Predominance of Antioxidants in Some Edible Plant Oils in Ameliorating Oxidative Stress and Testicular Toxicity Induced by Malathion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Groups and Treatments
2.2. Analysis of Blood Serum
2.3. Histopathological Examination
2.4. Bioinformatics Analysis
2.5. Molecular Docking Study
2.6. Analysis of The Statistics
3. Results
3.1. Assessment of Biochemical Markers
3.2. Histopathological Studies
3.3. Molecular Docking Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moridi, H.; Hosseini, S.A.; Shateri, H.; Kheiripour, N.; Kaki, A.; Hatami, M.; Ranjbar, A. Protective effect of cerium oxide nanoparticle on sperm quality and oxidative damage in malathion-induced testicular toxicity in rats: An experimental study. Int. J. Reprod. Biomed. 2018, 16, 261–266. [Google Scholar] [CrossRef]
- Badr, A.M. Organophosphate toxicity: Updates of malathion potential toxic effects in mammals and potential treatments. Environ. Sci. Pollut. Res. 2020, 27, 26036–26057. [Google Scholar] [CrossRef]
- Abo El-Atta, H.; Ahmed, D. Testicular dysfunction in malathion induced toxicity in male rats: Protective role of NAC and Silymarin. Mansoura J. Forensic Med. Clin. Toxicol. 2020, 28, 33–45. [Google Scholar] [CrossRef]
- Penna-Videau, S.; Bustos-Obregón, E.; Cermeño-Vivas, J.; Chirino, D. Malathion affects spermatogenic proliferation in mouse. Int. J. Morphol. 2012, 30, 1399–1407. [Google Scholar] [CrossRef] [Green Version]
- Slimen, S.; El Fazaa Saloua, G.N. Oxidative stress and cytotoxic potential of anticholinesterase insecticide, malathion in reproductive toxicology of male adolescent mice after acute exposure. Iran. J. Basic Med. Sci. 2014, 17, 522–530. [Google Scholar]
- Ojha, A.; Srivastava, N. In vitro studies on organophosphate pesticides induced oxidative DNA damage in rat lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 761, 10–17. [Google Scholar] [CrossRef]
- Jain, N.; Joshi, S. Toxic impact of malathion on epididymal function. Toxicol. Lett. 2009, 189, S149. [Google Scholar] [CrossRef]
- Almalki, D.A.; Alghamdi, S.A.; Al-Attar, A.M. Comparative study on the influence of some medicinal plants on diabetes induced by streptozotocin in male rats. BioMed Res. Int. 2019, 2019, 3596287. [Google Scholar] [CrossRef] [Green Version]
- Dórea, J.G.; da Costa, T.H.M. Is coffee a functional food? Br. J. Nutr. 2005, 93, 773–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrea, L.; Pugliese, G.; Frias-Toral, E.; El Ghoch, M.; Castellucci, B.; Chapela, S.P.; Carignano, M.A.; Laudisio, D.; Savastano, S.; Colao, A. Coffee consumption, health benefits and side effects: A narrative review and update for dietitians and nutritionists. Crit. Rev. Food Sci. Nutr. 2021, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, G.; Hiane, P.A.; Freitas, K.d.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R.d.C.A. Effects of olive oil and its minor components on cardiovascular diseases, inflammation, and gut microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [Green Version]
- Shadfan, M.; Lopez-Pajares, V.; Yuan, Z.-M. MDM2 and MDMX: Alone and together in regulation of p53. Transl. Cancer Res. 2012, 1, 88–89. [Google Scholar]
- Al-Asmari, K.M.; Zeid, I.M.A.; Altayb, H.N.; Al-Attar, A.M.; Alomar, M.Y. Alleviation of Malathion Toxicity Effect by Coffea arabica L. Oil and Olea europaea L. Oil on Lipid Profile: Physiological and In Silico Study. Plants 2021, 10, 2314. [Google Scholar] [CrossRef]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Al-Asmari, K.M.; Zeid, I.M.; Al-Thobaiti, S. Sciatic nerve crush in rats and its impact on soleus muscle. World Appl. Sci. J. 2020, 38, 195–203. [Google Scholar]
- Atatreh, N.; Ghattas, M.A.; Bardaweel, S.K.; Al Rawashdeh, S.; Al Sorkhy, M. Identification of new inhibitors of Mdm2–p53 interaction via pharmacophore and structure-based virtual screening. Drug Des. Dev. Ther. 2018, 12, 3741–3752. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.A.J.; Pablos, F.; González, A.G.; Valdenebro, M.a.S.; León-Camacho, M. Fatty acid profiles as discriminant parameters for coffee varieties differentiation. Talanta 2001, 54, 291–297. [Google Scholar] [CrossRef]
- Al-Asmari, K.M.; Zeid, I.M.A.; Al-Attar, A.M. Medicinal properties of Arabica coffee (Coffea arabica) oil: An overview. Adv. Life Sci. 2020, 8, 20–29. [Google Scholar]
- Al-Asmari, K.; Al-Attar, A.; Zeid, I.M. Potential health benefits and components of olive oil: An overview. Biosci. Res. 2020, 17, 2673–2687. [Google Scholar]
- Shieh, P.; Jan, C.-R.; Liang, W.-Z. The protective effects of the antioxidant N-acetylcysteine (NAC) against oxidative stress-associated apoptosis evoked by the organophosphorus insecticide malathion in normal human astrocytes. Toxicology 2019, 417, 1–14. [Google Scholar] [CrossRef]
- Geng, X.; Shao, H.; Zhang, Z.; Ng, J.C.; Peng, C. Malathion-induced testicular toxicity is associated with spermatogenic apoptosis and alterations in testicular enzymes and hormone levels in male Wistar rats. Environ. Toxicol. Pharmacol. 2015, 39, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Karabag-Coban, F.; Bulduk, I.; Liman, R.; Ince, S.; Cigerci, I.; Hazman, O. Oleuropein alleviates malathion-induced oxidative stress and DNA damage in rats. Toxicol. Environ. Chem. 2016, 98, 101–108. [Google Scholar] [CrossRef]
- Panda, V.S.; Naik, S.R. Evaluation of cardioprotective activity of Ginkgo biloba and Ocimum sanctum in rodents. Altern. Med. Rev. 2009, 14, 161–171. [Google Scholar]
- Al-Megrin, W.A.; El-Khadragy, M.F.; Hussein, M.H.; Mahgoub, S.; Abdel-Mohsen, D.M.; Taha, H.; Bakkar, A.A.; Abdel Moneim, A.E.; Amin, H.K. Green Coffea arabica extract ameliorates testicular injury in high-fat diet/streptozotocin-induced diabetes in rats. J. Diabetes Res. 2020, 2020, 6762709. [Google Scholar] [CrossRef]
- Calahorra, J.; Martínez-Lara, E.; Granadino-Roldán, J.M.; Martí, J.M.; Cañuelo, A.; Blanco, S.; Oliver, F.J.; Siles, E. Crosstalk between hydroxytyrosol, a major olive oil phenol, and HIF-1 in MCF-7 breast cancer cells. Sci. Rep. 2020, 10, 6361. [Google Scholar] [CrossRef]
- Lendhey, S.S.; Kale, T.; Seth, T.; Bhartiya, G.; Hudwekar, A. Effect of subgingival application of ozone oil versus olive oil as an adjunct to scaling and root planing in chronic periodontitis: A clinical and microbiological study. J. Oral Res. Rev. 2020, 12, 63–69. [Google Scholar] [CrossRef]
- Padma, V.V.; Sowmya, P.; Felix, T.A.; Baskaran, R.; Poornima, P. Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem. Toxicol. 2011, 49, 991–998. [Google Scholar] [CrossRef]
- Al-Attar, A.M.; Elnaggar, M.H.; Almalki, E.A. Protective effect of some plant oils on diazinon induced hepatorenal toxicity in male rats. Saudi J. Biol. Sci. 2017, 24, 1162–1171. [Google Scholar] [CrossRef] [Green Version]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2009, 674, 137–147. [Google Scholar] [CrossRef]
- Nieber, K. The impact of coffee on health. Planta Med. 2017, 83, 1256–1263. [Google Scholar] [CrossRef] [Green Version]
- Jemai, H.; El Feki, A.; Sayadi, S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J. Agric. Food Chem. 2009, 57, 8798–8804. [Google Scholar] [CrossRef]
- Ghorbel, I.; Elwej, A.; Jamoussi, K.; Boudawara, T.; Kamoun, N.G.; Zeghal, N. Potential protective effects of extra virgin olive oil on the hepatotoxicity induced by co-exposure of adult rats to acrylamide and aluminum. Food Funct. 2015, 6, 1126–1135. [Google Scholar] [CrossRef]
- Saber, T.; Farag, M.; Cooper, R. Ameliorative effect of extra virgin olive oil on hexavalent chromium-induced nephrotoxicity and genotoxicity in rats. Revue Méd. Vét. 2015, 166, 11–19. [Google Scholar]
- Rubiolo, J.A.; Mithieux, G.; Vega, F.V. Resveratrol protects primary rat hepatocytes against oxidative stress damage:: Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 2008, 591, 66–72. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Oke, O.V.; Owolabi, F.O.; Kayode, F.O.; Umanhonlen, E.E.; Aliyu, M. Chemical properties of Monodora myristica and its protective potentials against free radicals in vitro. Oxid. Antioxid. Med. Sci. 2012, 1, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, J.K.; Saraf, P.; Kumari, P.; Mittal, M.; Kumar, V. N-Acetyl-cysteine mediated inhibition of spermatogonial cells apoptosis against malathion exposure in testicular tissue. J. Biochem. Mol. Toxicol. 2018, 32, e22046. [Google Scholar] [CrossRef]
- Khayyat, L.I. Extra Virgin Olive Oil Protects the Testis and Blood from the Toxicity of Paracetamol (Overdose) in Adult Male Rats. Biology 2021, 10, 1042. [Google Scholar] [CrossRef]
- Momand, J.; Zambetti, G.P.; Olson, D.C.; George, D.; Levine, A.J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 1992, 69, 1237–1245. [Google Scholar] [CrossRef]
- Honda, R.; Yasuda, H. Activity of MDM2, a ubiquitin ligase, toward p53 or itself is dependent on the RING finger domain of the ligase. Oncogene 2000, 19, 1473–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayyaz, S.; Aydin, T.; Cakir, A.; Gasparri, M.L.; Benedetti Panici, P.; Ahmad Farooqi, A. Oleuropein mediated targeting of signaling network in cancer. Curr. Top. Med. Chem. 2016, 16, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- El-Khadragy, M.F.; Al-Megrin, W.A.; Alomar, S.; Alkhuriji, A.F.; Metwally, D.M.; Mahgoub, S.; Amin, H.K.; Habotta, O.A.; Moneim, A.E.A.; Albeltagy, R.S. Chlorogenic acid abates male reproductive dysfunction in arsenic-exposed mice via attenuation of testicular oxido-inflammatory stress and apoptotic responses. Chem. Biol. Interact. 2021, 333, 109333. [Google Scholar] [CrossRef]


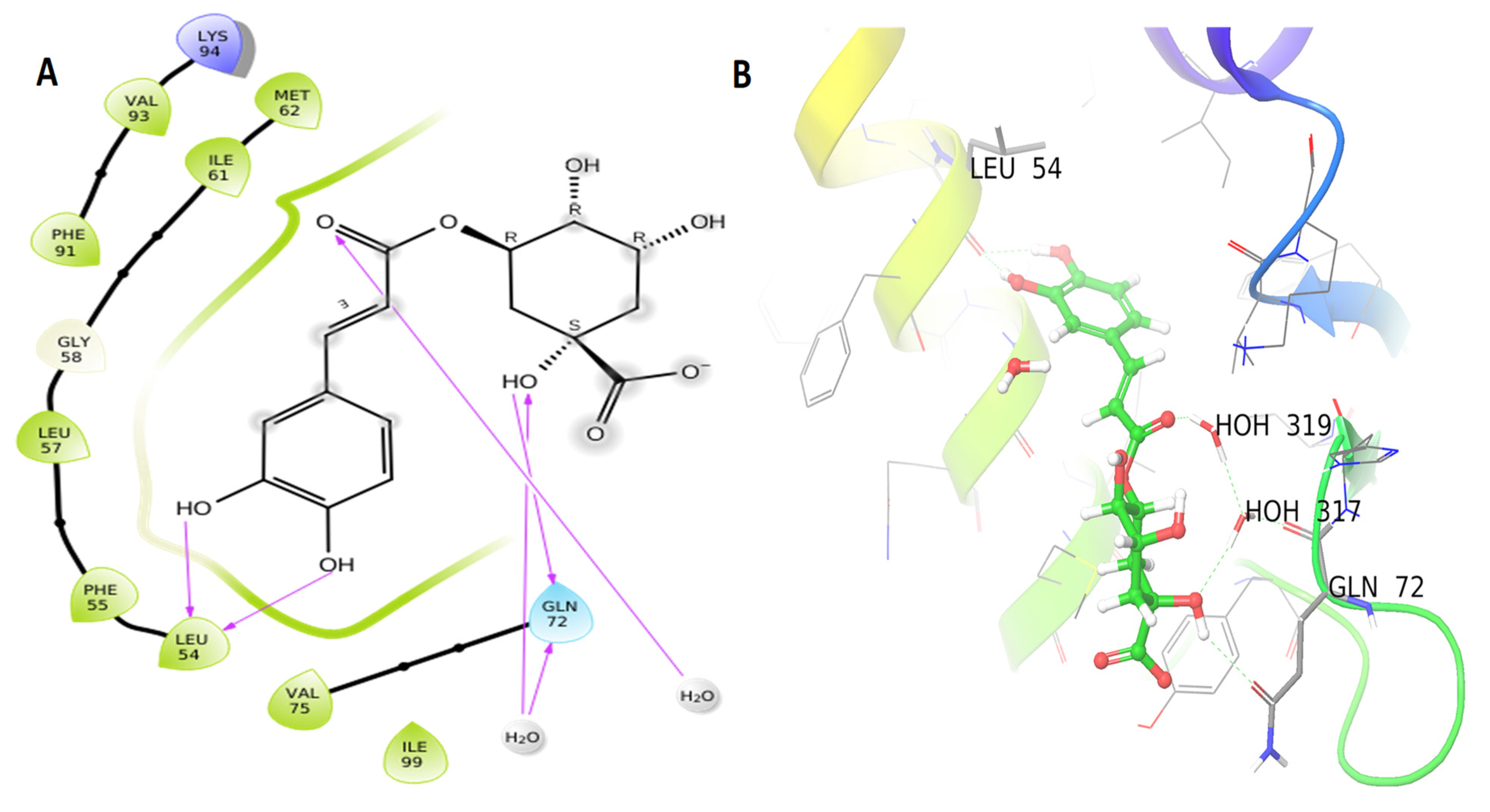
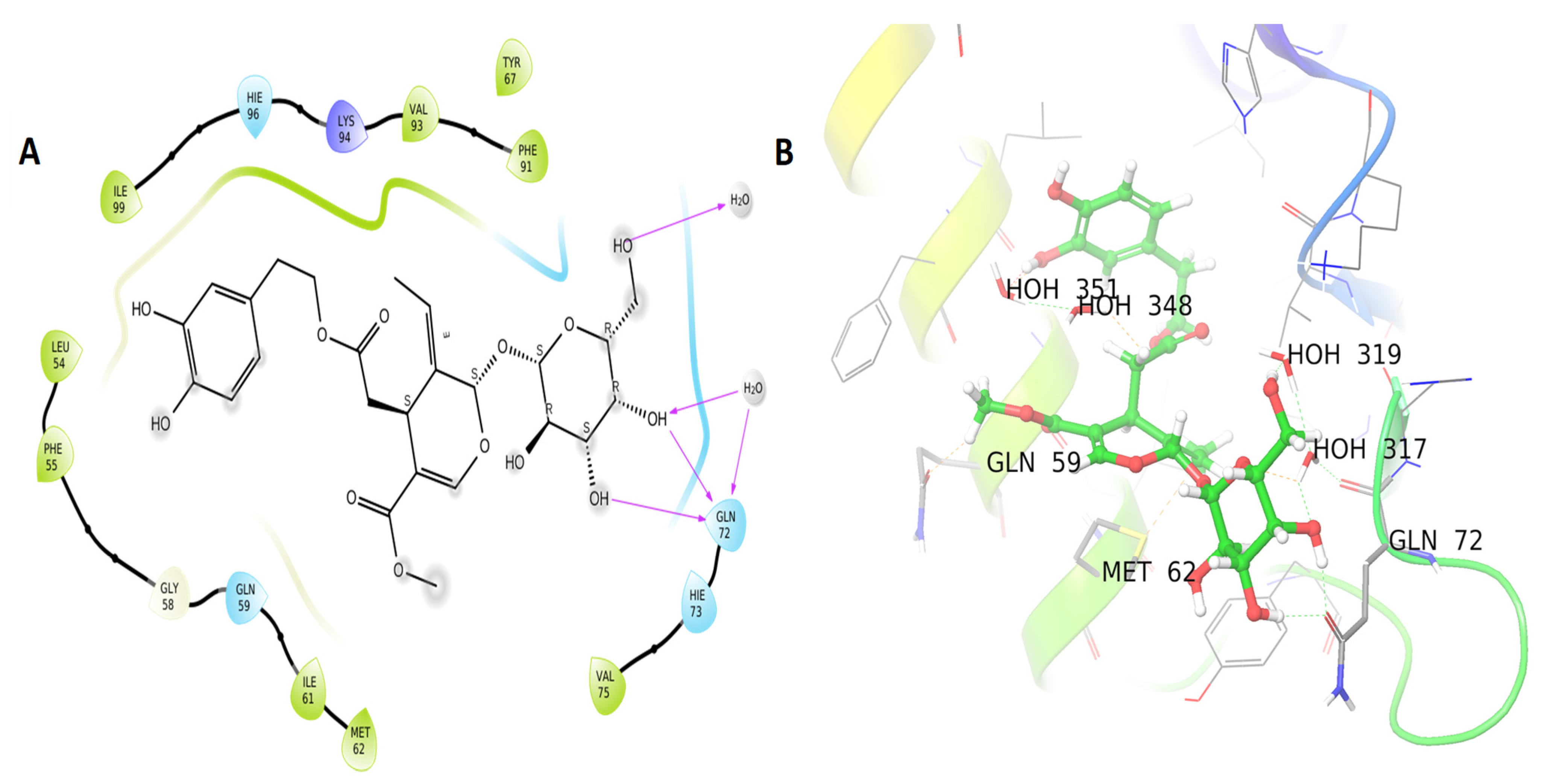
| Name | PubChem CID | XP GScore | Glide Energy | Glide Emodel | |
|---|---|---|---|---|---|
| MDM2 (6GGN) | coffee oil compounds | ||||
| Chlorogenic acid | 1794427 | −7.564 | −36.496 | −49.551 | |
| Kahweol | 114778 | −4.719 | −22.304 | −26.808 | |
| Cafestol | 108052 | −4.584 | −25.19 | −17.839 | |
| Oleic acid | 445639 | −4.204 | −31.943 | −34.117 | |
| Caffeine | 2519 | −4.024 | −24.182 | −30.139 | |
| Linoleic acid | 5280450 | −3.38 | −34.192 | −33.195 | |
| Palmitic acid | 985 | −2.895 | −26.24 | −30.925 | |
| olive oil compounds | |||||
| Oleuropein | 5281544 | −7.652 | −44.215 | −54.288 | |
| Hydroxytyrosol | 82755 | −5.91 | −21.452 | −22.75 | |
| Tyrosol | 10393 | −4.474 | −18.42 | −19.683 | |
| Squalene | 638072 | −3.949 | −33.026 | −35.897 | |
| Oleic acid | 445639 | −3.909 | −32.642 | −33.767 | |
| Linoleic acid | 5280450 | −3.38 | −34.192 | −33.195 | |
| Stearic acid | 5281 | −3.317 | −31.351 | −34.989 | |
| Palmitic acid | 985 | −3.263 | −27.325 | −29.101 | |
| Control | P53 and MDM2 Protein-Interaction-Inhibitor | 17754765 | −5.7 | −16.3 | −14.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Zeid, I.M.; Al-Asmari, K.M.; Altayb, H.N.; Al-Attar, A.M.; Qahl, S.H.; Alomar, M.Y. Predominance of Antioxidants in Some Edible Plant Oils in Ameliorating Oxidative Stress and Testicular Toxicity Induced by Malathion. Life 2022, 12, 350. https://doi.org/10.3390/life12030350
Abu Zeid IM, Al-Asmari KM, Altayb HN, Al-Attar AM, Qahl SH, Alomar MY. Predominance of Antioxidants in Some Edible Plant Oils in Ameliorating Oxidative Stress and Testicular Toxicity Induced by Malathion. Life. 2022; 12(3):350. https://doi.org/10.3390/life12030350
Chicago/Turabian StyleAbu Zeid, Isam M., Khalid M. Al-Asmari, Hisham N. Altayb, Atef M. Al-Attar, Safa H. Qahl, and Mohammed Y. Alomar. 2022. "Predominance of Antioxidants in Some Edible Plant Oils in Ameliorating Oxidative Stress and Testicular Toxicity Induced by Malathion" Life 12, no. 3: 350. https://doi.org/10.3390/life12030350
APA StyleAbu Zeid, I. M., Al-Asmari, K. M., Altayb, H. N., Al-Attar, A. M., Qahl, S. H., & Alomar, M. Y. (2022). Predominance of Antioxidants in Some Edible Plant Oils in Ameliorating Oxidative Stress and Testicular Toxicity Induced by Malathion. Life, 12(3), 350. https://doi.org/10.3390/life12030350






