Fatty Acid-Binding Proteins 4 and 5 Are Involved in the Pathogenesis of Retinal Vascular Diseases in Different Manners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Biochemical Measurements
2.3. Other Analytical Methods
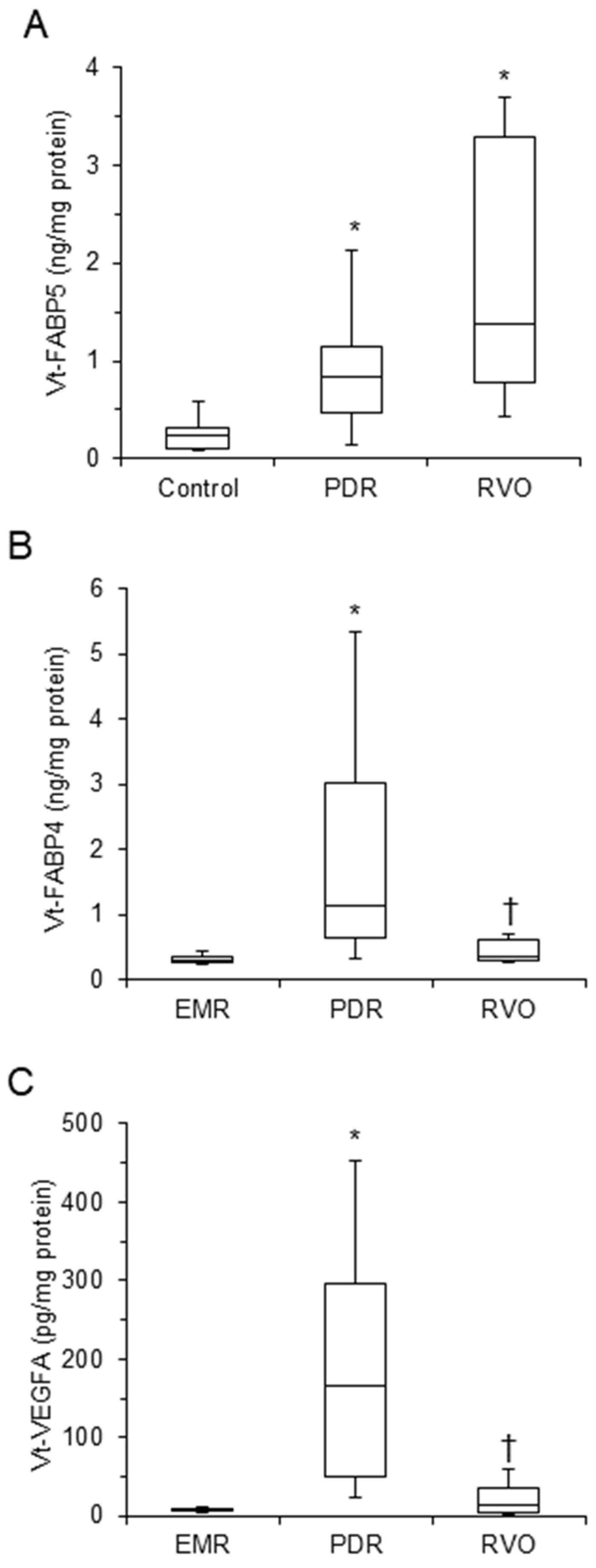
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotamisligil, G.S.; Bernlohr, D.A. Metabolic functions of FABPs--mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 2015, 11, 592–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuhashi, M. Fatty Acid-Binding Protein 4 in Cardiovascular and Metabolic Diseases. J. Atheroscler. Thromb. 2019, 26, 216–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotamisligil, G.S.; Johnson, R.S.; Distel, R.J.; Ellis, R.; Papaioannou, V.E.; Spiegelman, B.M. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science 1996, 274, 1377–1379. [Google Scholar] [CrossRef]
- Makowski, L.; Boord, J.B.; Maeda, K.; Babaev, V.R.; Uysal, K.T.; Morgan, M.A.; Parker, R.A.; Suttles, J.; Fazio, S.; Hotamisligil, G.S.; et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 2001, 7, 699–705. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Uysal, K.T.; Makowski, L.; Görgün, C.Z.; Atsumi, G.; Parker, R.A.; Brüning, J.; Hertzel, A.V.; Bernlohr, D.A.; Hotamisligil, G.S. Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes 2003, 52, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Babaev, V.R.; Runner, R.P.; Fan, D.; Ding, L.; Zhang, Y.; Tao, H.; Erbay, E.; Görgün, C.Z.; Fazio, S.; Hotamisligil, G.S.; et al. Macrophage Mal1 deficiency suppresses atherosclerosis in low-density lipoprotein receptor-null mice by activating peroxisome proliferator-activated receptor-γ-regulated genes. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1283–1290. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Cao, H.; Kono, K.; Gorgun, C.Z.; Furuhashi, M.; Uysal, K.T.; Cao, Q.; Atsumi, G.; Malone, H.; Krishnan, B.; et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005, 1, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Furuhashi, M.; Fucho, R.; Görgün, C.Z.; Tuncman, G.; Cao, H.; Hotamisligil, G.S. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J. Clin. Investig. 2008, 118, 2640–2650. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.; Wang, Y.; Xu, J.Y.; Stejskal, D.; Tam, S.; Zhang, J.; Wat, N.M.; Wong, W.K.; Lam, K.S. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin. Chem. 2006, 52, 405–413. [Google Scholar] [CrossRef]
- Ishimura, S.; Furuhashi, M.; Watanabe, Y.; Hoshina, K.; Fuseya, T.; Mita, T.; Okazaki, Y.; Koyama, M.; Tanaka, M.; Akasaka, H.; et al. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS ONE 2013, 8, e81318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, H.; Furuhashi, M.; Ishimura, S.; Koyama, M.; Okazaki, Y.; Mita, T.; Fuseya, T.; Yamashita, T.; Tanaka, M.; Yoshida, H.; et al. Elevation of fatty acid-binding protein 4 is predisposed by family history of hypertension and contributes to blood pressure elevation. Am. J. Hypertens. 2012, 25, 1124–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabré, A.; Lázaro, I.; Girona, J.; Manzanares, J.M.; Marimón, F.; Plana, N.; Heras, M.; Masana, L. Plasma fatty acid binding protein 4 is associated with atherogenic dyslipidemia in diabetes. J. Lipid Res. 2008, 49, 1746–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, D.C.; Xu, A.; Cheung, C.W.; Wat, N.M.; Yau, M.H.; Fong, C.H.; Chau, M.T.; Lam, K.S. Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1796–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, M.; Ma, L.; Fu, P. Role of Fatty Acid Binding Protein 4 (FABP4) in Kidney Disease. Curr. Med. Chem. 2020, 27, 3657–3664. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Matsumoto, M.; Murase, T.; Nakamura, T.; Higashiura, Y.; Koyama, M.; Tanaka, M.; Moniwa, N.; Ohnishi, H.; Saitoh, S.; et al. Independent links between plasma xanthine oxidoreductase activity and levels of adipokines. J. Diabetes Investig. 2019, 10, 1059–1067. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Calvo, R.; Girona, J.; Alegret, J.M.; Bosquet, A.; Ibarretxe, D.; Masana, L. Role of the fatty acid-binding protein 4 in heart failure and cardiovascular disease. J. Endocrinol. 2017, 233, R173–R184. [Google Scholar] [CrossRef]
- Yeung, D.C.; Wang, Y.; Xu, A.; Cheung, S.C.; Wat, N.M.; Fong, D.Y.; Fong, C.H.; Chau, M.T.; Sham, P.C.; Lam, K.S. Epidermal fatty-acid-binding protein: A new circulating biomarker associated with cardio-metabolic risk factors and carotid atherosclerosis. Eur. Heart J. 2008, 29, 2156–2163. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, R.; Qasim, A.N.; Mehta, N.N.; Terembula, K.; Kapoor, S.; Braunstein, S.; Schutta, M.; Iqbal, N.; Lehrke, M.; Reilly, M.P. Relation of plasma fatty acid binding proteins 4 and 5 with the metabolic syndrome, inflammation and coronary calcium in patients with type-2 diabetes mellitus. Am. J. Cardiol. 2010, 106, 1118–1123. [Google Scholar] [CrossRef] [Green Version]
- Itoh, K.; Furuhashi, M.; Ida, Y.; Ohguro, H.; Watanabe, M.; Suzuki, S.; Hikage, F. Detection of significantly high vitreous concentrations of fatty acid-binding protein 4 in patients with proliferative diabetic retinopathy. Sci. Rep. 2021, 11, 12382. [Google Scholar] [CrossRef]
- Hikage, F.; Furuhashi, M.; Ida, Y.; Ohguro, H.; Watanabe, M.; Suzuki, S.; Itoh, K. Fatty acid-binding protein 4 is an independent factor in the pathogenesis of retinal vein occlusion. PLoS ONE 2021, 16, e0245763. [Google Scholar] [CrossRef] [PubMed]
- Kingma, P.B.; Bok, D.; Ong, D.E. Bovine epidermal fatty acid-binding protein: Determination of ligand specificity and cellular localization in retina and testis. Biochemistry 1998, 37, 3250–3257. [Google Scholar] [CrossRef] [PubMed]
- Shinzawa, M.; Dogru, M.; Den, S.; Ichijima, T.; Higa, K.; Kojima, T.; Seta, N.; Nomura, T.; Tsubota, K.; Shimazaki, J. Epidermal Fatty Acid-Binding Protein: A Novel Marker in the Diagnosis of Dry Eye Disease in Sjögren Syndrome. Int. J. Mol. Sci. 2018, 19, 3463. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, T.; Araie, M.; Riva, C.E.; Schmetterer, L.; Orgul, S. Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol. 2010, 88, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Suzuma, K.; Matsumoto, M.; Tsuiki, E.; Fujikawa, A.; Harada, T.; Kitaoka, T. Retinal blood flow correlates to aqueous vascular endothelial growth factor in central retinal vein occlusion. Retina 2015, 35, 2037–2042. [Google Scholar] [CrossRef] [Green Version]
- Isono, H.; Kishi, S.; Kimura, Y.; Hagiwara, N.; Konishi, N.; Fujii, H. Observation of choroidal circulation using index of erythrocytic velocity. Arch Ophthalmol. 2003, 121, 225–231. [Google Scholar] [CrossRef]
- Hendrick, A.M.; Gibson, M.V.; Kulshreshtha, A. Diabetic Retinopathy. Prim. Care 2015, 42, 451–464. [Google Scholar] [CrossRef]
- Song, P.; Xu, Y.; Zha, M.; Zhang, Y.; Rudan, I. Global epidemiology of retinal vein occlusion: A systematic review and meta-analysis of prevalence, incidence, and risk factors. J. Glob. Health 2019, 9, 010427. [Google Scholar] [CrossRef]
- Rogers, S.; McIntosh, R.L.; Cheung, N.; Lim, L.; Wang, J.J.; Mitchell, P.; Kowalski, J.W.; Nguyen, H.; Wong, T.Y. The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010, 117, 313–319.e1. [Google Scholar] [CrossRef] [Green Version]
- Green, W.R.; Chan, C.C.; Hutchins, G.M.; Terry, J.M. Central retinal vein occlusion: A prospective histopathologic study of 29 eyes in 28 cases. Retina 1981, 1, 27–55. [Google Scholar] [CrossRef]
- Bertelsen, M.; Linneberg, A.; Christoffersen, N.; Vorum, H.; Gade, E.; Larsen, M. Mortality in patients with central retinal vein occlusion. Ophthalmology 2014, 121, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Elman, M.J.; Bhatt, A.K.; Quinlan, P.M.; Enger, C. The risk for systemic vascular diseases and mortality in patients with central retinal vein occlusion. Ophthalmology 1990, 97, 1543–1548. [Google Scholar] [CrossRef]
- Jonas, J.B.; Monés, J.; Glacet-Bernard, A.; Coscas, G. Retinal Vein Occlusions. Dev. Ophthalmol. 2017, 58, 139–167. [Google Scholar] [PubMed] [Green Version]
- Semeraro, F.; Morescalchi, F.; Cancarini, A.; Russo, A.; Rezzola, S.; Costagliola, C. Diabetic retinopathy, a vascular and inflammatory disease: Therapeutic implications. Diabetes Metab. 2019, 45, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Wirkkala, J.; Bloigu, R.; Hautala, N.M. Intravitreal bevacizumab improves the clearance of vitreous haemorrhage and visual outcomes in patients with proliferative diabetic retinopathy. BMJ Open Ophthalmol. 2019, 4, e000390. [Google Scholar] [CrossRef] [Green Version]
- Tadayoni, R.; Waldstein, S.M.; Boscia, F.; Gerding, H.; Gekkieva, M.; Barnes, E.; Das Gupta, A.; Wenzel, A.; Pearce, I. Sustained Benefits of Ranibizumab with or without Laser in Branch Retinal Vein Occlusion: 24-Month Results of the Brighter Study. Ophthalmology 2017, 124, 1778–1787. [Google Scholar] [CrossRef] [Green Version]
- Clark, W.L.; Boyer, D.S.; Heier, J.S.; Brown, D.M.; Haller, J.A.; Vitti, R.; Kazmi, H.; Berliner, A.J.; Erickson, K.; Chu, K.W.; et al. Intravitreal Aflibercept for Macular Edema Following Branch Retinal Vein Occlusion: 52-Week Results of the VIBRANT Study. Ophthalmology 2016, 123, 330–336. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Heier, J.S.; Feiner, L.; Gray, S.; Saroj, N.; Rundle, A.C.; Murahashi, W.Y.; Rubio, R.G. Ranibizumab for macular edema following branch retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology 2010, 117, 1102–1112.e1. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Lasave, A.F.; Wu, L.; Maia, M.; Diaz-Llopis, M.; Alezzandrini, A.A.; Brito, M. Intravitreal bevacizumab for proliferative diabetic retinopathy: Results From the Pan-American Collaborative Retina Study Group (PACORES) at 24 Months of Follow-up. Retina 2017, 37, 334–343. [Google Scholar] [CrossRef]
- Figueira, J.; Fletcher, E.; Massin, P.; Silva, R.; Bandello, F.; Midena, E.; Varano, M.; Sivaprasad, S.; Eleftheriadis, H.; Menon, G.; et al. Ranibizumab Plus Panretinal Photocoagulation versus Panretinal Photocoagulation Alone for High-Risk Proliferative Diabetic Retinopathy (PROTEUS Study). Ophthalmology 2018, 125, 691–700. [Google Scholar] [CrossRef]
- Achiron, A.; Lagstein, O.; Glick, M.; Gur, Z.; Bartov, E.; Burgansky-Eliash, Z. Quantifying metamorphopsia in patients with diabetic macular oedema and other macular abnormalities. Acta Ophthalmol. 2015, 93, e649–e653. [Google Scholar] [CrossRef] [PubMed]
- Manabe, K.; Tsujikawa, A.; Osaka, R.; Nakano, Y.; Fujita, T.; Shiragami, C.; Hirooka, K.; Uji, A.; Muraoka, Y. Metamorphopsia Associated with Branch Retinal Vein Occlusion. PLoS ONE 2016, 11, e0153817. [Google Scholar] [CrossRef] [PubMed]
- Osaka, R.; Manabe, K.; Manabe, S.; Nakano, Y.; Takasago, Y.; Shiragami, C.; Hirooka, K.; Muraoka, Y.; Tsujikawa, A. Persistent metamorphopsia associated with branch retinal vein occlusion. PLoS ONE 2018, 13, e0204015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiura, Y.; Okamoto, F.; Morikawa, S.; Okamoto, Y.; Hiraoka, T.; Oshika, T. Time course of changes in metamorphopsia following intravitreal ranibizumab injection for branch retinal vein occlusion. Retina 2018, 38, 1581–1587. [Google Scholar] [CrossRef]
- Adamson, J.; Morgan, E.A.; Beesley, C.; Mei, Y.; Foster, C.S.; Fujii, H.; Rudland, P.S.; Smith, P.H.; Ke, Y. High-level expression of cutaneous fatty acid-binding protein in prostatic carcinomas and its effect on tumorigenicity. Oncogene 2003, 22, 2739–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forootan, F.S.; Forootan, S.S.; Gou, X.; Yang, J.; Liu, B.; Chen, D.; Al Fayi, M.S.; Al-Jameel, W.; Rudland, P.S.; Hussain, S.A.; et al. Fatty acid activated PPARγ promotes tumorigenicity of prostate cancer cells by up regulating VEGF via PPAR responsive elements of the promoter. Oncotarget 2016, 7, 9322–9339. [Google Scholar] [CrossRef] [Green Version]
- Akanuma, S.; Hirose, S.; Tachikawa, M.; Hosoya, K. Localization of organic anion transporting polypeptide (Oatp) 1a4 and Oatp1c1 at the rat blood-retinal barrier. Fluids Barriers CNS 2013, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Scanlon, M.J.; Owada, Y.; Yamamoto, Y.; Porter, C.J.; Nicolazzo, J.A. Fatty Acid-Binding Protein 5 Facilitates the Blood-Brain Barrier Transport of Docosahexaenoic Acid. Mol. Pharm. 2015, 12, 4375–4385. [Google Scholar] [CrossRef]
- Mitchell, R.W.; On, N.H.; Del Bigio, M.R.; Miller, D.W.; Hatch, G.M. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J. Neurochem. 2011, 117, 735–746. [Google Scholar] [CrossRef]
- Elmasri, H.; Ghelfi, E.; Yu, C.W.; Traphagen, S.; Cernadas, M.; Cao, H.; Shi, G.P.; Plutzky, J.; Sahin, M.; Hotamisligil, G.; et al. Endothelial cell-fatty acid binding protein 4 promotes angiogenesis: Role of stem cell factor/c-kit pathway. Angiogenesis 2012, 15, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Elmasri, H.; Karaaslan, C.; Teper, Y.; Ghelfi, E.; Weng, M.; Ince, T.A.; Kozakewich, H.; Bischoff, J.; Cataltepe, S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009, 23, 3865–3873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.Y.; Wang, Y.; Vanhoutte, P.M. Senescence of cultured porcine coronary arterial endothelial cells is associated with accelerated oxidative stress and activation of NFkB. J. Vasc. Res. 2010, 47, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Fuseya, T.; Furuhashi, M.; Matsumoto, M.; Watanabe, Y.; Hoshina, K.; Mita, T.; Ishimura, S.; Tanaka, M.; Miura, T. Ectopic Fatty Acid-Binding Protein 4 Expression in the Vascular Endothelium is Involved in Neointima Formation After Vascular Injury. J. Am. Heart Assoc. 2017, 6, e006377. [Google Scholar] [CrossRef] [PubMed]

| Total | Control | PDR | RVO | p | |
|---|---|---|---|---|---|
| n | 48 | 18 | 20 | 10 | |
| Sex (Male/Female) | 19/29 | 6/12 | 10/10 | 3/7 | 0.453 |
| Age (years) | 65 ± 10 | 68 ± 8 | 62 ± 9 | 68 ± 15 | 0.210 |
| Body mass index | 23.8 ± 3.6 | 23.2 ± 3.4 | 24.0 ± 4.3 | 24.6 ± 2.7 | 0.592 |
| Systolic blood pressure (mmHg) | 137 ± 20 | 137 ± 17 | 137 ± 25 | 139 ± 18 | 0.965 |
| Diastolic blood pressure (mmHg) | 78 ± 11 | 80 ± 10 | 75 ± 12 | 82 ± 11 | 0.201 |
| Biochemical data | |||||
| Total cholesterol (mg/dL) | 201 ± 38 | 208 ± 40 | 195 ± 43 | 202 ± 18 | 0.545 |
| Triglycerides (mg/dL) | 149 (104–221) | 120 (96–222) | 157 (136–214) | 186 (9120–230) | 0.264 |
| Fasting glucose (mg/dL) | 126 (105–168) | 115 (101–147) | 167 (140–184) * | 111 (95–122) † | 0.011 |
| Hemoglobin A1c (%) | 6.4 ± 1.0 | 6.1 ± 0.9 | 6.9 ± 1.1 * | 6.0 ± 0.5 † | 0.006 |
| Blood urea nitrogen (mg/dL) | 17 ± 9 | 15 ± 4 | 21 ± 12 | 13 ± 4 † | 0.018 |
| Creatinine (mg/dL) | 0.7 (0.6–0.9) | 0.7 (0.6–0.8) | 0.8 (0.6–1.0) | 0.7 (0.6–0.8) | 0.275 |
| eGFR (mL/min/1.73 m2) | 67.9 ± 24.6 | 71.0 ± 17.4 | 63.3 ± 32.5 | 71.5 ± 16.9 | 0.562 |
| Uric acid (mg/dL) | 5.4 ± 1.3 | 5.3 ± 1.2 | 5.6 ± 1.3 | 4.9 ± 1.4 | 0.323 |
| AST (IU/L) | 21 (16–27) | 26 (20–33) | 17 (14–22) * | 24 (19–33) † | 0.016 |
| ALT (IU/L) | 20 (14–26) | 24 (16–29) | 16 (11–21) | 21 (15–30) | 0.071 |
| γGTP (IU/L) | 27 (16–51) | 26 (15–61) | 21 (15–45) | 36 (25–57) | 0.193 |
| hsCRP (mg/dL) | 0.06 (0.04–0.13) | 0.06 (0.04–0.12) | 0.05 (0.03–0.12) | 0.10 (0.05–0.17) | 0.488 |
| Vt–FABP4 (ng/mg protein) | 0.50 (0.30–0.98) | 0.30 (0.26–0.35) | 1.14 (0.65–3.03) * | 0.36 (0.30–0.61) † | <0.001 |
| Vt–FABP5 (ng/mg protein) | 0.24 (0.12–0.48) | 0.24 (0.11–0.32) | 0.84 (0.47–1.14) * | 1.38 (0.77–3.29) * | <0.001 |
| Vt–VEGFA (pg/mg protein) | 18.6 (6.2–111.5) | 6.8 (5.8––8.4) | 166.4 (50.3–295.1) * | 12.9 (3.6–35.2) † | <0.001 |
| Log Vt-FABP5 | Log Vt-FABP4 | Log Vt-VEGFA | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| M(A) | −0.57 | <0.001 | −0.43 | 0.007 | −0.35 | 0.032 |
| M(V) | −0.62 | <0.001 | −0.48 | 0.003 | −0.35 | 0.035 |
| M(T) | −0.37 | 0.026 | −0.14 | 0.399 | −0.13 | 0.438 |
| M(V)-M(T) | −0.62 | <0.001 | −0.53 | 0.001 | −0.37 | 0.023 |
| M(M) | −0.34 | 0.037 | −0.08 | 0.639 | −0.20 | 0.231 |
| Log Vt-FABP5 | Log Vt-FABP4 | Log Vt-VEGFA | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age | −0.11 | 0.449 | −0.28 | 0.051 | −0.24 | 0.096 |
| Body mass index | 0.09 | 0.564 | 0.13 | 0.396 | −0.04 | 0.767 |
| Systolic blood pressure | 0.13 | 0.375 | 0.04 | 0.807 | −0.01 | 0.958 |
| Diastolic blood pressure | 0.01 | 0.966 | −0.24 | 0.101 | −0.18 | 0.221 |
| Biochemical data | ||||||
| Log AST | −0.15 | 0.315 | −0.31 | 0.032 | −0.42 | 0.003 |
| Log ALT | 0.04 | 0.764 | −0.17 | 0.236 | −0.24 | 0.095 |
| Log γGTP | 0.14 | 0.355 | −0.16 | 0.265 | −0.14 | 0.329 |
| BUN | −0.03 | 0.864 | 0.26 | 0.072 | 0.24 | 0.102 |
| Log Creatinine | 0.11 | 0.457 | 0.34 | 0.019 | 0.22 | 0.141 |
| eGFR | −0.08 | 0.612 | −0.24 | 0.107 | −0.07 | 0.648 |
| Uric acid | 0.00 | 0.978 | 0.12 | 0.421 | 0.24 | 0.107 |
| Total cholesterol | −0.13 | 0.388 | −0.03 | 0.825 | −0.11 | 0.451 |
| Log Triglycerides | 0.31 | 0.031 | 0.20 | 0.178 | 0.23 | 0.116 |
| Log Fasting glucose | 0.23 | 0.111 | 0.33 | 0.023 | 0.39 | 0.007 |
| Hemoglobin A1c | 0.28 | 0.055 | 0.26 | 0.070 | 0.44 | 0.002 |
| Log hsCRP | 0.23 | 0.118 | 0.10 | 0.516 | 0.07 | 0.643 |
| Vitreous humor | ||||||
| Log Vt-FABP4 | 0.38 | 0.008 | - | - | 0.68 | <0.001 |
| Log Vt-VEGFA | 0.35 | 0.015 | 0.68 | <0.001 | - | - |
| Log Vt-FABP5 | - | - | 0.38 | 0.008 | 0.35 | 0.015 |
| Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |||
| Age | −0.054 | 0.687 | Age | −0.037 | 0.793 | Age | −0.021 | 0.878 |
| Sex (Male) | 0.146 | 0.298 | Sex (Male) | 0.189 | 0.238 | Sex (Male) | 0.186 | 0.242 |
| M(V)-M(T) | −0.583 | <0.001 | M(V)-M(T) | −0.501 | 0.006 | M(V)-M(T) | −0.488 | 0.008 |
| Log Vt-FABP4 | 0.174 | 0.432 | Log Vt-FABP4 | 0.176 | 0.425 | |||
| Log Vt-VEGFA | −0.054 | 0.786 | Log Vt-VEGFA | −0.096 | 0.636 | |||
| Log Triglycerides | 0.171 | 0.236 | ||||||
| (R2 = 0.412, AIC = 92) | (R2 = 0.443, AIC = 97) | (R2 = 0.452, AIC = 98) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashide, M.; Furuhashi, M.; Watanabe, M.; Itoh, K.; Suzuki, S.; Umetsu, A.; Tsugeno, Y.; Ida, Y.; Hikage, F.; Ohguro, H. Fatty Acid-Binding Proteins 4 and 5 Are Involved in the Pathogenesis of Retinal Vascular Diseases in Different Manners. Life 2022, 12, 467. https://doi.org/10.3390/life12040467
Higashide M, Furuhashi M, Watanabe M, Itoh K, Suzuki S, Umetsu A, Tsugeno Y, Ida Y, Hikage F, Ohguro H. Fatty Acid-Binding Proteins 4 and 5 Are Involved in the Pathogenesis of Retinal Vascular Diseases in Different Manners. Life. 2022; 12(4):467. https://doi.org/10.3390/life12040467
Chicago/Turabian StyleHigashide, Megumi, Masato Furuhashi, Megumi Watanabe, Kaku Itoh, Soma Suzuki, Araya Umetsu, Yuri Tsugeno, Yosuke Ida, Fumihito Hikage, and Hiroshi Ohguro. 2022. "Fatty Acid-Binding Proteins 4 and 5 Are Involved in the Pathogenesis of Retinal Vascular Diseases in Different Manners" Life 12, no. 4: 467. https://doi.org/10.3390/life12040467
APA StyleHigashide, M., Furuhashi, M., Watanabe, M., Itoh, K., Suzuki, S., Umetsu, A., Tsugeno, Y., Ida, Y., Hikage, F., & Ohguro, H. (2022). Fatty Acid-Binding Proteins 4 and 5 Are Involved in the Pathogenesis of Retinal Vascular Diseases in Different Manners. Life, 12(4), 467. https://doi.org/10.3390/life12040467







