [119] | TNF, DNA fragmentation | ✓ | |||
| [120] | ANXA5, BAX, BCL2, CASP1, CASP3, CASP8, FAS, FASL, FASR, TP53, DNA fragmentation, membrane cell permeability | ✓ | ✓ | ✓ | |
| [121] | BCL2, CASP1, CASP3, CASP8, FASL, TP53, | ✓ | |||
| [122] | DNA fragmentation | ✓ | |||
| Protein | Detected or Increased | Absent, Low or Decreased | ||||
|---|---|---|---|---|---|---|
| PV | PF | PV and PF | PV | PF | PV and PF | |
| BAX | [120] * | [84] | ||||
| BCL2 | [65] * | [120] * | ||||
| CASP-1 | [120] * | |||||
| CASP-3 | [105] * [109] [80] [120] * | [64] [89] * | [63] * | |||
| CASP-8 | [120] * [89] * | [63] * | ||||
| CXCL-10 | [117] | |||||
| EGFR | [19] * | |||||
| FAS | [105] * [61] [113] [120] * | [65] * | ||||
| FAS-L | [100] * [105] * [120] * | [107] | [107] + | |||
| Fractin | [63] * | |||||
| IFN-G | [117] | [65] * | ||||
| IL-1B | [65] * | |||||
| iNOS | [65] * | |||||
| LIVIN | [118] + | |||||
| ST18 | [112] | |||||
| PARP | [63] * | |||||
| TNF | [92] [83] [74] [108] [80] [75] # | [65] * | [81] | [75] #,+ [117] [102] [83] + | ||
| TP53 | [109] [120] * | |||||
| XIAP | [118] + | |||||
| Type | Time | Detected or Increased | Absent, without Difference | ||||
|---|---|---|---|---|---|---|---|
| PV | PF | PV and PF | PV | PF | PV and PF | ||
| HUMANS | After blister formation in patients | [105] * [120] * [80] [84] [82] | [65] * | [107] [62]* | [68] [64] | [122] * | [63] * |
| CELL LINES | After treatment with PF/PV IgG (before keratinocytes dissociation) | [120] * [17] | [68] | ||||
| After keratinocytes dissociation | [76] [24] [120] * [104] [104] + [89] * [19] * [19] +,* [121] * [96] [114] | [107] | [68] [64] | ||||
| After some therapeutic intervention in cell lines | [17] [107] | [19] * [24] [120] * [96] | [107] | ||||
| MOUSE MODELS | After injection with PF/PV IgG (before blistering formation) | [61] [106] | [95] | [68] | |||
| After blistering formation | [24] | [17] | [68] | ||||
| After some therapeutic intervention | [24] [106] | [95] | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bumiller-Bini Hoch, V.; Schneider, L.; Pumpe, A.E.; Lüders, E.; Hundt, J.E.; Boldt, A.B.W. Marked to Die-Cell Death Mechanisms for Keratinocyte Acantholysis in Pemphigus Diseases. Life 2022, 12, 329. https://doi.org/10.3390/life12030329
Bumiller-Bini Hoch V, Schneider L, Pumpe AE, Lüders E, Hundt JE, Boldt ABW. Marked to Die-Cell Death Mechanisms for Keratinocyte Acantholysis in Pemphigus Diseases. Life. 2022; 12(3):329. https://doi.org/10.3390/life12030329
Chicago/Turabian StyleBumiller-Bini Hoch, Valéria, Larissa Schneider, Anna Elisabeth Pumpe, Emelie Lüders, Jennifer Elisabeth Hundt, and Angelica Beate Winter Boldt. 2022. "Marked to Die-Cell Death Mechanisms for Keratinocyte Acantholysis in Pemphigus Diseases" Life 12, no. 3: 329. https://doi.org/10.3390/life12030329
APA StyleBumiller-Bini Hoch, V., Schneider, L., Pumpe, A. E., Lüders, E., Hundt, J. E., & Boldt, A. B. W. (2022). Marked to Die-Cell Death Mechanisms for Keratinocyte Acantholysis in Pemphigus Diseases. Life, 12(3), 329. https://doi.org/10.3390/life12030329







