The Maternal–Fetal Gut Microbiota Axis: Physiological Changes, Dietary Influence, and Modulation Possibilities
Abstract
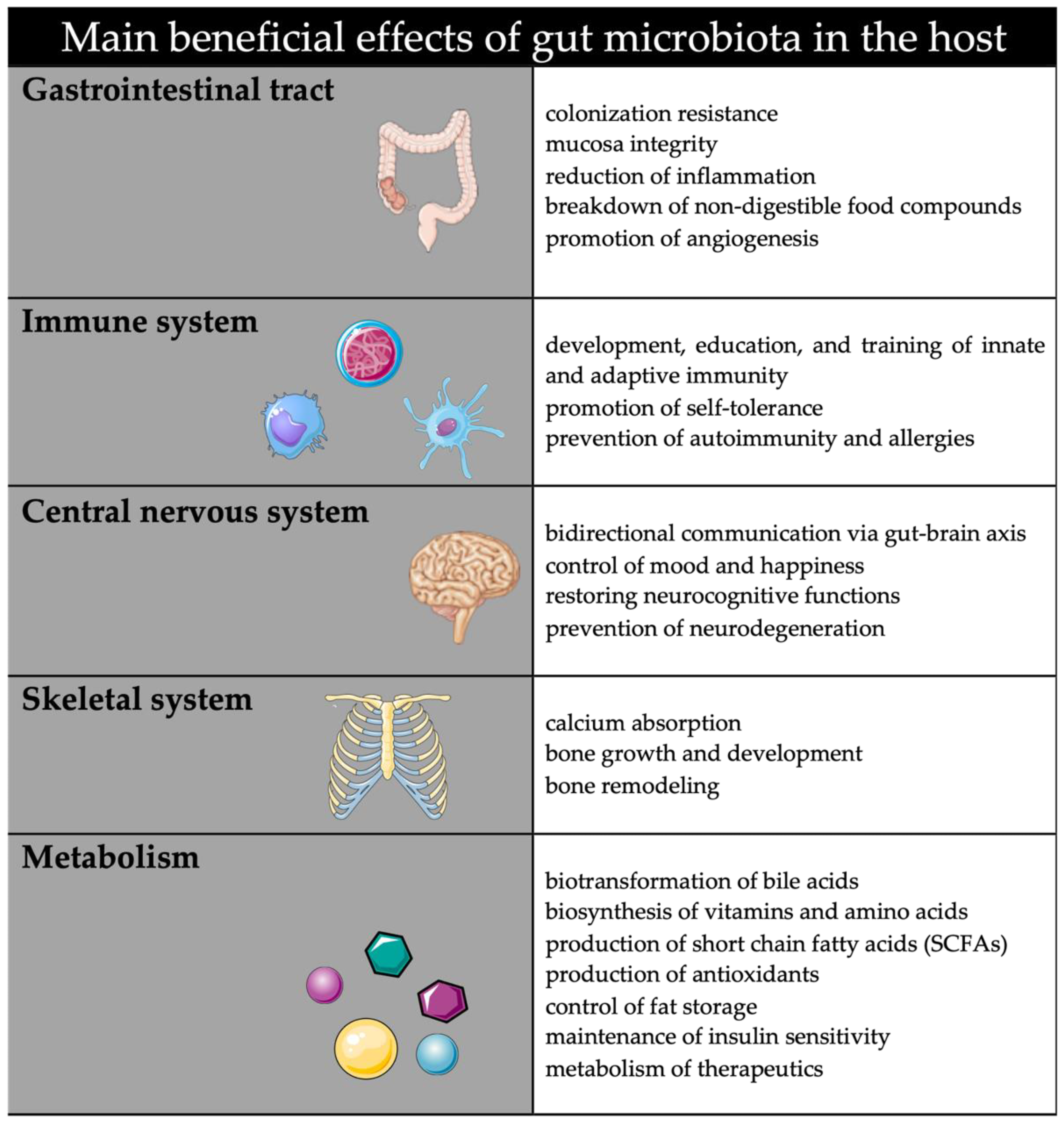
:1. Introduction
2. Maternal Gut Microbiota
3. Establishment of the Maternal–Fetal Gut Microbiota Axis
3.1. Placental Microbiota
3.2. Effects of Microbiota-Derived Molecules
4. Effects of Maternal Nutritional Factors on Gut Microbiota and Offspring’s Health
4.1. High-Fat Diet and Maternal Obesity
Gestational Diabetes Mellitus: A Special Case
4.2. Vegetarian Diet
4.3. Artificial Sweeteners
4.4. Alcohol
| Author, Year | Study Population | Investigated Fetal Side Microbiota | Method | Main Findings |
|---|---|---|---|---|
| Maternal High-Fat diet, Obesity | ||||
| Collado et al., 2010 [30] | Infants of obese mothers (n = 16) vs. infants of normal-weight mothers (n = 26) | Infant fecal samples at 1 and 6 months of age | FISH qPCR | Higher weights of mothers were correlated with higher concentrations of Bacteroides, Clostridium, and Staphylococcus, and lower concentrations of the Bifidobacterium group prevalence of Akkermansia muciniphila, Staphylococcus, and Clostridium difficile groups were lower in infants of normal-weight mothers |
| Galley et al. 2014 [126] | Children of obese (n = 26) vs. nonobese mothers | Fecal samples from children 18–27 months of age | 16S ribosomal RNA (rRNA) sequencing) | Effects of maternal obesity on offspring’s gut microbiota were stronger among children of mothers of higher socioeconomic status Higher alpha and beta diversity in children of obese vs. nonobese mothers Children born to obese vs. nonobese mothers had greater abundances of Parabacteroides spp. and Oscillibacter spp., as well as lower Blautia spp. and Eubacterium spp. |
| Mueller et al, 2016 [128] | Neonates (n = 18) born vaginally (5 to overweight mothers), neonates (n = 56) by elective C-section (26 to overweight mothers) | Second-day fecal samples from neonates | 16S ribosomal RNA (rRNA) sequencing | Compared to neonates delivered vaginally to normal-weight mothers, microbiota of neonates born to overweight or obese mothers were enriched in Bacteroides and depleted in Enterococcus, Acinetobacter, Pseudomonas, and Hydrogenophilus |
| Gestational Diabetes Mellitus | ||||
| Hu et al., 2013 [140] | Newborns (n = 23): 5 from mothers with DM, 5 from mothers with GDM, 13 from mothers with no diabetes | Meconium samples | 16S ribosomal RNA (rRNA) sequencing | The phylum Bacteroidota and the genus Parabacteriodes were enriched in the meconium in the DM group compared to the nondiabetes group |
| Bassols et al., 2016 [44,155] | Placentas from women with GDM (n = 11) and from control women (n = 11) | Placenta | 16S ribosomal RNA (rRNA) sequencing | Pseudomonadales and Acinetobacter showed lower relative abundance in women with GDM compared to control Increase in placental Acinetobacter ratio was associated with a more adverse metabolic and inflammatory phenotype |
| Wang et al., 2018 [139] | Pregnant women and their neonates with and without GDM | Oral, pharyngeal, meconium, and amniotic fluid samples | 16S ribosomal RNA (rRNA) sequencing | In the amniotic fluid of the GDM group, a lower relative abundance of Anoxybacillus and a higher relative abundance of Corynebacterium were detected In the meconium of the GDM group, a lower relative abundance of Corynebacterium and a higher relative abundance of Enterobacter were detected Microbes varied by the same trend between the maternal and neonatal microbiota |
| Vegetarian Diet | ||||
| None | ||||
| Artificial Sweeteners | ||||
| Laforest-Lapointe et al., 2021 [155] | Infants (n = 100) selected based on maternal sweetener consumption during pregnancy (50 nonconsumers and 50 daily consumers) | Infant fecal samples at 3 and 12 months of age | 16S ribosomal RNA (rRNA) sequencing | Maternal sweetener consumption did not differ between clusters reflecting the maturation of gut microbiota but was associated with community-level shifts in infant’s gut bacterial taxonomy structure and depletion of several Bacteroides sp. in a certain cluster Nine bacterial taxa from Bacteroides sp. were enriched or depleted at high levels of maternal sweetener consumption at 12 months of age. Daily maternal sweetener consumption is associated with higher infant weight and altered microbiota composition |
| Alcohol Consumption | ||||
| Wang et al., 2021 [166] | Pregnant women and their neonates with (n = 10) and without (n = 19) alcohol consumption | Fecal samples of newborns within 48 h | 16S ribosomal RNA (rRNA) sequencing | A positive relationship showed between Megamonas and newborns with maternal alcohol consumption |
5. Modulation of Maternal Gut Microbiota for Offspring’s Benefits
5.1. Probiotics
5.2. Prebiotics
6. Conclusions
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the Microbiome in Human Development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; van Hylckama Vlieg, J.E.T. Fate, Activity, and Impact of Ingested Bacteria within the Human Gut Microbiota. Trends Microbiol. 2015, 23, 354–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hara, A.M.; Shanahan, F. The Gut Flora as a Forgotten Organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dethlefsen, L.; Relman, D.A. Incomplete Recovery and Individualized Responses of the Human Distal Gut Microbiota to Repeated Antibiotic Perturbation. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4554–4561. [Google Scholar] [CrossRef] [Green Version]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2018, 76, 473–493. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Kobyliak, N.; Virchenko, O.; Falalyeyeva, T. Pathophysiological Role of Host Microbiota in the Development of Obesity. Nutr. J. 2016, 15, 43. [Google Scholar] [CrossRef] [Green Version]
- Laukens, D.; Brinkman, B.M.; Raes, J.; de Vos, M.; Vandenabeele, P. Heterogeneity of the Gut Microbiome in Mice: Guidelines for Optimizing Experimental Design. FEMS Microbiol. Rev. 2016, 40, 117. [Google Scholar] [CrossRef] [Green Version]
- Coscia, A.; Bardanzellu, F.; Caboni, E.; Fanos, V.; Peroni, D.G. When a Neonate Is Born, So Is a Microbiota. Life 2021, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; de Vos, W.M.; Distefano, P.S.; Doré, J.; Huttenhower, C.; Knight, R.; Lawley, T.D.; Raes, J.; Turnbaugh, P. Translating the Human Microbiome. Nat. Biotechnol. 2013, 31, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Front. Microbiol. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H. Diet and Gut Microbiome and the “Chicken or Egg” Problem. Front. Nutr. 2021, 8, 828630. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F.; Ghosh, T.S.; O’Toole, P.W. The Healthy Microbiome—What Is the Definition of a Healthy Gut Microbiome? Gastroenterology 2021, 160, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Freemark, M. Regulation of Maternal Metabolism by Pituitary and Placental Hormones: Roles in Fetal Development and Metabolic Programming. Horm. Res. 2006, 65, 41–49. [Google Scholar] [CrossRef]
- Arck, P.C.; Hecher, K. Fetomaternal Immune Cross-Talk and Its Consequences for Maternal and Offspring’s Health. Nat. Med. 2013, 19, 548–556. [Google Scholar] [CrossRef]
- Erlebacher, A. Why Isn’t the Fetus Rejected? Curr. Opin. Immunol. 2001, 13, 590–593. [Google Scholar] [CrossRef]
- Belo, L.; Santos-Silva, A.; Rocha, S.; Caslake, M.; Cooney, J.; Pereira-Leite, L.; Quintanilha, A.; Rebelo, I. Fluctuations in C-Reactive Protein Concentration and Neutrophil Activation during Normal Human Pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 123, 46–51. [Google Scholar] [CrossRef]
- Fink, N.R.; Chawes, B.; Bønnelykke, K.; Thorsen, J.; Stokholm, J.; Rasmussen, M.A.; Brix, S.; Bisgaard, H. Levels of Systemic Low-Grade Inflammation in Pregnant Mothers and Their Offspring Are Correlated. Sci. Rep. 2019, 9, 3043. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesa, M.D.; Loureiro, B.; Iglesia, I.; Gonzalez, S.F.; Olivé, E.L.; Algar, O.G.; Solana, M.J.; Perez, M.J.C.; Sainz, T.; Martinez, L.; et al. The Evolving Microbiome from Pregnancy to Early Infancy: A Comprehensive Review. Nutrients 2020, 12, 133. [Google Scholar] [CrossRef] [Green Version]
- Wallace, J.G.; Bellissimo, C.J.; Yeo, E.; Fei Xia, Y.; Petrik, J.J.; Surette, M.G.; Bowdish, D.M.E.; Sloboda, D.M. Obesity during Pregnancy Results in Maternal Intestinal Inflammation, Placental Hypoxia, and Alters Fetal Glucose Metabolism at Mid-Gestation. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Koren, O. The Pregnancy Microbiome. Nestle Nutr. Inst. Workshop Ser. 2017, 88, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Peelen, M.J.; Luef, B.M.; Lamont, R.F.; de Milliano, I.; Jensen, J.S.; Limpens, J.; Hajenius, P.J.; Jørgensen, J.S.; Menon, R. The Influence of the Vaginal Microbiota on Preterm Birth: A Systematic Review and Recommendations for a Minimum Dataset for Future Research. Placenta 2019, 79, 30–39. [Google Scholar] [CrossRef] [Green Version]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.A.; Wong, R.J.; Shawa, G.; et al. Temporal and Spatial Variation of the Human Microbiota during Pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef] [Green Version]
- Santacruz, A.; Collado, M.C.; García-Valdés, L.; Segura, M.T.; Marítn-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut Microbiota Composition Is Associated with Body Weight, Weight Gain and Biochemical Parameters in Pregnant Women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct Composition of Gut Microbiota during Pregnancy in Overweight and Normal-Weight Women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Effect of Mother’s Weight on Infant’s Microbiota Acquisition, Composition, and Activity during Early Infancy: A Prospective Follow-up Study Initiated in Early Pregnancy. Am. J. Clin. Nutr. 2010, 92, 1023–1030. [Google Scholar] [CrossRef]
- García-Mantrana, I.; Alcántara, C.; Selma-Royo, M.; Boix-Amorós, A.; Dzidic, M.; Gimeno-Alcañiz, J.; Úbeda-Sansano, I.; Sorribes-Monrabal, I.; Escuriet, R.; Gil-Raga, F.; et al. MAMI: A Birth Cohort Focused on Maternal-Infant Microbiota during Early Life. BMC Pediatrics 2019, 19, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Olivier-Van Stichelen, S.; Rother, K.I.; Hanover, J.A. Maternal Exposure to Non-Nutritive Sweeteners Impacts Progeny’s Metabolism and Microbiome. Front. Microbiol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wankhade, U.D.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Piccolo, B.D.; Mercer, K.E.; Andres, A.; Thakali, K.M.; Shankar, K. Maternal High-Fat Diet Programs Offspring Liver Steatosis in a Sexually Dimorphic Manner in Association with Changes in Gut Microbial Ecology in Mice. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D.; Tremellen, A.; Tobin, J.; Wilkinson, S.; McSweeney, C.; et al. Connections Between the Gut Microbiome and Metabolic Hormones in Early Pregnancy in Overweight and Obese Women. Diabetes 2016, 65, 2214–2223. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the Barriers: The Gut Microbiome, Intestinal Permeability and Stress-Related Psychiatric Disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhmoud, T.; Kumar, A.; Lo, C.C.; Al-Sadi, R.; Clegg, S.; Alomari, I.; Zmeili, T.; Gleasne, C.D.; Mcmurry, K.; Dichosa, A.E.K.; et al. Investigating Intestinal Permeability and Gut Microbiota Roles in Acute Coronary Syndrome Patients. Hum. Microbiome, J. 2019, 13, 100059. [Google Scholar] [CrossRef]
- Escherich, T.H. The Intestinal Bacteria of the Neonate and Breast-Fed Infant 1885. Rev. Infect. Dis. 1989, 11, 352–356. [Google Scholar] [CrossRef]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A Critical Assessment of the “Sterile Womb” and “Utero Colonization” Hypotheses: Implications for Research on the Pioneer Infant Microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Stout, M.J.; Conlon, B.; Landeau, M.; Lee, I.; Bower, C.; Zhao, Q.; Roehl, K.A.; Nelson, D.M.; MacOnes, G.A.; Mysorekar, I.U. Identification of Intracellular Bacteria in the Basal Plate of the Human Placenta in Term and Preterm Gestations. Am. J. Obstet. Gynecol. 2013, 208, 226.e1–226.e7. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Xiao, X.; Zhang, Q.; Mao, L.; Yu, M.; Xu, J. The Placental Microbiome Varies in Association with Low Birth Weight in Full-Term Neonates. Nutrients 2015, 7, 6924–6937. [Google Scholar] [CrossRef] [PubMed]
- Parnell, L.A.; Briggs, C.M.; Cao, B.; Delannoy-Bruno, O.; Schrieffer, A.E.; Mysorekar, I.U. Microbial Communities in Placentas from Term Normal Pregnancy Exhibit Spatially Variable Profiles. Sci. Rep. 2017, 7, 11200. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human Gut Colonisation May Be Initiated in Utero by Distinct Microbial Communities in the Placenta and Amniotic Fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassols, J.; Serino, M.; Carreras-Badosa, G.; Burcelin, R.; Blasco-Baque, V.; Lopez-Bermejo, A.; Fernandez-Real, J.M. Gestational Diabetes Is Associated with Changes in Placental Microbiota and Microbiome. Pediatr. Res. 2016, 80, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Mysorekar, I.U. Intracellular Bacteria in Placental Basal Plate Localize to Extravillous Trophoblasts. Placenta 2014, 35, 139–142. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [Green Version]
- Younge, N.; McCann, J.R.; Ballard, J.; Plunkett, C.; Akhtar, S.; Araújo-Pérez, F.; Murtha, A.; Brandon, D.; Seed, P.C. Fetal Exposure to the Maternal Microbiota in Humans and Mice. JCI Insight 2019, 4, e127806. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. Contributions of the Maternal Oral and Gut Microbiome to Placental Microbial Colonization in Overweight and Obese Pregnant Women. Sci. Rep. 2017, 7, 2860. [Google Scholar] [CrossRef]
- Perez, P.F.; Doré, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J.; Donnet-Hughes, A. Bacterial Imprinting of the Neonatal Immune System: Lessons from Maternal Cells? Pediatrics 2007, 119, e724–e732. [Google Scholar] [CrossRef]
- Hohlweg, U.; Doerfler, W. On the Fate of Plant or Other Foreign Genes upon the Uptake in Food or after Intramuscular Injection in Mice. Mol. Genet. Genom. 2001, 265, 225–233. [Google Scholar] [CrossRef]
- Schubbert, R.; Hohlweg, U.; Renz, D.; Doerfler, W. On the Fate of Orally Ingested Foreign DNA in Mice: Chromosomal Association and Placental Transmission to the Fetus. Mol. Genet. Genom. 1998, 259, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Is Meconium from Healthy Newborns Actually Sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; Fernández, L.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Isolation of Commensal Bacteria from Umbilical Cord Blood of Healthy Neonates Born by Cesarean Section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Borgo, P.V.; Rodrigues, V.A.A.; Feitosa, A.C.R.; Xavier, K.C.B.; Avila-Campos, M.J. Association between Periodontal Condition and Subgingival Microbiota in Women during Pregnancy: A Longitudinal Study. J. Appl. Oral Sci. 2014, 22, 528. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Tsuruda, K.; Iwamoto, Y.; Kato, F.; Odaki, T.; Yamane, N.; Hori, Y.; Harashima, Y.; Sakoda, A.; Tagaya, A.; et al. Significant Increase of Oral Bacteria in the Early Pregnancy Period in Japanese Women. J. Investig. Clin. Dent. 2017, 8, e12189. [Google Scholar] [CrossRef]
- Arce, R.M.; Barros, S.P.; Wacker, B.; Peters, B.; Moss, K.; Offenbacher, S. Increased TLR4 Expression in Murine Placentas after Oral Infection with Periodontal Pathogens. Placenta 2009, 30, 156. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Smith, M.A.; Elter, J.; Champagne, C.; Downey, C.L.; Beck, J.; Offenbacher, S. Porphyromonas Gingivalis Infection in Pregnant Mice Is Associated with Placental Dissemination, an Increase in the Placental Th1/Th2 Cytokine Ratio, and Fetal Growth Restriction. Infect. Immun. 2003, 71, 5163–5168. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.W.; Redline, R.W.; Li, M.; Yin, L.; Hill, G.B.; McCormick, T.S. Fusobacterium Nucleatum Induces Premature and Term Stillbirths in Pregnant Mice: Implication of Oral Bacteria in Preterm Birth. Infect. Immun. 2004, 72, 2272. [Google Scholar] [CrossRef] [Green Version]
- Prince, A.L.; Antony, K.M.; Chu, D.M.; Aagaard, K.M. The Microbiome, Parturition, and Timing of Birth: More Questions than Answers. J. Reprod. Immunol. 2014, 104–105, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Mysorekar, I.U.; Cao, B. Microbiome in Parturition and Preterm Birth. Semin. Reprod. Med. 2014, 32, 50–55. [Google Scholar] [CrossRef]
- Jefferson, K.K. The Bacterial Etiology of Preterm Birth. Adv. Appl. Microbiol. 2012, 80, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.S.; Romero, R.; Hillier, S.L.; Eschenbach, D.A.; Sweet, R.L. A Review of Premature Birth and Subclinical Infection. Am. J. Obstet. Gynecol. 1992, 166, 1515–1528. [Google Scholar] [CrossRef]
- Neu, J. The Microbiome during Pregnancy and Early Postnatal Life. Semin. Fetal. Neonatal. Med. 2016, 21, 373–379. [Google Scholar] [CrossRef] [PubMed]
- DiGiulio, D.B. Diversity of Microbes in Amniotic Fluid. Semin. Fetal. Neonatal. Med. 2012, 17, 2–11. [Google Scholar] [CrossRef]
- Mishra, A.; Lai, G.C.; Yao, L.J.; Aung, T.T.; Shental, N.; Rotter-Maskowitz, A.; Shepherdson, E.; Singh, G.S.N.; Pai, R.; Shanti, A.; et al. Microbial Exposure during Early Human Development Primes Fetal Immune Cells. Cell 2021, 184, 3394–3409.e20. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Bellissimo, C.J.; Breznik, J.A.; Barrett, J.; Braun, T.; Bushman, F.D.; de Goffau, M.; Elovitz, M.A.; Heimesaat, M.M.; Konnikova, L.; et al. Over-Celling Fetal Microbial Exposure. Cell 2021, 184, 5839–5841. [Google Scholar] [CrossRef]
- Salter, S.J.; Cox, M.J.; Turek, E.M.; Calus, S.T.; Cookson, W.O.; Moffatt, M.F.; Turner, P.; Parkhill, J.; Loman, N.J.; Walker, A.W. Reagent and Laboratory Contamination Can Critically Impact Sequence-Based Microbiome Analyses. BMC Biol. 2014, 12, 87. [Google Scholar] [CrossRef] [Green Version]
- Gschwind, R.; Fournier, T.; Kennedy, S.; Tsatsaris, V.; Cordier, A.G.; Barbut, F.; Butel, M.J.; WydauDematteis, S. Evidence for Contamination as the Origin for Bacteria Found in Human Placenta Rather than a Microbiota. PLoS ONE 2020, 15, e0237232. [Google Scholar] [CrossRef]
- Lauder, A.P.; Roche, A.M.; Sherrill-Mix, S.; Bailey, A.; Laughlin, A.L.; Bittinger, K.; Leite, R.; Elovitz, M.A.; Parry, S.; Bushman, F.D. Comparison of Placenta Samples with Contamination Controls Does Not Provide Evidence for a Distinct Placenta Microbiota. Microbiome 2016, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Accuracy of Microbial Community Diversity Estimated by Closed- and Open-Reference OTUs. PeerJ 2017, 2017, e3889. [Google Scholar] [CrossRef] [Green Version]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.P.; Ricciardi-Castagnoli, P. Dendritic Cells Express Tight Junction Proteins and Penetrate Gut Epithelial Monolayers to Sample Bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Uhr, T. Induction of Protective IgA by Intestinal Dendritic Cells Carrying Commensal Bacteria. Science 2004, 303, 1662–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelsall, B. Recent Progress in Understanding the Phenotype and Function of Intestinal Dendritic Cells and Macrophages. Mucosal Immunol. 2008, 1, 460–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolen, K.K.; Ruck, C.E.; Fortuno, E.S.; Ho, K.; Dimitriu, P.; Mohn, W.W.; Speert, D.P.; Cooper, P.J.; Esser, M.; Goetghebuer, T.; et al. Pattern Recognition Receptor-Mediated Cytokine Response in Infants across 4 Continents. J. Allergy Clin. Immunol. 2014, 133, 818–826. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like Receptor and RIG-I-like Receptor Signaling. Ann. N. Y. Acad. Sci. 2008, 1143, 1–20. [Google Scholar] [CrossRef]
- Strunk, T.; Currie, A.; Richmond, P.; Simmer, K.; Burgner, D. Innate Immunity in Human Newborn Infants: Prematurity Means More than Immaturity. J. Matern. Fetal Neonatal. Med. 2011, 24, 25–31. [Google Scholar] [CrossRef]
- Sadeghi, K.; Berger, A.; Langgartner, M.; Prusa, A.R.; Hayde, M.; Herkner, K.; Pollak, A.; Spittler, A.; Förster-Waldl, E. Immaturity of Infection Control in Preterm and Term Newborns Is Associated with Impaired Toll-like Receptor Signaling. J. Infect. Dis. 2007, 195, 296–302. [Google Scholar] [CrossRef]
- Leeansyah, E.; Loh, L.; Nixon, D.F.; Sandberg, J.K. Acquisition of Innate-like Microbial Reactivity in Mucosal Tissues during Human Fetal MAIT-Cell Development. Nat. Commun. 2014, 5, 3143. [Google Scholar] [CrossRef] [Green Version]
- Schreurs, R.R.C.E.; Baumdick, M.E.; Sagebiel, A.F.; Kaufmann, M.; Mokry, M.; Klarenbeek, P.L.; Schaltenberg, N.; Steinert, F.L.; van Rijn, J.M.; Drewniak, A.; et al. Human Fetal TNF-α-Cytokine-Producing CD4 + Effector Memory T Cells Promote Intestinal Development and Mediate Inflammation Early in Life. Immunity 2019, 50, 462–476. [Google Scholar] [CrossRef] [Green Version]
- Blümer, N.; Pfefferle, P.I.; Renz, H. Development of Mucosal Immune Function in the Intrauterine and Early Postnatal Environment. Curr. Opin. Gastroenterol. 2007, 23, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; MacIa, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence That Asthma Is a Developmental Origin Disease Influenced by Maternal Diet and Bacterial Metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Bale, T.L. Prenatal and Postnatal Contributions of the Maternal Microbiome on Offspring Programming. Front. Neuroendocrinol. 2019, 55. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Payne, M.S.; Keelan, J.A. Planting the Seed: Origins, Composition, and Postnatal Health Significance of the Fetal Gastrointestinal Microbiota. Crit. Rev. Microbiol. 2017, 43, 352–369. [Google Scholar] [CrossRef]
- Hapfelmeier, S.; Lawson, M.A.E.; Slack, E.; Kirundi, J.K.; Stoel, M.; Heikenwalder, M.; Cahenzli, J.; Velykoredko, Y.; Balmer, M.L.; Endt, K.; et al. Reversible Microbial Colonization of Germ-Free Mice Reveals the Dynamics of IgA Immune Responses. Science 2010, 328, 1705–1709. [Google Scholar] [CrossRef] [Green Version]
- Gomez de Agüero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The Maternal Microbiota Drives Early Postnatal Innate Immune Development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef]
- Stockinger, B.; Meglio, P.D.; Gialitakis, M.; Duarte, J.H. The Aryl Hydrocarbon Receptor: Multitasking in the Immune System. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef]
- Ziętek, M.; Celewicz, Z.; Szczuko, M. Short-Chain Fatty Acids, Maternal Microbiota and Metabolism in Pregnancy. Nutrients 2021, 13, 1244. [Google Scholar] [CrossRef]
- Hayward, L.; Watkins, J.; Bautista, B.; Lin, C.; Malphurs, W.; Zubcevic, J. Nicotine Exposure during Pregnancy Alters the Maternal Gut Microbiome and Both Cecal and Plasma Short Chain Fatty Acids in Sprague Dawley Rats. FASEB J. 2020, 34, 1-1. [Google Scholar] [CrossRef]
- Nilsen, M.; Saunders, C.M.; Angell, I.L.; Arntzen, M.; Lødrup Carlsen, K.C.; Carlsen, K.H.; Haugen, G.; Hagen, L.H.; Carlsen, M.H.; Hedlin, G.; et al. Butyrate Levels in the Transition from an Infant- to an Adult-Like Gut Microbiota Correlate with Bacterial Networks Associated with Eubacterium Rectale and Ruminococcus Gnavus. Genes 2020, 11, 1245. [Google Scholar] [CrossRef]
- Soderborg, T.K.; Carpenter, C.M.; Janssen, R.C.; Weir, T.L.; Robertson, C.E.; Ir, D.; Young, B.E.; Krebs, N.F.; Hernandez, T.L.; Barbour, L.A.; et al. Gestational Diabetes Is Uniquely Associated With Altered Early Seeding of the Infant Gut Microbiota. Front. Endocrinol. 2020, 11, 603021. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.E.K.; O’Hely, M.; Ranganathan, S.; Sly, P.D.; Vuillermin, P. The Maternal Diet, Gut Bacteria, and Bacterial Metabolites during Pregnancy Influence Offspring Asthma. Front. immunol. 2017, 8, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, I.; Miyamoto, J.; Ohue-Kitano, R.; Watanabe, K.; Yamada, T.; Onuki, M.; Aoki, R.; Isobe, Y.; Kashihara, D.; Inoue, D.; et al. Maternal Gut Microbiota in Pregnancy Influences Offspring Metabolic Phenotype in Mice. Science 2020, 367, eaaw8429. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fujikado, N.; Kolodin, D.; Benoist, C.; Mathis, D. Immune Tolerance. Regulatory T Cells Generated Early in Life Play a Distinct Role in Maintaining Self-Tolerance. Science 2015, 348, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-Chain Fatty Acids Induce Both Effector and Regulatory T Cells by Suppression of Histone Deacetylases and Regulation of the MTOR-S6K Pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [Green Version]
- Georgieff, M.K. Nutrition and the Developing Brain: Nutrient Priorities and Measurement. Am. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar] [CrossRef]
- Burbridge, S.; Stewart, I.; Placzek, M. Development of the Neuroendocrine Hypothalamus. Compr. Physiol. 2016, 6, 623–643. [Google Scholar] [CrossRef]
- Jašarević, E.; Howard, C.D.; Morrison, K.; Misic, A.; Weinkopff, T.; Scott, P.; Hunter, C.; Beiting, D.; Bale, T.L. The Maternal Vaginal Microbiome Partially Mediates the Effects of Prenatal Stress on Offspring Gut and Hypothalamus. Nat. Neurosci. 2018, 21, 1061–1071. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Oh, J.Z.; Ravindran, R.; Chassaing, B.; Carvalho, F.A.; Maddur, M.S.; Bower, M.; Hakimpour, P.; Gill, K.P.; Nakaya, H.I.; Yarovinsky, F.; et al. TLR5-Mediated Sensing of Gut Microbiota Is Necessary for Antibody Responses to Seasonal Influenza Vaccination. Immunity 2014, 41, 478–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamura, C.; Bouladoux, N.; Belkaid, Y.; Sher, A.; Jankovic, D. Sensing of the Microbiota by NOD1 in Mesenchymal Stromal Cells Regulates Murine Hematopoiesis. Blood 2017, 129, 171–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchimura, Y.; Fuhrer, T.; Li, H.; Lawson, M.A.; Zimmermann, M.; Yilmaz, B.; Zindel, J.; Ronchi, F.; Sorribas, M.; Hapfelmeier, S.; et al. Antibodies Set Boundaries Limiting Microbial Metabolite Penetration and the Resultant Mammalian Host Response. Immunity 2018, 49, 545–559.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganal-Vonarburg, S.C.; Hornef, M.W.; Macpherson, A.J. Microbial-Host Molecular Exchange and Its Functional Consequences in Early Mammalian Life. Science 2020, 368, 604–607. [Google Scholar] [CrossRef]
- Williams, P.J.; Searle, R.F.; Robson, S.C.; Innes, B.A.; Bulmer, J.N. Decidual Leucocyte Populations in Early to Late Gestation Normal Human Pregnancy. J. Reprod. Immunol. 2009, 82, 24–31. [Google Scholar] [CrossRef]
- Brugman, S.; Perdijk, O.; van Neerven, R.J.J.; Savelkoul, H.F.J. Mucosal Immune Development in Early Life: Setting the Stage. Arch. Immunol. Ther. Exp. (Warsz.) 2015, 63, 251–268. [Google Scholar] [CrossRef] [Green Version]
- Romano-Keeler, J.; Weitkamp, J.H. Maternal Influences on Fetal Microbial Colonization and Immune Development. Pediatr. Res. 2015, 77, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Hsu, P.; Nanan, R. Foetal Immune Programming: Hormones, Cytokines, Microbes and Regulatory T Cells. J. Reprod. Immunol. 2014, 104–105, 2–7. [Google Scholar] [CrossRef]
- D’Argenio, V. The Prenatal Microbiome: A New Player for Human Health. High-Throughput 2018, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of Lifetime Health around the Time of Conception: Causes and Consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Bolte, E.E.; Moorshead, D.; Aagaard, K.M. Maternal and Early Life Exposures and Their Potential to Influence Development of the Microbiome. Genome Med. 2022, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Michaelsen, K.F.; Rasmussen, K.M.; Sørensen, T.I.A. Maternal Prepregnant Body Mass Index, Duration of Breastfeeding, and Timing of Complementary Food Introduction Are Associated with Infant Weight Gain. Am. J. Clin. Nutr. 2004, 80, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.L.; Regnault, T.R.H. Nutrition in Pregnancy: Optimising Maternal Diet and Fetal Adaptations to Altered Nutrient Supply. Nutrients 2016, 8, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlop, A.L.; Mulle, J.G.; Ferranti, E.P.; Edwards, S.; Dunn, A.B.; Corwin, E.J. Maternal Microbiome and Pregnancy Outcomes That Impact Infant Health: A Review. Adv. Neonatal. Care 2015, 15, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Luoto, R.; Collado, M.C.; Salminen, S.; Isolauri, E. Reshaping the Gut Microbiota at an Early Age: Functional Impact on Obesity Risk? Ann. Nutr. Metab. 2013, 63, 17–26. [Google Scholar] [CrossRef]
- Gaudet, L.; Ferraro, Z.M.; Wen, S.W.; Walker, M. Maternal Obesity and Occurrence of Fetal Macrosomia: A Systematic Review and Meta-Analysis. Biomed Res. Int. 2014, 2014, 640291. [Google Scholar] [CrossRef]
- Papachatzi, E.; Dimitriou, G.; Dimitropoulos, K.; Vantarakis, A. Pre-Pregnancy Obesity: Maternal, Neonatal and Childhood Outcomes. J. Neonatal-Perinat. Med. 2013, 6, 203–216. [Google Scholar] [CrossRef]
- Flenady, V.; Koopmans, L.; Middleton, P.; Frøen, J.F.; Smith, G.C.; Gibbons, K.; Coory, M.; Gordon, A.; Ellwood, D.; McIntyre, H.D.; et al. Major Risk Factors for Stillbirth in High-Income Countries: A Systematic Review and Meta-Analysis. Lancet 2011, 377, 1331–1340. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Chu, S.Y.; Kim, S.Y.; Schmid, C.H.; Lau, J. Maternal Obesity and Risk of Neural Tube Defects: A Metaanalysis. Am. J. Obstet. Gynecol. 2008, 198, 611–619. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Collado, M.C. Obesity and Overweight: Impact on Maternal and Milk Microbiome and Their Role for Infant Health and Nutrition. Mol. Nutr. Food Res. 2016, 60, 1865–1875. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, M.; Knight, R.; Leibel, R.L. The Gut Microbiota in Human Energy Homeostasis and Obesity. Trends Endocrinol. Metab. 2015, 26, 493–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-Analyses of Human Gut Microbes Associated with Obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, H.A.; Bomhof, M.R.; Vogel, H.J.; Reimer, R.A. Diet-Induced Changes in Maternal Gut Microbiota and Metabolomic Profiles Influence Programming of Offspring Obesity Risk in Rats. Sci. Rep. 2016, 6, 20683. [Google Scholar] [CrossRef] [PubMed]
- le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Roÿtiö, H.; Mokkala, K.; Vahlberg, T.; Laitinen, K. Dietary Intake of Fat and Fibre According to Reference Values Relates to Higher Gut Microbiota Richness in Overweight Pregnant Women. Br. J. Nutr. 2017, 118, 343–352. [Google Scholar] [CrossRef]
- Galley, J.D.; Bailey, M.; Dush, C.K.; Schoppe-Sullivan, S.; Christian, L.M. Maternal Obesity Is Associated with Alterations in the Gut Microbiome in Toddlers. PLoS ONE 2014, 9, e113026. [Google Scholar] [CrossRef]
- Ma, J.; Prince, A.L.; Bader, D.; Hu, M.; Ganu, R.; Baquero, K.; Blundell, P.; Alan Harris, R.; Frias, A.E.; Grove, K.L.; et al. High-Fat Maternal Diet during Pregnancy Persistently Alters the Offspring Microbiome in a Primate Model. Nat. Commun. 2014, 5, 3889. [Google Scholar] [CrossRef]
- Mueller, N.T.; Shin, H.; Pizoni, A.; Werlang, I.C.; Matte, U.; Goldani, M.Z.; Goldani, H.A.S.; Dominguez-Bello, M.G. Birth Mode-Dependent Association between Pre-Pregnancy Maternal Weight Status and the Neonatal Intestinal Microbiome. Sci. Rep. 2016, 6, 23133. [Google Scholar] [CrossRef]
- Selma-Royo, M.; García-Mantrana, I.; Calatayud, M.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M.C. Maternal Diet during Pregnancy and Intestinal Markers Are Associated with Early Gut Microbiota. Eur. J. Nutr. 2021, 60, 1429–1442. [Google Scholar] [CrossRef]
- Chu, D.M.; Meyer, K.M.; Prince, A.L.; Aagaard, K.M. Impact of Maternal Nutrition in Pregnancy and Lactation on Offspring Gut Microbial Composition and Function. Gut Microbes 2016, 7, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Kozyrskyj, A.L.; Kalu, R.; Koleva, P.T.; Bridgman, S.L. Fetal Programming of Overweight through the Microbiome: Boys Are Disproportionately Affected. J. Dev. Orig. Health Dis. 2016, 7, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Olabi, B.; Bhopal, R. Diagnosis of Diabetes Using the Oral Glucose Tolerance Test. BMJ 2009, 339, 1268. [Google Scholar] [CrossRef] [PubMed]
- Marchi, J.; Berg, M.; Dencker, A.; Olander, E.K.; Begley, C. Risks Associated with Obesity in Pregnancy, for the Mother and Baby: A Systematic Review of Reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Medici Dualib, P.; Ogassavara, J.; Mattar, R.; Mariko Koga da Silva, E.; Atala Dib, S.; de Almeida Pititto, B. Gut Microbiota and Gestational Diabetes Mellitus: A Systematic Review. Diabetes Res. Clin. Pract. 2021, 180, 109078. [Google Scholar] [CrossRef]
- Mokkala, K.; Houttu, N.; Vahlberg, T.; Munukka, E.; Rönnemaa, T.; Laitinen, K. Gut Microbiota Aberrations Precede Diagnosis of Gestational Diabetes Mellitus. Acta Diabetol. 2017, 54, 1147–1149. [Google Scholar] [CrossRef]
- Wang, J.; Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Shi, W.; Du, N.; Xu, X.; Zhang, Y.; Ji, P.; Zhang, F.; Jia, Z.; Wang, Y.; et al. Original Article: Dysbiosis of Maternal and Neonatal Microbiota Associated with Gestational Diabetes Mellitus. Gut 2018, 67, 1614. [Google Scholar] [CrossRef]
- Hu, J.; Nomura, Y.; Bashir, A.; Fernandez-Hernandez, H.; Itzkowitz, S.; Pei, Z.; Stone, J.; Loudon, H.; Peter, I. Diversified Microbiota of Meconium Is Affected by Maternal Diabetes Status. PLoS ONE 2013, 8, e78257. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.P.; Laster, J.; Parag, B.; Shah, N.U. Multiple Health Benefits and Minimal Risks Associated with Vegetarian Diets. Curr. Nutr. Rep. 2019, 8, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Bozidis, P.; Touzios, C.; Kolios, D.; Athanasiou, G.; Athanasopoulou, E.; Gerou, I.; Gartzonika, C. Nutritional Status and the Influence of the Vegan Diet on the Gut Microbiota and Human Health. Medicina 2020, 56, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashtanova, D.A.; Popenko, A.S.; Tkacheva, O.N.; Tyakht, A.B.; Alexeev, D.G.; Boytsov, S.A. Association between the Gut Microbiota and Diet: Fetal Life, Early Childhood, and Further Life. Nutrition 2016, 32, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Barrett, H.L.; Gomez-Arango, L.F.; Wilkinson, S.A.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. A Vegetarian Diet Is a Major Determinant of Gut Microbiota Composition in Early Pregnancy. Nutrients 2018, 10, 890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylvetsky, A.C.; Rother, K.I. Trends in the Consumption of Low-Calorie Sweeteners. Physiol. Behav. 2016, 164, 446–450. [Google Scholar] [CrossRef] [Green Version]
- Nettleton, J.E.; Cho, N.A.; Klancic, T.; Nicolucci, A.C.; Shearer, J.; Borgland, S.L.; Johnston, L.A.; Ramay, H.R.; Noye Tuplin, E.; Chleilat, F.; et al. Maternal Low-Dose Aspartame and Stevia Consumption with an Obesogenic Diet Alters Metabolism, Gut Microbiota and Mesolimbic Reward System in Rat Dams and Their Offspring. Gut 2020, 69, 1807–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. Gut Microbiome Response to Sucralose and Its Potential Role in Inducing Liver Inflammation in Mice. Front. physiol. 2017, 8, 487. [Google Scholar] [CrossRef] [Green Version]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. The Artificial Sweetener Acesulfame Potassium Affects the Gut Microbiome and Body Weight Gain in CD-1 Mice. PLoS ONE 2017, 12, e0178426. [Google Scholar] [CrossRef]
- Abou-Donia, M.B.; El-Masry, E.M.; Abdel-Rahman, A.A.; McLendon, R.E.; Schiffman, S.S. Splenda Alters Gut Microflora and Increases Intestinal P-Glycoprotein and Cytochrome p-450 in Male Rats. Journal of toxicology and environmental health. J. Toxicol. Environ. Health Part A 2008, 71, 1415–1429. [Google Scholar] [CrossRef]
- Palmnäs, M.S.A.; Cowan, T.E.; Bomhof, M.R.; Su, J.; Reimer, R.A.; Vogel, H.J.; Hittel, D.S.; Shearer, J. Low-Dose Aspartame Consumption Differentially Affects Gut Microbiota-Host Metabolic Interactions in the Diet-Induced Obese Rat. PLoS ONE 2014, 9, e109841. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia Muciniphila Inversely Correlates with the Onset of Inflammation, Altered Adipose Tissue Metabolism and Metabolic Disorders during Obesity in Mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, C.H.F.; Krych, L.; Nielsen, D.S.; Vogensen, F.K.; Hansen, L.H.; Sørensen, S.J.; Buschard, K.; Hansen, A.K. Early Life Treatment with Vancomycin Propagates Akkermansia Muciniphila and Reduces Diabetes Incidence in the NOD Mouse. Diabetologia 2012, 55, 2285–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [Green Version]
- Laforest-Lapointe, I.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; Sears, M.R.; Subbarao, P.; Sycuro, L.K.; Azad, M.B.; Arrieta, M.C. Maternal Consumption of Artificially Sweetened Beverages during Pregnancy Is Associated with Infant Gut Microbiota and Metabolic Modifications and Increased Infant Body Mass Index. Gut Microbes 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Ouellette, E.M.; Rosett, H.L.; Rosman, N.P.; Weiner, L. Adverse Effects on Offspring of Maternal Alcohol Abuse during Pregnancy. N. Engl. J. Med. 1977, 297, 528–530. [Google Scholar] [CrossRef] [Green Version]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Alcohol Consumption during Pregnancy and Birth Outcomes: The Kyushu Okinawa Maternal and Child Health Study. BMC Pregnancy Childbirth 2014, 14, 79. [Google Scholar] [CrossRef] [Green Version]
- Bode, J.C.; Bode, C.; Heidelbach, R.; Dürr, H.K.; Martini, G.A. Jejunal Microflora in Patients with Chronic Alcohol Abuse. Hepatogastroenterology 1984, 31, 30–34. [Google Scholar]
- Lucey, M.R. Management of Alcoholic Liver Disease. Clin. Liver Dis. 2009, 13, 267–275. [Google Scholar] [CrossRef]
- Bode, C.; Bode, J.C. Effect of Alcohol Consumption on the Gut. Best practice & research. J. Clin. Gastroenterol. 2003, 17, 575–592. [Google Scholar] [CrossRef]
- Dubinkina, V.B.; Tyakht, A.V.; Odintsova, V.Y.; Yarygin, K.S.; Kovarsky, B.A.; Pavlenko, A.V.; Ischenko, D.S.; Popenko, A.S.; Alexeev, D.G.; Taraskina, A.Y.; et al. Links of Gut Microbiota Composition with Alcohol Dependence Syndrome and Alcoholic Liver Disease. Microbiome 2017, 5, 141. [Google Scholar] [CrossRef] [PubMed]
- Bull-Otterson, L.; Feng, W.; Kirpich, I.; Wang, Y.; Qin, X.; Liu, Y.; Gobejishvili, L.; Joshi-Barve, S.; Ayvaz, T.; Petrosino, J.; et al. Metagenomic Analyses of Alcohol Induced Pathogenic Alterations in the Intestinal Microbiome and the Effect of Lactobacillus Rhamnosus GG Treatment. PLoS ONE 2013, 8, e53028. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; White, M.B.; Monteith, P.; Noble, N.A.; Unser, A.B.; Daita, K.; Fisher, A.R.; et al. Altered Profile of Human Gut Microbiome Is Associated with Cirrhosis and Its Complications. J. Hepatol. 2014, 60, 940–947. [Google Scholar] [CrossRef] [Green Version]
- Mutlu, E.A.; Gillevet, P.M.; Rangwala, H.; Sikaroodi, M.; Naqvi, A.; Engen, P.A.; Kwasny, M.; Lau, C.K.; Keshavarzian, A. Colonic Microbiome Is Altered in Alcoholism. American journal of physiology. Am. J. Physiol. Gastrointest. 2012, 302, G966–G978. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, M.T.; Malone, D.; Caldwell, K.E.; Allan, A.M. Impact of Ethanol and Saccharin on Fecal Microbiome in Pregnant and Non-Pregnant Mice. J. Pregnancy Child Health 2015, 2, 1000193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xie, T.; Wu, Y.; Liu, Y.; Zou, Z.; Bai, J. Impacts of Maternal Diet and Alcohol Consumption during Pregnancy on Maternal and Infant Gut Microbiota. Biomolecules 2021, 11, 369. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Ciafrè, S.; Ferraguti, G.; Greco, A.; Polimeni, A.; Ralli, M.; Ceci, F.M.; Ceccanti, M.; Fiore, M. Alcohol as an Early Life Stressor: Epigenetics, Metabolic, Neuroendocrine and Neurobehavioral Implications. Neurosci. Biobehav. Rev. 2020, 118, 654–668. [Google Scholar] [CrossRef]
- Lees, B.; Mewton, L.; Jacobus, J.; Valadez, E.A.; Stapinski, L.A.; Teesson, M.; Tapert, S.F.; Squeglia, L.M. Association of Prenatal Alcohol Exposure With Psychological, Behavioral, and Neurodevelopmental Outcomes in Children From the Adolescent Brain Cognitive Development Study. Am. J. Psychiatry 2020, 177, 1060–1072. [Google Scholar] [CrossRef]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the Gut Microbiota in Intestinal Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Dotterud, C.K.; Storrø, O.; Johnsen, R.; Øien, T. Probiotics in Pregnant Women to Prevent Allergic Disease: A Randomized, Double-Blind Trial. Br. J. Dermatol. Suppl. 2010, 163, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Dotterud, C.K.; Avershina, E.; Sekelja, M.; Simpson, M.R.; Rudi, K.; Storrø, O.; Johnsen, R.; Eien, T. Does Maternal Perinatal Probiotic Supplementation Alter the Intestinal Microbiota of Mother and Child? J. Pediatr. Gastroenterol. Nutr. 2015, 61, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Kallio, S.; Kukkonen, A.K.; Savilahti, E.; Kuitunen, M. Perinatal Probiotic Intervention Prevented Allergic Disease in a Caesarean-Delivered Subgroup at 13-Year Follow-Up. Clin. Exp. Allergy 2019, 49, 506–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enomoto, T.; Sowa, M.; Nishimori, K.; Shimazu, S.; Yoshida, A.; Yamada, K.; Furukawa, F.; Nakagawa, T.; Yanagisawa, N.; Iwabuchi, N.; et al. Effects of Bifidobacterial Supplementation to Pregnant Women and Infants in the Prevention of Allergy Development in Infants and on Fecal Microbiota. Allergol. Int. 2014, 63, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Rautava, S.; Collado, M.C.; Salminen, S.; Isolauri, E. Probiotics Modulate Host-Microbe Interaction in the Placenta and Fetal Gut: A Randomized, Double-Blind, Placebo-Controlled Trial. Neonatology 2012, 102, 178–184. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kwon, J.H.; Ahn, S.H.; Lee, S.I.; Han, Y.S.; Choi, Y.O.; Lee, S.Y.; Ahn, K.M.; Ji, G.E. Effect of Probiotic Mix (Bifidobacterium Bifidum, Bifidobacterium Lactis, Lactobacillus Acidophilus) in the Primary Prevention of Eczema: A Double-Blind, Randomized, Placebo-Controlled Trial. Pediatr. Allergy Immunol. 2010, 21, e386–e393. [Google Scholar] [CrossRef]
- Niers, L.; Martín, R.; Rijkers, G.; Sengers, F.; Timmerman, H.; van Uden, N.; Smidt, H.; Kimpen, J.; Hoekstra, M. The Effects of Selected Probiotic Strains on the Development of Eczema (the PandA Study). Allergy 2009, 64, 1349–1358. [Google Scholar] [CrossRef]
- Simpson, M.R.; Dotterud, C.K.; Storrø, O.; Johnsen, R.; Øien, T. Perinatal Probiotic Supplementation in the Prevention of Allergy Related Disease: 6 Year Follow up of a Randomised Controlled Trial. BMC Dermatol. 2015, 15, 13. [Google Scholar] [CrossRef] [Green Version]
- Tannock, G.W.; Fuller, R.; Smith, S.L.; Hall, M.A. Plasmid Profiling of Members of the Family Enterobacteriaceae, Lactobacilli, and Bifidobacteria to Study the Transmission of Bacteria from Mother to Infant. J. Clin. Microbiol. 1990, 28, 1225–1228. [Google Scholar] [CrossRef] [Green Version]
- Krebs, N.F.; Westcott, J.E.; Butler, N.; Robinson, C.; Bell, M.; Hambidge, K.M. Meat as a First Complementary Food for Breastfed Infants: Feasibility and Impact on Zinc Intake and Status. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 207–214. [Google Scholar] [CrossRef]
- Schultz, M.; Göttl, C.; Young, R.J.; Iwen, P.; Vanderhoof, J.A. Administration of Oral Probiotic Bacteria to Pregnant Women Causes Temporary Infantile Colonization. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Barthow, C.; Wickens, K.; Stanley, T.; Mitchell, E.A.; Maude, R.; Abels, P.; Purdie, G.; Murphy, R.; Stone, P.; Kang, J.; et al. The Probiotics in Pregnancy Study (PiP Study): Rationale and Design of a Double-Blind Randomised Controlled Trial to Improve Maternal Health during Pregnancy and Prevent Infant Eczema and Allergy. BMC Pregnancy Childbirth 2016, 16, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahamsson, T.R.; Jakobsson, T.; Böttcher, M.F.; Fredrikson, M.; Jenmalm, M.C.; Björkstén, B.; Oldaeus, G. Probiotics in Prevention of IgE-Associated Eczema: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Allergy Clin. Immunol. 2007, 119, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, R.J.; Brantsæter, A.L.; Magnus, M.C.; Haugen, M.; Myhre, R.; Jacobsson, B.; Longnecker, M.P.; Meltzer, H.M.; London, S.J. Probiotic Milk Consumption in Pregnancy and Infancy and Subsequent Childhood Allergic Diseases. J. Allergy Clin. Immunol. 2014, 133, 165–171.e1-8. [Google Scholar] [CrossRef] [Green Version]
- Wickens, K.; Stanley, T.V.; Mitchell, E.A.; Barthow, C.; Fitzharris, P.; Purdie, G.; Siebers, R.; Black, P.N.; Crane, J. Early Supplementation with Lactobacillus Rhamnosus HN001 Reduces Eczema Prevalence to 6 Years: Does It Also Reduce Atopic Sensitization? Clin. Exp. Allergy 2013, 43, 1048–1057. [Google Scholar] [CrossRef]
- Ou, C.Y.; Kuo, H.C.; Wang, L.; Hsu, T.Y.; Chuang, H.; Liu, C.A.; Chang, J.C.; Yu, H.R.; Yang, K.D. Prenatal and Postnatal Probiotics Reduces Maternal but Not Childhood Allergic Diseases: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Exp. Allergy 2012, 42, 1386–1396. [Google Scholar] [CrossRef]
- Luoto, R.; Kalliomäki, M.; Laitinen, K.; Isolauri, E. The Impact of Perinatal Probiotic Intervention on the Development of Overweight and Obesity: Follow-up Study from Birth to 10 Years. Int. J. Obes. (Lond) 2010, 34, 1531–1537. [Google Scholar] [CrossRef] [Green Version]
- Wickens, K.L.; Barthow, C.A.; Murphy, R.; Abels, P.R.; Maude, R.M.; Stone, P.R.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.L.; Kang, J.M.; et al. Early Pregnancy Probiotic Supplementation with Lactobacillus Rhamnosus HN001 May Reduce the Prevalence of Gestational Diabetes Mellitus: A Randomised Controlled. Br. J. Nutr. 2017, 117, 804–813. [Google Scholar] [CrossRef] [Green Version]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a Multispecies Probiotic Mixture on Glycemic Control and Inflammatory Status in Women with Gestational Diabetes: A Randomized Controlled Clinical Trial. J. Nutr. Metab. 2016, 2, 5190846. [Google Scholar] [CrossRef] [Green Version]
- Hajifaraji, M.; Jahanjou, F.; Abbasalizadeh, F. Effect of Probiotic Supplementation on Blood Pressure of Females with Gestational Diabetes Mellitus: A Randomized Double Blind Controlled Clinical Trial. Iran. Red Crescent Med. J. 2017, 9, 6. [Google Scholar] [CrossRef]
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Abbasi, M.M. Is There a Value for Probiotic Supplements in Gestational Diabetes Mellitus? A Randomized Clinical Trial. J. Health Popul. Nutr. 2015, 33, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Jafari, P.; Rahmani, E.; Asemi, Z. The Effects of Probiotic Supplementation on Biomarkers of Inflammation, Oxidative Stress and Pregnancy Outcomes in Gestational Diabetes. J. Matern.-Fetal Neonatal Med. 2018, 31, 1128–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamilian, M.; Bahmani, F.; Vahedpoor, Z.; Salmani, A.; Tajabadi-Ebrahimi, M.; Jafari, P.; Dizaji, S.H.; Asemi, Z. Effects of Probiotic Supplementation on Metabolic Status in Pregnant Women: A Randomized, Double-Blind, Placebo-Controlled Trial. Arch. Iran. Med. 2016, 19, 687–692. [Google Scholar] [PubMed]
- Buddington, R.K.; Williams, C.H.; Kostek, B.M.; Buddington, K.K.; Kullen, M.J. Maternal-to-Infant Transmission of Probiotics: Concept Validation in Mice, Rats, and Pigs. Neonatology 2009, 97, 250–256. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sakata, S.; Kalliomäki, M.; Isolauri, E.; Benno, Y.; Salminen, S. Effect of Maternal Consumption of Lactobacillus GG on Transfer and Establishment of Fecal Bifidobacterial Microbiota in Neonates. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 166–170. [Google Scholar]
- Lahtinen, S.J.; Boyle, R.J.; Kivivuori, S.; Oppedisano, F.; Smith, K.R.; Robins-Browne, R.; Salminen, S.J.; Tang, M.L.K. Prenatal Probiotic Administration Can Influence Bifidobacterium Microbiota Development in Infants at High Risk of Allergy. J. Allergy Clin. Immunol. 2009, 123, 499–501. [Google Scholar] [CrossRef]
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of Probiotic Supplements on Insulin Resistance in Gestational Diabetes Mellitus: A Double-blind Randomized Controlled Trial. J. Diabetes Investig. 2019, 10, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Shadid, R.; Haarman, M.; Knol, J.; Theis, W.; Beermann, C.; Rjosk-Dendorfer, D.; Schendel, D.J.; Schendel, D.V.; Krauss-Etschmann, S. Effects of Galactooligosaccharide and Long-Chain Fructooligosaccharide Supplementation during Pregnancy on Maternal and Neonatal Microbiota and Immunity--a Randomized, Double-Blind, Placebo-Controlled Study. Am. J. Clin. Nutr. 2007, 86, 1426–1437. [Google Scholar] [CrossRef]
- Fujiwara, R.; Takemura, N.; Watanabe, J.; Sonoyama, K. Maternal Consumption of Fructo-Oligosaccharide Diminishes the Severity of Skin Inflammation in Offspring of NC/Nga Mice. Br. J. Nutr. 2009, 103, 530–538. [Google Scholar] [CrossRef] [Green Version]
- Thum, C.; Cookson, A.L.; Otter, D.E.; McNabb, W.C.; Hodgkinson, A.J.; Dyer, J.; Roy, N.C. Can Nutritional Modulation of Maternal Intestinal Microbiota Influence the Development of the Infant Gastrointestinal Tract? J. Nutr. 2012, 142, 1921–1928. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, R.; Watanabe, J.; Sonoyama, K. Assessing Changes in Composition of Intestinal Microbiota in Neonatal BALB/c Mice through Cluster Analysis of Molecular Markers. Br. J. Nutr. 2008, 99, 1174–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desbuards, N.; Gourbeyre, P.; Haure-Mirande, V.; Darmaun, D.; Champ, M.; Bodinier, M. Impact of Perinatal Prebiotic Consumption on Gestating Mice and Their Offspring: A Preliminary Report. Br. J. Nutr. 2012, 107, 1245–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal Microbial Colonization Programs the Hypothalamic–Pituitary–Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miko, E.; Csaszar, A.; Bodis, J.; Kovacs, K. The Maternal–Fetal Gut Microbiota Axis: Physiological Changes, Dietary Influence, and Modulation Possibilities. Life 2022, 12, 424. https://doi.org/10.3390/life12030424
Miko E, Csaszar A, Bodis J, Kovacs K. The Maternal–Fetal Gut Microbiota Axis: Physiological Changes, Dietary Influence, and Modulation Possibilities. Life. 2022; 12(3):424. https://doi.org/10.3390/life12030424
Chicago/Turabian StyleMiko, Eva, Andras Csaszar, Jozsef Bodis, and Kalman Kovacs. 2022. "The Maternal–Fetal Gut Microbiota Axis: Physiological Changes, Dietary Influence, and Modulation Possibilities" Life 12, no. 3: 424. https://doi.org/10.3390/life12030424
APA StyleMiko, E., Csaszar, A., Bodis, J., & Kovacs, K. (2022). The Maternal–Fetal Gut Microbiota Axis: Physiological Changes, Dietary Influence, and Modulation Possibilities. Life, 12(3), 424. https://doi.org/10.3390/life12030424






