Response of Circulating Inflammatory Markers to Intermittent Hypoxia-Hyperoxia Training in Healthy Elderly People and Patients with Mild Cognitive Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Participants
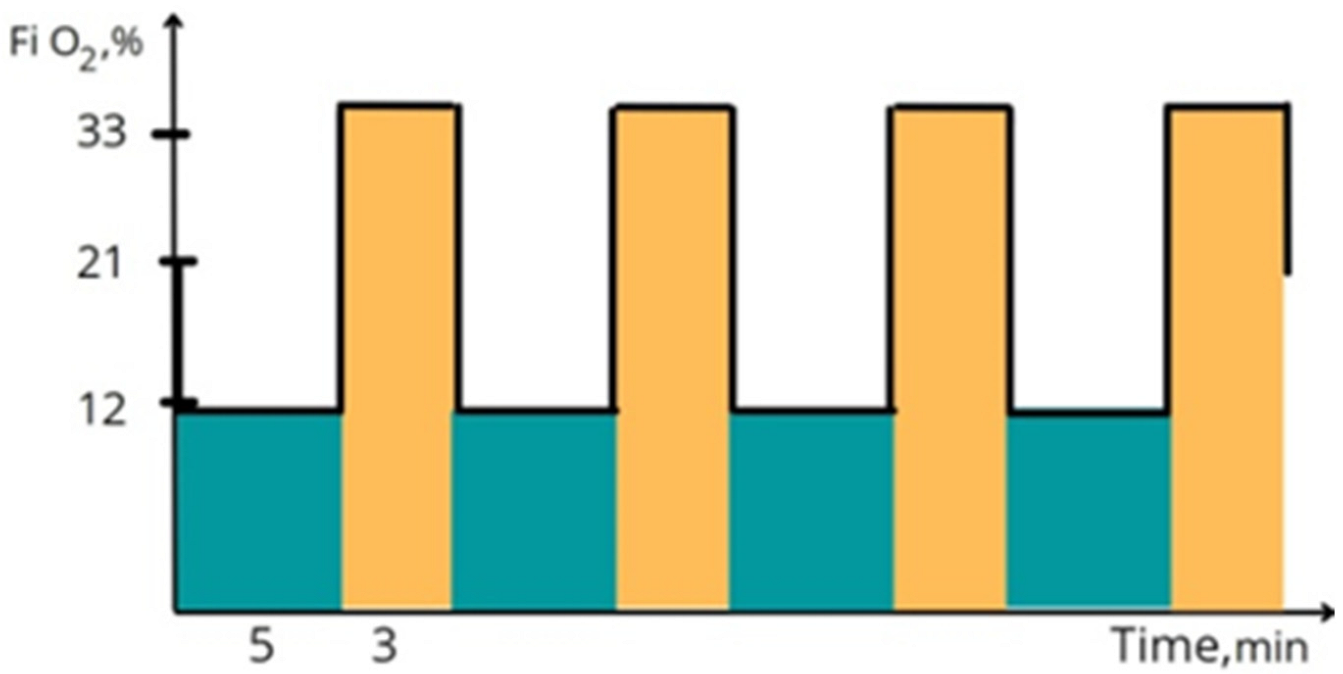
2.2. Protocol of IHHT
2.3. Cognitive Function Assessment
2.4. NETs Evaluation
2.5. Western Blot Analysis
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Analysis of MMPs Activity
2.8. Detection of Circulating lncRNA HIF1α-AS
2.9. Statistical Analysis
3. Results
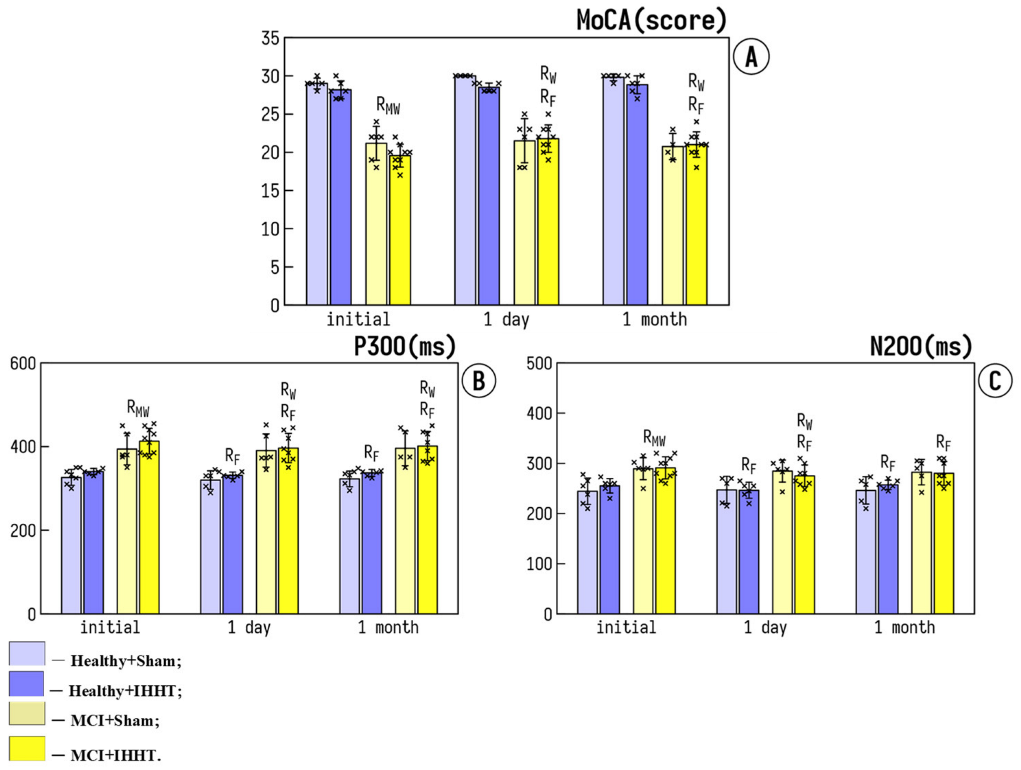
3.1. Cognitive Function
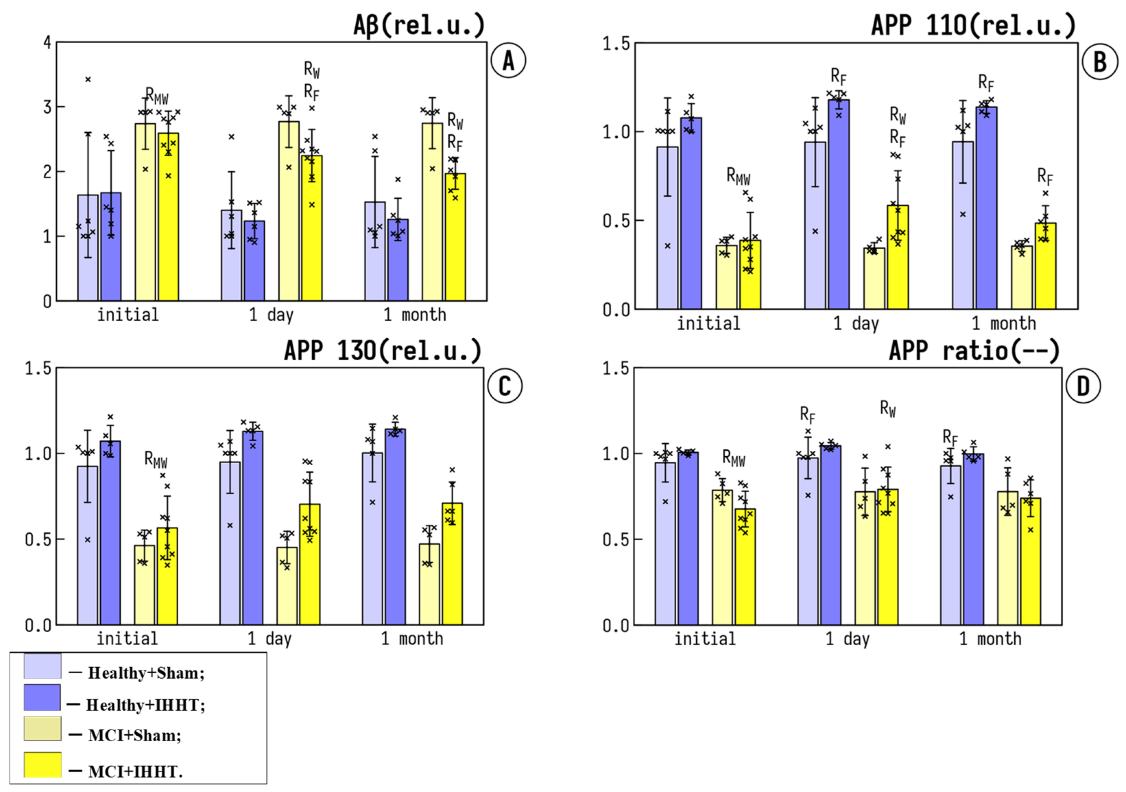
3.2. Circulating AD Markers in Platelets: Amyloid Precursor Proteins (APP) and Amyloid Beta (Aβ)
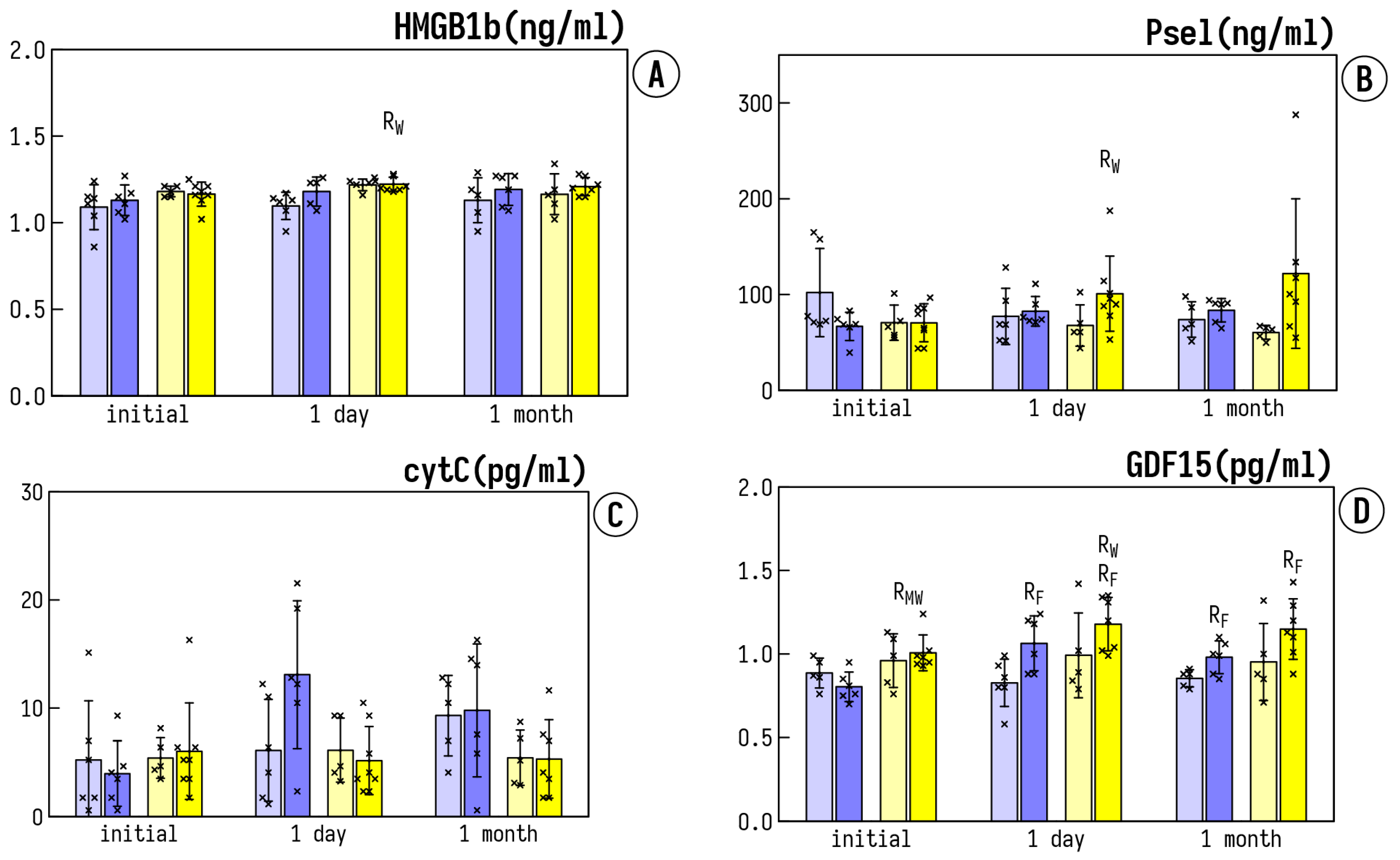
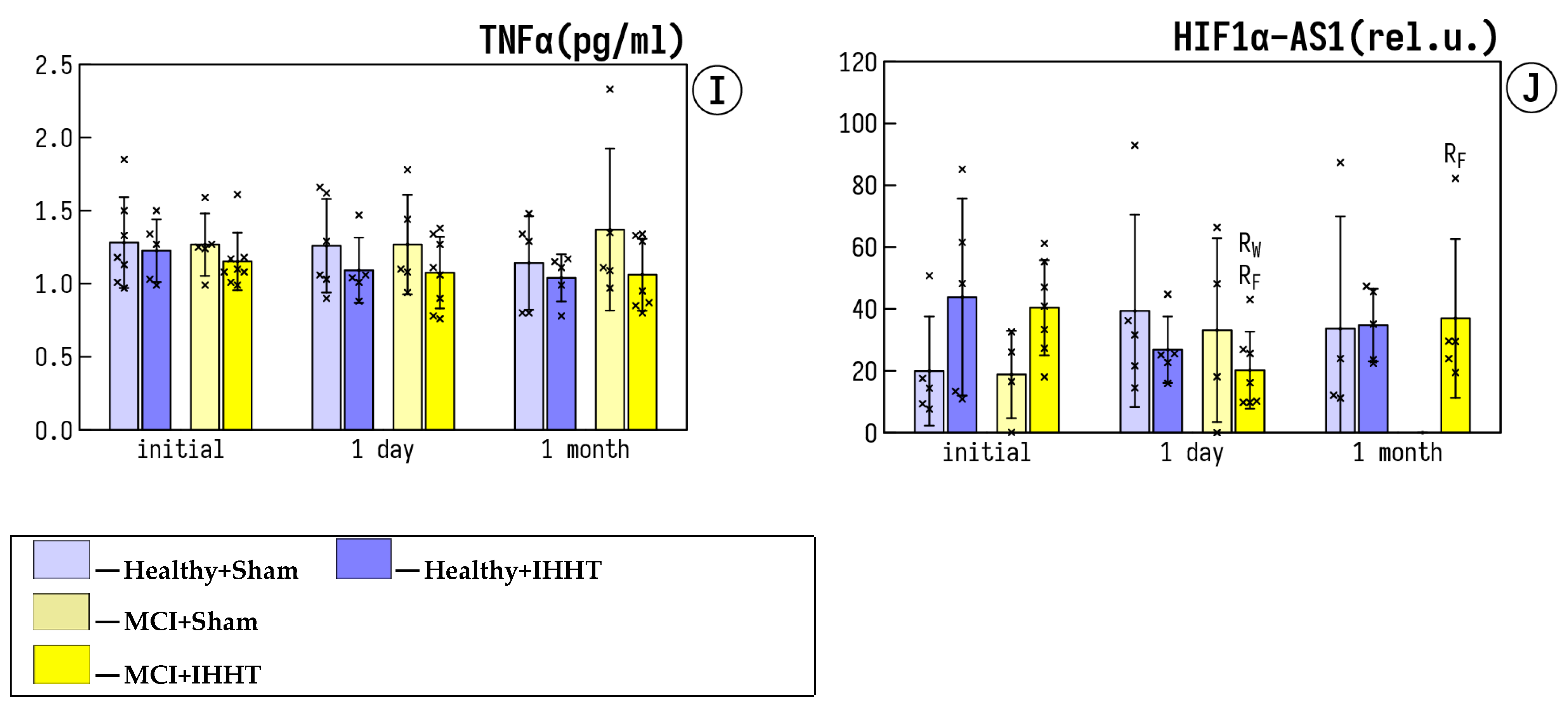
3.3. Circulating Inflammatory Markers
3.4. Expression of lncRNA HIF1a-AS1 in Plasma
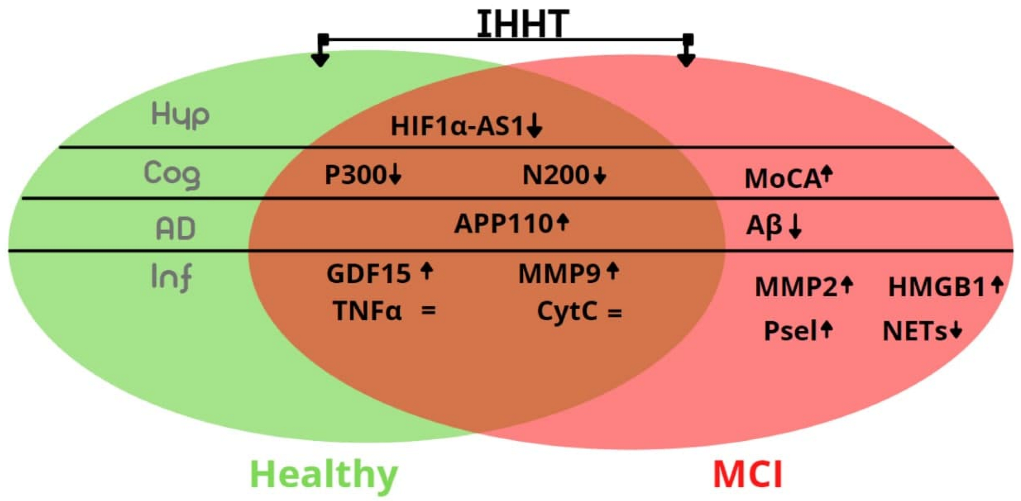
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zainaghi, I.A.; Talib, L.L.; Diniz, B.S.; Gattaz, W.F.; Forlenza, O.V. Reduced platelet amyloid precursor protein ratio (APP ratio) predicts conversion from mild cognitive impairment to Alzheimer’s disease. J. Neural. Transm. 2012, 119, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, L.E.; Erickson, K.I.; Mackey, R.; Klunk, W.E.; Aizenstein, H.; Lopresti, B.J.; Kuller, L.H.; Lopez, O.L.; Snitz, B.E. Peripheral inflammatory biomarkers predict the deposition and progression of amyloid-beta in cognitively unimpaired older adults. Brain Behav. Immun. 2021, 95, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Khan, A.; Alshahrani, S.; Rashid, H.; Qadri, M.; Rashid, S.; Alsaffar, R.M.; Kamal, M.A.; Rehman, M.U. Inflammation and Alzheimer’s Disease: Mechanisms and Therapeutic Implications by Natural Products. Mediators Inflamm. 2021, 2021, 9982954. [Google Scholar] [CrossRef] [PubMed]
- Calabro, M.; Rinaldi, C.; Santoro, G.; Crisafulli, C. The biological pathways of Alzheimer disease: A review. AIMS Neurosci. 2021, 8, 86–132. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, J.; Wang, S.; Ayoub, I.M.; Kolarova, J.D.; Levine, R.F.; Gazmuri, R.J. Circulating levels of cytochrome c after resuscitation from cardiac arrest: A marker of mitochondrial injury and predictor of survival. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H767–H775. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Liakopoulos, V.; Stefanidis, I. Cytochrome c as a Potentially Clinical Useful Marker of Mitochondrial and Cellular Damage. Front. Immunol. 2016, 7, 279. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Chen, C.; Chen, Y.; Li, Y.; Tang, F.; Huang, H.; He, W.; Zhang, R.; Shen, L. High-Mobility Group Box 1 (HMGB1) and Autophagy in Acute Lung Injury (ALI): A Review. Med. Sci. Monit. 2019, 25, 1828–1837. [Google Scholar] [CrossRef]
- Murdaca, G.; Allegra, A.; Paladin, F.; Calapai, F.; Musolino, C.; Gangemi, S. Involvement of Alarmins in the Pathogenesis and Progression of Multiple Myeloma. Int. J. Mol. Sci. 2021, 22, 9039. [Google Scholar] [CrossRef]
- Masai, K.; Kuroda, K.; Isooka, N.; Kikuoka, R.; Murakami, S.; Kamimai, S.; Wang, D.; Liu, K.; Miyazaki, I.; Nishibori, M.; et al. Neuroprotective Effects of Anti-high Mobility Group Box-1 Monoclonal Antibody Against Methamphetamine-Induced Dopaminergic Neurotoxicity. Neurotox. Res. 2021, 39, 1511–1523. [Google Scholar] [CrossRef]
- Papatheodorou, A.; Stein, A.; Bank, M.; Sison, C.P.; Gibbs, K.; Davies, P.; Bloom, O. High-Mobility Group Box 1 (HMGB1) Is Elevated Systemically in Persons with Acute or Chronic Traumatic Spinal Cord Injury. J. Neurotrauma 2017, 34, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Harb, H.; Stephen-Victor, E.; Crestani, E.; Benamar, M.; Massoud, A.; Cui, Y.; Charbonnier, L.M.; Arbag, S.; Baris, S.; Cunnigham, A.; et al. A regulatory T cell Notch4-GDF15 axis licenses tissue inflammation in asthma. Nat. Immunol. 2020, 21, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, L.; Li, W.; Xu, C.; Zhang, J.; Wang, D.; Dou, K.; Zhuang, R.; Jin, B.; Zhang, W. GDF15 induces immunosuppression via CD48 on regulatory T cells in hepatocellular carcinoma. J. Immunother. Cancer 2021, 9, e002787. [Google Scholar] [CrossRef] [PubMed]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Liu, Y.; Chen, R.; Zhao, D.; McAlister, V.; Mele, T.; Liu, K.; Zheng, X. GDF15 Regulates Malat-1 Circular RNA and Inactivates NFkappaB Signaling Leading to Immune Tolerogenic DCs for Preventing Alloimmune Rejection in Heart Transplantation. Front. Immunol. 2018, 9, 2407. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Lee, M.S. GDF15 as a central mediator for integrated stress response and a promising therapeutic molecule for metabolic disorders and NASH. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129834. [Google Scholar] [CrossRef]
- Ahmed, D.S.; Isnard, S.; Lin, J.; Routy, B.; Routy, J.P. GDF15/GFRAL Pathway as a Metabolic Signature for Cachexia in Patients with Cancer. J. Cancer 2021, 12, 1125–1132. [Google Scholar] [CrossRef]
- Buchholz, K.; Antosik, P.; Grzanka, D.; Gagat, M.; Smolinska, M.; Grzanka, A.; Gzil, A.; Kasperska, A.; Klimaszewska-Wisniewska, A. Expression of the Body-Weight Signaling Players: GDF15, GFRAL and RET and their clinical relevance in Gastric Cancer. J. Cancer 2021, 12, 4698–4709. [Google Scholar] [CrossRef]
- Sabatini, P.V.; Frikke-Schmidt, H.; Arthurs, J.; Gordian, D.; Patel, A.; Rupp, A.C.; Adams, J.M.; Wang, J.; Beck Jorgensen, S.; Olson, D.P.; et al. GFRAL-expressing neurons suppress food intake via aversive pathways. Proc. Natl. Acad. Sci. USA 2021, 118, e2021357118. [Google Scholar] [CrossRef]
- Conte, M.; Sabbatinelli, J.; Chiariello, A.; Martucci, M.; Santoro, A.; Monti, D.; Arcaro, M.; Galimberti, D.; Scarpini, E.; Bonfigli, A.R.; et al. Disease-specific plasma levels of mitokines FGF21, GDF15, and Humanin in type II diabetes and Alzheimer’s disease in comparison with healthy aging. Geroscience 2021, 43, 985–1001. [Google Scholar] [CrossRef]
- McGrath, E.R.; Himali, J.J.; Levy, D.; Conner, S.C.; DeCarli, C.; Pase, M.P.; Ninomiya, T.; Ohara, T.; Courchesne, P.; Satizabal, C.L.; et al. Growth Differentiation Factor 15 and NT-proBNP as Blood-Based Markers of Vascular Brain Injury and Dementia. J. Am. Heart Assoc. 2020, 9, e014659. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.F.; Zhang, X.H.; Zhou, P.; Yin, R.; Zhou, X.T.; Zhang, W. Growth Differentiation Factor 15 Is Associated With Alzheimer’s Disease Risk. Front. Genet. 2021, 12, 700371. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, D.; Lim, H.; Choi, S.J.; Oh, W.; Yang, Y.S.; Chang, J.H.; Jeon, H.B. Effect of growth differentiation factor-15 secreted by human umbilical cord blood-derived mesenchymal stem cells on amyloid beta levels in in vitro and in vivo models of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2018, 504, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, G.C.; Bumiller-Bini, V.; Gasparetto Filho, M.A.; Zonta, Y.R.; Yu, K.S.T.; de Souza, R.L.R.; Dias-Melicio, L.A.; Boldt, A.B.W. Neutrophil Extracellular Traps: A Perspective of Neuroinflammation and Complement Activation in Alzheimer’s Disease. Front. Mol. Biosci. 2021, 8, 630869. [Google Scholar] [CrossRef]
- Zahedipour, F.; Hosseini, S.A.; Henney, N.C.; Barreto, G.E.; Sahebkar, A. Phytochemicals as inhibitors of tumor necrosis factor alpha and neuroinflammatory responses in neurodegenerative diseases. Neural Regen. Res. 2022, 17, 1675–1684. [Google Scholar]
- Markulin, I.; Matasin, M.; Turk, V.E.; Salković-Petrisic, M. Challenges of repurposing tetracyclines for the treatment of Alzheimer’s and Parkinson’s disease. J. Neural Transm. 2022, Epub ahead of print. [Google Scholar] [CrossRef]
- Serebrovs’ka, T.V.; Kolesnikova, I.e.E.; Karaban’, I.M. Respiratory regulation during adaptation to intermittent hypoxia in patients with Parkinson disease. Fiziol. Zh. 2003, 49, 95–103. [Google Scholar]
- Sajjadi, E.; Seven, Y.B.; Ehrbar, J.G.; Wymer, J.P.; Mitchell, G.S.; Smith, B.K. Acute intermittent hypoxia and respiratory muscle recruitment in people with amyotrophic lateral sclerosis: A preliminary study. Exp. Neurol. 2022, 347, 113890. [Google Scholar] [CrossRef]
- Mansur, A.P.; Alvarenga, G.S.; Kopel, L.; Gutierrez, M.A.; Consolim-Colombo, F.M.; Abrahão, L.H.; Lage, S.G. Cerebral blood flow changes during intermittent acute hypoxia in patients with heart failure. J. Int. Med. Res. 2018, 46, 4214–4225. [Google Scholar] [CrossRef]
- Serebrovska, T.V.; Shatilo, V.B. Remote ischemic preconditioning versus intermittent hypoxia training: A comparative analysis for cardioprotection. Fiziol. Zh. 2015, 61, 99–117. [Google Scholar] [CrossRef] [Green Version]
- Faulhaber, M.; Gatterer, H.; Haider, T.; Linser, T.; Netzer, N.; Burtscher, M. Heart rate and blood pressure responses during hypoxic cycles of a 3-week intermittent hypoxia breathing program in patients at risk for or with mild COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 339–345. [Google Scholar] [PubMed] [Green Version]
- Muangritdech, N.; Hamlin, M.J.; Sawanyawisuth, K.; Prajumwongs, P.; Saengjan, W.; Wonnabussapawich, P.; Manimmanakorn, N.; Manimmanakorn, A. Hypoxic training improves blood pressure, nitric oxide and hypoxia-inducible factor-1 alpha in hypertensive patients. Eur. J. Appl. Physiol. 2020, 120, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Törpel, A.; Peter, B.; Hamacher, D.; Schega, L. Dose–response relationship of intermittent normobaric hypoxia to stimulate erythropoietin in the context of health promotion in young and old people. Eur. J. Appl. Physiol. 2019, 119, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Tobin, B.; Costalat, G.; Renshaw, G.M.C. Intermittent not continuous hypoxia provoked haematological adaptations in healthy seniors: Hypoxic pattern may hold the key. Eur. J. Appl. Physiol. 2020, 120, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Schega, L.; Peter, B.; Brigadski, T.; Leßmann, V.; Isermann, B.; Hamacher, D.; Törpel, A. Effect of intermittent normobaric hypoxia on aerobic capacity and cognitive function in older people. J. Sci. Med. Sport 2016, 19, 941–945. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, M.; Li, H.; Yuan, J.; Li, J.; Wu, F.; Zhang, Y. Hypoxia preconditioning attenuates lung injury after thoracoscopic lobectomy in patients with lung cancer: A prospective randomized controlled trial. BMC Anesthesiol. 2019, 19, 209. [Google Scholar] [CrossRef]
- Susta, D.; Glazachev, O.S.; Zapara, M.A.; Dudnik, E.N.; Samartseva, V.G. Redox Homeostasis in Humans Exposed to Intermittent Hypoxia–Normoxia and to Intermittent Hypoxia–Hyperoxia. High Alt. Med. Biol. 2020, 21, 45–51. [Google Scholar] [CrossRef]
- Serebrovska, T.V.; Portnychenko, A.G.; Portnichenko, V.I.; Xi, L.; Egorov, E.; Antoniuk-Shcheglova, I.; Naskalova, S.; Shatylo, V.B. Effects of intermittent hypoxia training on leukocyte pyruvate dehydrogenase kinase 1, (PDK-1) mRNA expression and blood insulin level in prediabetes patients. Eur. J. Appl. Physiol. 2019, 119, 813–823. [Google Scholar] [CrossRef]
- Ramezani, R.J.; Stacpoole, P.W. Sleep disorders associated with primary mitochondrial diseases. J. Clin. Sleep Med. 2014, 10, 1233–1239. [Google Scholar] [CrossRef] [Green Version]
- Tietjens, J.R.; Claman, D.; Kezirian, E.J.; De Marco, T.; Mirzayan, A.; Sadroonri, B.; Goldberg, A.N.; Long, C.; Gerstenfeld, E.P.; Yeghiazarians, Y. Obstructive Sleep Apnea in Cardiovascular Disease: A Review of the Literature and Proposed Multidisciplinary Clinical Management Strategy. J. Am. Heart Assoc. 2019, 8, e010440. [Google Scholar] [CrossRef] [Green Version]
- Ryou, M.G.; Sun, J.; Oguayo, K.N.; Manukhina, E.B.; Downey, H.F.; Mallet, R.T. (2008) Hypoxic conditioning suppresses nitric oxide production upon myocardial reperfusion. Exp. Biol. Med. 2008, 233, 766–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serebrovskaya, T.V.; Xi, L. Intermittent hypoxia in childhood: The harmful consequences versus potential benefits of therapeutic uses. Front. Pediatr. 2015, 3, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serebrovskaya, T.V.; Xi, L. Intermittent hypoxia training as non-pharmacologic therapy for cardiovascular diseases: Practical analysis on methods and equipment. Exp. Biol. Med. 2016, 241, 1708–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprick, J.D.; Mallet, R.T.; Przyklenk, K.; Rickards, C.A. Ischaemic and hypoxic conditioning: Potential for protection of vital organs. Exp. Physiol. 2019, 104, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Serebrovska, Z.O.; Serebrovska, T.V.; Kholin, V.A.; Tumanovska, L.V.; Shysh, A.M.; Pashevin, D.A.; Goncharov, S.V.; Stroy, D.; Grib, O.N.; Shatylo, V.B. Intermittent Hypoxia-Hyperoxia Training Improves Cognitive Function and Decreases Circulating Biomarkers of Alzheimer’s Disease in Patients with Mild Cognitive Impairment: A Pilot Study. Int. J. Mol. Sci. 2019, 20, 5405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belonwu, S.A.; Li, Y.; Bunis, D.; Rao, A.A.; Solsberg, C.W.; Tang, A.; Fragiadakis, G.K.; Dubal, D.B.; Oskotsky, T.; Sirota, M. Sex-Stratified Single-Cell RNA-Seq Analysis Identifies Sex-Specific and Cell Type-Specific Transcriptional Responses in Alzheimer’s Disease Across Two Brain Regions. Mol. Neurobiol. 2022, 59, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Rabipour, S.; Rajagopal, S.; Pasvanis, S.; Rajah, M.N.; PREVENT-AD Research Group. Generalization of memory-related brain function in asymptomatic older women with a family history of late onset Alzheimer’s Disease: Results from the PREVENT-AD Cohort. Neurobiol. Aging 2021, 104, 42–56. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Dawes, P.; Pye, A.; Reeves, D.; Yeung, W.K.; Sheikh, S.; Thodi, C.; Charalambous, A.P.; Gallant, K.; Nasreddine, Z.; Leroi, I. Protocol for the development of versions of the Montreal Cognitive Assessment (MoCA) for people with hearing or vision impairment. BMJ Open 2019, 9, e026246. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Milani, S.A.; Marsiske, M.; Cottler, L.B.; Chen, X.; Striley, C.W. Optimal cutoffs for the Montreal Cognitive Assessment vary by race and ethnicity. Alzheimers Dement. 2018, 10, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int. Psychogeriatr. 1997, 9, 173–176, discussion 177–178. [Google Scholar] [CrossRef] [PubMed]
- Serebrovska, T.V.; Portnychenko, A.G.; Drevytska, T.I.; Portnichenko, V.I.; Xi, L.; Egorov, E.; Gavalko, A.V.; Naskalova, S.; Chizhova, V.; Shatylo, V.B. Intermittent hypoxia training in prediabetes patients: Beneficial effects on glucose homeostasis, hypoxia tolerance and gene expression. Exp. Biol. Med. 2017, 242, 1542–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunerli, D.; Emek-Savas, D.D.; Cavusoglu, B.; Donmez Colakoglu, B.; Ada, E.; Yener, G.G. Mild cognitive impairment in Parkinson’s disease is associated with decreased P300 amplitude and reduced putamen volume. Clin. Neurophysiol. 2019, 130, 1208–1217. [Google Scholar] [CrossRef]
- Lai, C.L.; Lin, R.T.; Liou, L.M.; Liu, C.K. The role of event-related potentials in cognitive decline in Alzheimer’s disease. Clin. Neurophysiol. 2010, 121, 194–199. [Google Scholar] [CrossRef]
- Vaitkevicius, A.; Kaubrys, G.; Audronyte, E. Distinctive Effect of Donepezil Treatment on P300 and N200 Subcomponents of Auditory Event-Related Evoked Potentials in Alzheimer Disease Patients. Med. Sci. Monit. 2015, 21, 1920–1927. [Google Scholar] [CrossRef] [Green Version]
- Pashevin, D.O.; Nagibin, V.S.; Tumanovska, L.V.; Moibenko, A.A.; Dosenko, V.E. Proteasome Inhibition Diminishes the Formation of Neutrophil Extracellular Traps and Prevents the Death of Cardiomyocytes in Coculture with Activated Neutrophils during Anoxia-Reoxygenation. Pathobiology 2015, 82, 290–298. [Google Scholar] [CrossRef]
- Barancik, M.; Bohacova, V.; Gibalova, L.; Sedlak, J.; Sulova, Z.; Breier, A. Potentiation of anticancer drugs: Effects of pentoxifylline on neoplastic cells. Int. J. Mol. Sci. 2012, 13, 369–382. [Google Scholar] [CrossRef] [Green Version]
- Muller, U.C.; Zheng, H. Physiological functions of APP family proteins. Cold Spring Harb. Perspect. Med. 2012, 2, a006288. [Google Scholar] [CrossRef] [Green Version]
- Montagna, E.; Dorostkar, M.M.; Herms, J. The Role of APP in Structural Spine Plasticity. Front. Mol. Neurosci. 2017, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Visconte, C.; Canino, J.; Guidetti, G.F.; Zara, M.; Seppi, C.; Abubaker, A.A.; Pula, G.; Torti, M.; Canobbio, I. Amyloid precursor protein is required for in vitro platelet adhesion to amyloid peptides and potentiation of thrombus formation. Cell Signal 2018, 52, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.V.; Yoon, S.; Domingues, S.; Guimaraes, S.; Goltsev, A.V.; da Cruz, E.S.E.F.; Mendes, J.F.; da Cruz, E.S.O.A.; Fardilha, M. Amyloid precursor protein interaction network in human testis: Sentinel proteins for male reproduction. BMC Bioinform. 2015, 16, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borroni, B.; Agosti, C.; Marcello, E.; Di Luca, M.; Padovani, A. Blood cell markers in Alzheimer Disease: Amyloid Precursor Protein form ratio in platelets. Exp. Gerontol. 2010, 45, 53–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluta, R.; Ulamek-Koziol, M.; Januszewski, S.; Czuczwar, S.J. Platelets, lymphocytes and erythrocytes from Alzheimer’s disease patients: The quest for blood cell-based biomarkers. Folia Neuropathol. 2018, 56, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Gao, Y.; Li, Y.; Zheng, L.; Xu, F.; Li, X. Inhibition of HIF1A-AS1 promoted starvation-induced hepatocellular carcinoma cell apoptosis by reducing HIF-1alpha/mTOR-mediated autophagy. World J. Surg. Oncol. 2020, 18, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Guo, X.; Hu, J.; Li, B. Long Noncoding RNA Hypoxia-Inducible Factor-1 Alpha-Antisense RNA 1 Regulates Vascular Smooth Muscle Cells to Promote the Development of Thoracic Aortic Aneurysm by Modulating Apoptotic Protease-Activating Factor 1 and Targeting let-7g. J. Surg. Res. 2020, 255, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Ringland, C.; Schweig, J.E.; Eisenbaum, M.; Paris, D.; Ait-Ghezala, G.; Mullan, M.; Crawford, F.; Abdullah, L.; Bachmeier, C. MMP9 modulation improves specific neurobehavioral deficits in a mouse model of Alzheimer’s disease. BMC Neurosci. 2021, 22, 39. [Google Scholar] [CrossRef]
- Ashok, A.; Rai, N.K.; Raza, W.; Pandey, R.; Bandyopadhyay, S. Chronic cerebral hypoperfusion-induced impairment of Abeta clearance requires HB-EGF-dependent sequential activation of HIF1alpha and MMP9. Neurobiol. Dis. 2016, 95, 179–193. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Xin, X.; Sun, D.; Huang, H.; Chen, Y.; Zhang, T.; Zhang, Y. Hypoxia stimulates the migration and invasion of osteosarcoma via up-regulating the NUSAP1 expression. Open Med. 2021, 16, 1083–1089. [Google Scholar] [CrossRef]
- Choi, J.B.; Cho, K.J.; Kim, J.C.; Pae, C.U.; Koh, J.S. An open-label, single-arm pilot study to evaluate the efficacy of daily low dose tadalafil on depression in patients with erectile dysfunction. Transl. Androl. Urol. 2019, 8, 501–506. [Google Scholar] [CrossRef]
- Ries, M.; Sastre, M. Mechanisms of Abeta Clearance and Degradation by Glial Cells. Front. Aging Neurosci. 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.S.; Chen, L.Y.; Fu, L.L.; Chen, M.L.; Wong, M.K. Effects of moderate and severe intermittent hypoxia on vascular endothelial function and haemodynamic control in sedentary men. Eur. J. Appl. Physiol. 2007, 100, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ling, Y.; Lin, J.; Du, X.; Fang, Y.; Wu, J. Force-dependent calcium signaling and its pathway of human neutrophils on P-selectin in flow. Protein Cell 2017, 8, 103–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issekutz, A.C.; Nakazato, S.; Issekutz, T.B. Differential roles of VLA-4(CD49d/CD29) and LFA-1(CD11a/CD18) integrins and E- and P-selectin during developing and established active or adoptively transferred adjuvant arthritis in the rat. Immunol. Cell Biol. 2003, 81, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Kato, T.; Nemoto, N.; Araki, A.; Gazi, M.Y.; Nara, H.; Asao, H. Systemic neutrophil migration and rapid consumption of neutrophils in the spleen. Data Brief 2018, 20, 680–682. [Google Scholar] [CrossRef]

| Groups | N | Gender (Female/Male) | Age (Years) | BMI (kg/m2) | SBP (mmHg) | DBP (mmHg) |
|---|---|---|---|---|---|---|
| Healthy+Sham | 7 | 5/2 | 65 ± 8.1 | 26.6 ± 3.8 | 133.8 ± 18.1 | 82.1 ± 8.0 |
| Healthy+IHHT | 6 | 5/1 | 67.5 ± 7 | 26.5 ± 4.7 | 129.0 ± 15.4 | 82.4 ± 8.0 |
| MCI+Sham | 6 | 6/0 | 70.8 ± 9.3 | 26.5 ± 4.3 | 136.4 ± 17.6 | 82.1 ± 14.0 |
| MCI+IHHT | 8 | 7/1 | 65.4 ± 6.2 | 26.8 ± 5.1 | 135.2 ± 15.4 | 82.7 ± 9.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serebrovska, Z.O.; Xi, L.; Tumanovska, L.V.; Shysh, A.M.; Goncharov, S.V.; Khetsuriani, M.; Kozak, T.O.; Pashevin, D.A.; Dosenko, V.E.; Virko, S.V.; et al. Response of Circulating Inflammatory Markers to Intermittent Hypoxia-Hyperoxia Training in Healthy Elderly People and Patients with Mild Cognitive Impairment. Life 2022, 12, 432. https://doi.org/10.3390/life12030432
Serebrovska ZO, Xi L, Tumanovska LV, Shysh AM, Goncharov SV, Khetsuriani M, Kozak TO, Pashevin DA, Dosenko VE, Virko SV, et al. Response of Circulating Inflammatory Markers to Intermittent Hypoxia-Hyperoxia Training in Healthy Elderly People and Patients with Mild Cognitive Impairment. Life. 2022; 12(3):432. https://doi.org/10.3390/life12030432
Chicago/Turabian StyleSerebrovska, Zoya O., Lei Xi, Lesya V. Tumanovska, Angela M. Shysh, Sergii V. Goncharov, Michael Khetsuriani, Taisia O. Kozak, Denis A. Pashevin, Victor E. Dosenko, Sergii V. Virko, and et al. 2022. "Response of Circulating Inflammatory Markers to Intermittent Hypoxia-Hyperoxia Training in Healthy Elderly People and Patients with Mild Cognitive Impairment" Life 12, no. 3: 432. https://doi.org/10.3390/life12030432
APA StyleSerebrovska, Z. O., Xi, L., Tumanovska, L. V., Shysh, A. M., Goncharov, S. V., Khetsuriani, M., Kozak, T. O., Pashevin, D. A., Dosenko, V. E., Virko, S. V., Kholin, V. A., Grib, O. N., Utko, N. A., Egorov, E., Polischuk, A. O., & Serebrovska, T. V. (2022). Response of Circulating Inflammatory Markers to Intermittent Hypoxia-Hyperoxia Training in Healthy Elderly People and Patients with Mild Cognitive Impairment. Life, 12(3), 432. https://doi.org/10.3390/life12030432






