Environmental and Human Microbiome for Health
Abstract
:1. Introduction
2. Diversity of Microbes in the Environment and Human Body
3. Beneficial Microbes Present in the Human Body
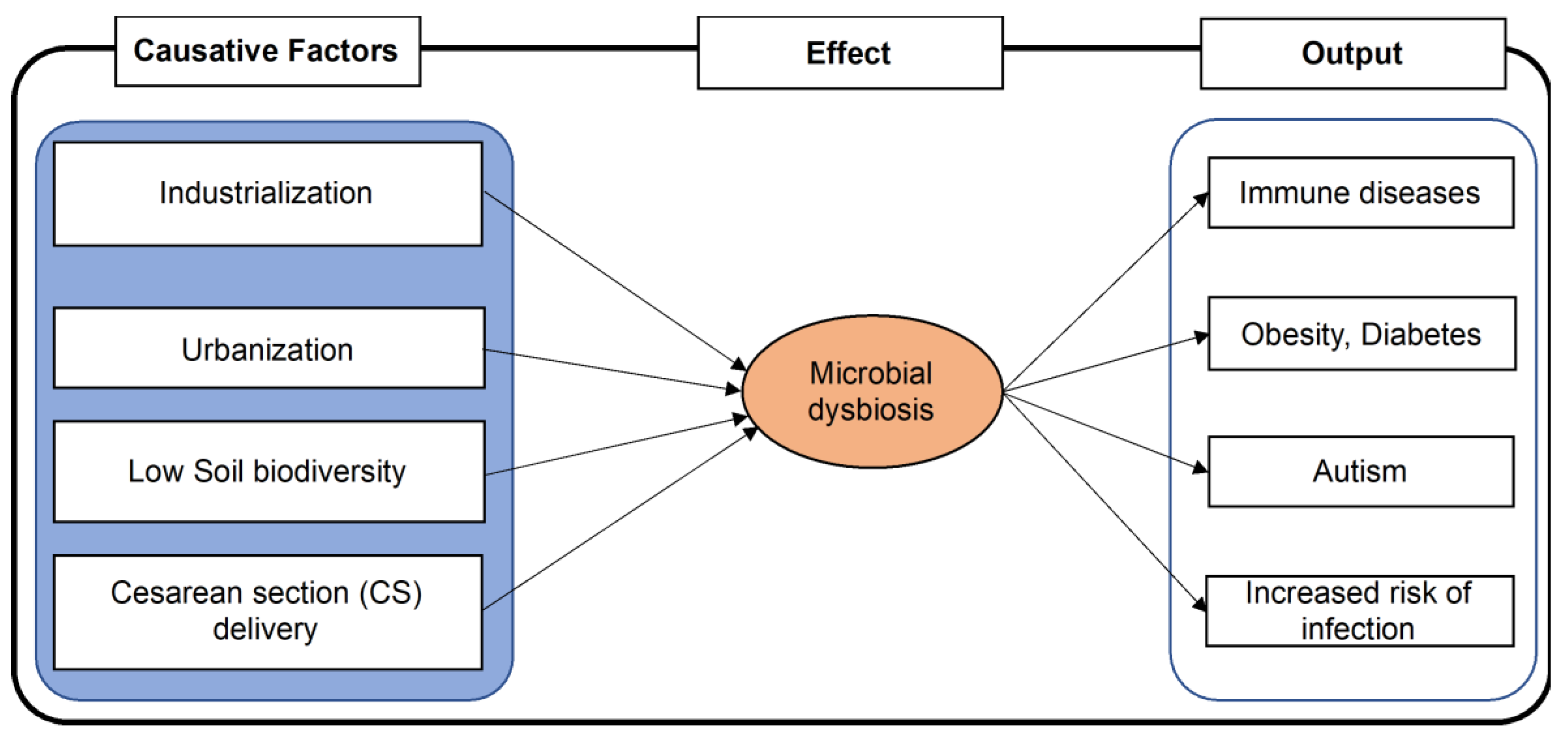
4. Factors Associated with Microbial Dysbiosis and Its Impact on Human Health
5. Factors Associated with Microbial Diversity in the Human
6. Environment-Host Dynamics
7. Improving Health: Living with Environment
8. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bitton, G. Role of Microorganisms in Biogeochemical Cycles. In Wastewater Microbiology; Bitton, G., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Gougoulias, C.; Clark, J.; Shaw, L. The role of soil microbes in the global carbon cycle: Tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLOS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fijan, S. Microorganisms with Claimed Probiotic Properties: An Overview of Recent Literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.R. Microbial diversity: The importance of exploration and conservation. J. Ind. Microbiol. Biotechnol. 1997, 18, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, V.; Øvreås, L. Microbial diversity and function in soil: From genes to ecosystems. Curr. Opin. Microbiol. 2002, 5, 240–245. [Google Scholar] [CrossRef]
- Rappé, M.S.; Giovannoni, S.J. The Uncultured Microbial Majority. Annu. Rev. Microbiol. 2003, 57, 369–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, W.E.H.; Zechmeister-Boltenstern, S.; Keiblinger, K.M. Does Soil Contribute to the Human Gut Microbiome? Microorganisms 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raynaud, X.; Nunan, N. Spatial Ecology of Bacteria at the Microscale in Soil. PLoS ONE 2014, 9, e87217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torsvik, V.; Sørheim, R.; Goksøyr, J. Total bacterial diversity in soil and sediment communities—A review. J. Ind. Microbiol. Biotechnol. 1996, 17, 170–178. [Google Scholar] [CrossRef]
- Bickel, S.; Or, D. Soil bacterial diversity mediated by microscale aqueous-phase processes across biomes. Nat. Commun. 2020, 11, 116–119. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.; Wei, G. Linking phylogenetic niche conservatism to soil archaeal biogeography, community assembly and species coexistence. Glob. Ecol. Biogeogr. 2021, 30, 1488–1501. [Google Scholar] [CrossRef]
- LaMartina, E.L.; Mohaimani, A.A.; Newton, R.J. Urban wastewater bacterial communities assemble into seasonal steady states. Microbiome 2021, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.J.; McLellan, S.L.; Dila, D.K.; Vineis, J.H.; Morrison, H.G.; Eren, A.M.; Sogin, M.L. Sewage Reflects the Microbiomes of Human Populations. MBio 2015, 6, e02574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iraola, G.; Kumar, N. Surveying what’s flushed away. Nat. Rev. Genet. 2018, 16, 456. [Google Scholar] [CrossRef]
- Yan, T.; O’Brien, P.; Shelton, J.M.; Whelen, A.C.; Pagaling, E. Municipal Wastewater as a Microbial Surveillance Platform for Enteric Diseases: A Case Study for Salmonella and Salmonellosis. Environ. Sci. Technol. 2018, 52, 4869–4877. [Google Scholar] [CrossRef]
- Rackaityte, E.; Lynch, S.V. The human microbiome in the 21st century. Nat. Commun. 2020, 11, 5256. [Google Scholar] [CrossRef] [PubMed]
- Heederik, D.; von Mutius, E. Does diversity of environmental microbial exposure matter for the occurrence of allergy and asthma? J. Allergy Clin. Immunol. 2012, 130, 44–50. [Google Scholar] [CrossRef]
- Kelley, S.T.; Gilbert, J.A. Studying the microbiology of the indoor environment. Genome Biol. 2013, 14, 202. [Google Scholar] [CrossRef] [Green Version]
- Ursell, L.K.; Clemente, J.C.; Rideout, J.R.; Gevers, D.; Caporaso, J.G.; Knight, R. The interpersonal and intrapersonal diversity of human-associated microbiota in key body sites. J. Allergy Clin. Immunol. 2012, 129, 1204–1208. [Google Scholar] [CrossRef] [Green Version]
- Marco, M.L. Defining how microorganisms benefit human health. Microb. Biotechnol. 2021, 14, 35–40. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome–An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.A.; Gallo, R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassis, C.M.; Tang, A.L.; Young, V.B.; Pynnonen, M.A. The nasal cavity microbiota of healthy adults. Microbiome 2014, 2, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wei, C.-X.; Min, L.; Zhu, L.-Y. Good or bad: Gut bacteria in human health and diseases. Biotechnol. Biotechnol. Equip. 2018, 32, 1075–1080. [Google Scholar] [CrossRef] [Green Version]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.-J.M.; Wells, J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Liver Physiol. 2010, 298, G851–G859. [Google Scholar] [CrossRef] [Green Version]
- Zong, X.; Fu, J.; Xu, B.; Wang, Y.; Jin, M. Interplay between gut microbiota and antimicrobial peptides. Anim. Nutr. 2020, 6, 389–396. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [Green Version]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4578–4585. [Google Scholar] [CrossRef] [Green Version]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the oral microbiome by whole-genome sequencing and resistome analysis: The complexity of the healthy picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef]
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Wellness 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Kumpitsch, C.; Koskinen, K.; Schöpf, V.; Moissl-Eichinger, C. The microbiome of the upper respiratory tract in health and disease. BMC Biol. 2019, 17, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, W.H.; De Steenhuijsen Piters, W.A.A.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal Microbiome: Rethinking Health and Disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.-A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Veyver, I.V.D.; Milosavljevic, A.; et al. A Metagenomic Approach to Characterization of the Vaginal Microbiome Signature in Pregnancy. PLoS ONE 2012, 7, e36466. [Google Scholar] [CrossRef] [PubMed]
- Timm, C.M.; Loomis, K.; Stone, W.; Mehoke, T.; Brensinger, B.; Pellicore, M.; Staniczenko, P.P.; Charles, C.; Nayak, S.; Karig, D.K. Isolation and characterization of diverse microbial representatives from the human skin microbiome. Microbiome 2020, 8, 58. [Google Scholar] [CrossRef]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 2016, 7, 1204. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Picard, C.; Fioramonti, J.; Francois, A.; Robinson, T.; Neant, F.; Matuchansky, C. Review article: Bifidobacteria as probiotic agents—Physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005, 22, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; Van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, N.; Bai, C.; Song, S.; Zhang, Y.; Wang, B.; Li, Z. Bifidobacterium plays a protective role in TNF-α-induced inflammatory response in Caco-2 cell through NF-κB and p38MAPK pathways. Mol. Cell. Biochem. 2019, 464, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Halder, C.V.; de Sousa Faria, A.V.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martin, R.; Rossi, O.; Bermudez-Humaran, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhu, C.; Quan, Y.; Yang, J.; Yuan, W.; Yang, Z.; Wu, S.; Luo, W.; Tan, B.; Wang, X. Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. J. Gastroenterol. Hepatol. 2018, 33, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Shen, Z.; Deng, M.; Li, X.; Tan, B.; Xiao, M.; Wu, S.; Yang, Z.; Zhu, C.; Tian, L.; et al. Roseburia intestinalis supernatant ameliorates colitis induced in mice by regulating the immune response. Mol. Med. Rep. 2019, 20, 1007–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slattery, C.; Cotter, P.D.; O’Toole, P.W. Analysis of Health Benefits Conferred by Lactobacillus Species from Kefir. Nutrients 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.U.; Nayab, H.; Shafique, F.; Williamson, M.P.; Almansouri, T.S.; Asim, N.; Shafi, N.; Attacha, S.; Khalid, M.; Ali, N.; et al. Probiotic Properties of Lactobacillus helveticus and Lactobacillus plantarum Isolated from Traditional Pakistani Yoghurt. BioMed Res. Int. 2020, 2020, 8889198. [Google Scholar] [CrossRef] [PubMed]
- Bik, E.M.; Long, C.D.; Armitage, G.C.; Loomer, P.; Emerson, J.; Mongodin, E.F.; Nelson, K.E.; Gill, S.R.; Fraser-Liggett, C.M.; Relman, D.A. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010, 4, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Wicaksono, D.P.; Washio, J.; Abiko, Y.; Domon, H.; Takahashi, N. Nitrite Production from Nitrate and Its Link with Lactate Metabolism in Oral Veillonella spp. Appl. Environ. Microbiol. 2020, 86, 86. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.M.; Chatterjee, D.; Ghosh, R. Role of probiotics in respiratory tract diseases with special reference to COVID-19: A review. Asian J. Med. Sci. 2020, 11, 64–70. [Google Scholar] [CrossRef]
- Picó-Monllor, J.A.; Ruzafa-Costas, B.; Núñez-Delegido, E.; Sánchez-Pellicer, P.; Peris-Berraco, J.; Navarro-Lopez, V. Selection of Probiotics in the Prevention of Respiratory Tract Infections and Their Impact on Occupational Health: Scoping Review. Nutrients 2021, 13, 4419. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, K.; Razi, B.; Darand, M.; Dehghani, A.; Janmohammadi, P.; Alizadeh, S. Effect of probiotic fermented dairy products on incidence of respiratory tract infections: A systematic review and meta-analysis of randomized clinical trials. Nutr. J. 2021, 20, 61. [Google Scholar] [CrossRef]
- Ling, Z.; Kong, J.; Liu, F.; Zhu, H.; Chen, X.; Wang, Y.; Li, L.; Nelson, K.E.; Xia, Y.; Xiang, C. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genom. 2010, 11, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghartey, J.P.; Smith, B.C.; Chen, Z.; Buckley, N.; Lo, Y.; Ratner, A.; Herold, B.C.; Burk, R.D. Lactobacillus crispatus Dominant Vaginal Microbiome Is Associated with Inhibitory Activity of Female Genital Tract Secretions against Escherichia coli. PLoS ONE 2014, 9, e96659. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef]
- Van Rensburg, J.J.; Lin, H.; Gao, X.; Toh, E.; Fortney, K.R.; Ellinger, S.; Zwickl, B.; Janowicz, D.M.; Katz, B.P.; Nelson, D.E.; et al. The Human Skin Microbiome Associates with the Outcome of and Is Influenced by Bacterial Infection. MBio 2015, 6, e01315-15. [Google Scholar] [CrossRef] [Green Version]
- Tsai, W.-H.; Chou, C.-H.; Chiang, Y.-J.; Lin, C.-G.; Lee, C.-H. Regulatory effects of Lactobacillus plantarum-GMNL6 on human skin health by improving skin microbiome. Int. J. Med. Sci. 2021, 18, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Delanghe, L.; Spacova, I.; Van Malderen, J.; Oerlemans, E.; Claes, I.; Lebeer, S. The role of lactobacilli in inhibiting skin pathogens. Biochem. Soc. Trans. 2021, 49, 617–627. [Google Scholar] [CrossRef]
- Fredricks, D.N.; Fiedler, T.L.; Marrazzo, J. Molecular Identification of Bacteria Associated with Bacterial Vaginosis. N. Engl. J. Med. 2005, 353, 1899–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdes, A.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, F.; Rühlemann, M.; Bang, C.; Höppner, M.; Rehman, A.; Kaleta, C.; Schmitt-Kopplin, P.; Dempfle, A.; Weidinger, S.; Ellinghaus, E.; et al. Microbiomarkers in inflammatory bowel diseases: Caveats come with caviar. Gut 2017, 66, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Roslund, M.I.; Puhakka, R.; Grönroos, M.; Nurminen, N.; Oikarinen, S.; Gazali, A.M.; Cinek, O.; Kramná, L.; Siter, N.; Vari, H.K.; et al. Biodiversity intervention enhances immune regulation and health-associated commensal microbiota among daycare children. Sci. Adv. 2020, 6, eaba2578. [Google Scholar] [CrossRef]
- Kirjavainen, P.V.; Karvonen, A.M.; Adams, R.I.; Täubel, M.; Roponen, M.; Tuoresmäki, P.; Loss, G.; Jayaprakash, B.; Depner, M.; Ege, M.J.; et al. Farm-like indoor microbiota in non-farm homes protects children from asthma development. Nat. Med. 2019, 25, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Depner, M.; PASTURE Study Group; Taft, D.H.; Kirjavainen, P.V.; Kalanetra, K.M.; Karvonen, A.M.; Peschel, S.; Schmausser-Hechfellner, E.; Roduit, C.; Frei, R.; et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat. Med. 2020, 26, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, N.; Scheu, S.; Jousset, A. Bacterial Diversity Stabilizes Community Productivity. PLoS ONE 2012, 7, e34517. [Google Scholar] [CrossRef] [Green Version]
- Bernardo-Cravo, A.P.; Schmeller, D.S.; Chatzinotas, A.; Vredenburg, V.T.; Loyau, A. Environmental Factors and Host Microbiomes Shape Host–Pathogen Dynamics. Trends Parasitol. 2020, 36, 616–633. [Google Scholar] [CrossRef]
- Kates, A.E.; Jarrett, O.; Skarlupka, J.H.; Sethi, A.; Duster, M.; Watson, L.; Suen, G.; Poulsen, K.; Safdar, N. Household Pet Ownership and the Microbial Diversity of the Human Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Sears, M.R.; Becker, A.B.; Scott, J.A.A.; Kozyrskyj, A.L.; CHILD Study Investigators. Infant gut microbiota and the hygiene hypothesis of allergic disease: Impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin. Immunol. 2013, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tun, H.M.; Konya, T.; Takaro, T.K.; Brook, J.R.; Chari, R.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; et al. Exposure to household furry pets influences the gut microbiota of infants at 3–4 months following various birth scenarios. Microbiome 2017, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haahtela, T. A biodiversity hypothesis. Allergy 2019, 74, 1445–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, C.; Gascon, M.; Osornio-Vargas, A.R.; Shier, C.; Guttman, D.S.; Becker, A.B.; Azad, M.B.; Sears, M.R.; Lefebvre, D.L.; Moraes, T.J.; et al. Natural environments in the urban context and gut microbiota in infants. Environ. Int. 2020, 142, 105881. [Google Scholar] [CrossRef] [PubMed]
- Hanski, I.; von Hertzen, L.; Fyhrquist, N.; Koskinen, K.; Torppa, K.; Laatikainen, T.; Karisola, P.; Auvinen, P.; Paulin, L.; Mäkelä, M.J.; et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. USA 2012, 109, 8334–8339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Mutius, E. The microbial environment and its influence on asthma prevention in early life. J. Allergy Clin. Immunol. 2016, 137, 680–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fall, T.; Lundholm, C.; Örtqvist, A.K.; Fall, K.; Fang, F.; Hedhammar, Å.; Kämpe, O.; Ingelsson, E.; Almqvist, C. Early Exposure to Dogs and Farm Animals and the Risk of Childhood Asthma. JAMA Pediatr. 2015, 169, e153219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dannemiller, K.C.; Mendell, M.J.; Macher, J.M.; Kumagai, K.; Bradman, A.; Holland, N.; Harley, K.; Eskenazi, B.; Peccia, J. Next-generation DNA sequencing reveals that low fungal diversity in house dust is associated with childhood asthma development. Indoor Air 2014, 24, 236–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ege, M.J.; Mayer, M.; Normand, A.-C.; Genuneit, J.; Cookson, W.O.; Braun-Fahrländer, C.; Heederik, D.; Piarroux, R.; von Mutius, E. Exposure to Environmental Microorganisms and Childhood Asthma. N. Engl. J. Med. 2011, 364, 701–709. [Google Scholar] [CrossRef]
- Tischer, C.; Weikl, F.; Probst, A.; Standl, M.; Heinrich, J.; Pritsch, K. Urban Dust Microbiome: Impact on Later Atopy and Wheezing. Environ. Health Perspect. 2016, 124, 1919–1923. [Google Scholar] [CrossRef] [Green Version]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Flies, E.J.; Skelly, C.; Lovell, R.; Breed, M.; Phillips, D.; Weinstein, P. Cities, biodiversity and health: We need healthy urban microbiome initiatives. Cities Health 2018, 2, 143–150. [Google Scholar] [CrossRef]
- Mosca, A.; Leclerc, M.; Hugot, J.-P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef] [Green Version]
- Biasucci, G.; Rubini, M.; Riboni, S.; Morelli, L.; Bessi, E.; Retetangos, C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum. Dev. 2010, 86 (Suppl. S1), 13–15. [Google Scholar] [CrossRef]
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nature 2015, 528, 69–76. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; Lopez-Contreras, B.E.; Villarruel-Vazquez, R.; Ocampo-Medina, E.; Macias-Kauffer, L.; Martinez-Medina, J.N.; Villamil-Ramirez, H.; León-Mimila, P.; Del Rio-Navarro, B.E.; Ibarra-Gonzalez, I.; et al. Environmental and intrinsic factors shaping gut microbiota composition and diversity and its relation to metabolic health in children and early adolescents: A population-based study. Gut Microbes 2020, 11, 900–917. [Google Scholar] [CrossRef]
- De la Cuesta-Zuluaga, J.; Kelley, S.T.; Chen, Y.; Escobar, J.S.; Mueller, N.T.; Ley, R.E.; McDonald, D.; Huang, S.; Swafford, A.D.; Knight, R.; et al. Age- and Sex-Dependent Patterns of Gut Microbial Diversity in Human Adults. Msystems 2019, 4, e00261-19. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Chen, W.; Liu, S.; Wu, J.; Zhu, Y.; Qin, L.; Zhu, B. Beneficial Relationships Between Endophytic Bacteria and Medicinal Plants. Front. Plant Sci. 2021, 12, 646146. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, T.-Y.; He, Y.; Gou, W.; Zuo, L.-S.-Y.; Fu, Y.; Miao, Z.; Shuai, M.; Xu, F.; Xiao, C.; et al. Dietary fruit and vegetable intake, gut microbiota, and type 2 diabetes: Results from two large human cohort studies. BMC Med. 2020, 18, 371. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.J.; Bates, K.A.; King, K.C. Host microbiota can facilitate pathogen infection. PLOS Pathog. 2021, 17, e1009514. [Google Scholar] [CrossRef] [PubMed]
- Taştan, R.; Can, A.A. One health approach to decreasing biodiversity and the problem of emerging zoonotic diseases. Biol. Divers. Conserv. 2019, 12, 95–102. [Google Scholar] [CrossRef]
- Trinh, P.; Zaneveld, J.R.; Safranek, S.; Rabinowitz, P.M. One Health Relationships Between Human, Animal, and Environmental Microbiomes: A Mini-Review. Front. Public Health 2018, 6, 235. [Google Scholar] [CrossRef] [PubMed]
| Body Sites | Common Phyla | Common Genera | Positive Effects of Beneficial Genera |
|---|---|---|---|
| Gut [39] | Actinobacteria | Corynebacterium * | |
| Bifidobacterium | Stimulates immune system, Gut homeostasis, Protection against gastrointestinal infection [40,41,42,43,44], Protective role in TNF-α induced inflammatory response [45]. | ||
| Atopobium | |||
| Firmicutes | Faecalibacterium | Prevention of Inflammatory bowel disease and colorectal cancer, Protection of colon, control of metabolism [46], Immune response/balancing immunity in intestine [46,47]. | |
| Clostridium * | |||
| Roseburia | Immunity maintenance, Anti-inflammatory response [48,49,50]. | ||
| Ruminococcus | |||
| Dialister | |||
| Lactobacillus | Anti-microbial activity [51,52], Cholesterol metabolism, immunomodulation, anti-allergic effects, anti-diabetic effects [51]. | ||
| Enterococcus * | |||
| Staphylococcus | |||
| Bacteroidetes | Sphingobacterium | ||
| Bacteroides * | |||
| Tannerella | |||
| Parabacteroides | |||
| Alistipes | |||
| Prevotella | |||
| Proteobacteria | Escherichia | ||
| Shigella | |||
| Desulfovibrio | |||
| Bilophila | |||
| Helicobacter | |||
| Fusobacteria | Fusobacterium | ||
| Verrucomicrobia | Akkermansia * | ||
| Oral cavity [53] | Actinobacteria | Actinomyces | |
| Atopobium | |||
| Corynebacterium * | |||
| Rothia | |||
| Proteobacteria | Campylobacter | ||
| Haemophilus | |||
| Neisseria | |||
| Bacteroidetes | Bergeyella | ||
| Capnocytophaga | |||
| Prevotella | |||
| Firmicutes | Granulicatella | ||
| Streptococcus | |||
| Veillonella | Lactate metabolism, NO2 production, Maintain oral health and general health [54] | ||
| Saccharibacteria | |||
| Fusobacteria | Fusobacterium | ||
| Respiratory tract [25,33] | Actinobacteria | Corynebacterium * | |
| Cutibacterium | |||
| Bifidobacterium | Reduction in respiratory tract infections [55,56,57] Reduces the colonization of pathogenic bacteria [55] | ||
| Rothia | |||
| Firmicutes | Dolosigranulum | ||
| Staphylococcus | |||
| Veillonella * | |||
| Lachnospiraceae | |||
| Streptococcus | |||
| Bacteriodetes | Prevotella | ||
| Fusobacteria | |||
| Proteobacteria | |||
| Vagina [58] | Actinobacteria | Gardnerella | |
| Atopobium | |||
| Eggerthella | |||
| Firmicutes | Alloiococcus | ||
| Papillibacter | |||
| Megasphaera | |||
| Aerococcus | |||
| Lactobacillus | Immunomodulation and restoration of healthy microflora in the vagina, The first line of defense against vaginal pathogens [59,60]. | ||
| Streptococcus | |||
| Bacteroidetes | Prevotella | ||
| Fusobacteria | |||
| Skin [61] | Actinobacteria | Propionibacterium | |
| Corynebacterium | |||
| Micrococcus | |||
| Mycobacterium | |||
| Kocuria | |||
| Rothia | |||
| Firmicutes | Staphylococcus | ||
| Streptococcus | |||
| Lactobacillus | Improves skin moisture, color, texture, pores, wrinkles, UV spots, and brown spots [62] Antipathogenic function [63] | ||
| Finegoldia | |||
| Aerococcus | |||
| Anaerococcus | |||
| Proteobacteria | Paracoccus | ||
| Haematobcter | |||
| Sphingomonas | |||
| Hemophilus | |||
| Bacteroidetes | Flavobacterium | ||
| Prevotella |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panthee, B.; Gyawali, S.; Panthee, P.; Techato, K. Environmental and Human Microbiome for Health. Life 2022, 12, 456. https://doi.org/10.3390/life12030456
Panthee B, Gyawali S, Panthee P, Techato K. Environmental and Human Microbiome for Health. Life. 2022; 12(3):456. https://doi.org/10.3390/life12030456
Chicago/Turabian StylePanthee, Bimala, Saroj Gyawali, Pratiksha Panthee, and Kuaanan Techato. 2022. "Environmental and Human Microbiome for Health" Life 12, no. 3: 456. https://doi.org/10.3390/life12030456
APA StylePanthee, B., Gyawali, S., Panthee, P., & Techato, K. (2022). Environmental and Human Microbiome for Health. Life, 12(3), 456. https://doi.org/10.3390/life12030456







