Self-Assembled Nanomicellar Formulation of Docetaxel as a Potential Breast Cancer Chemotherapeutic System
Abstract
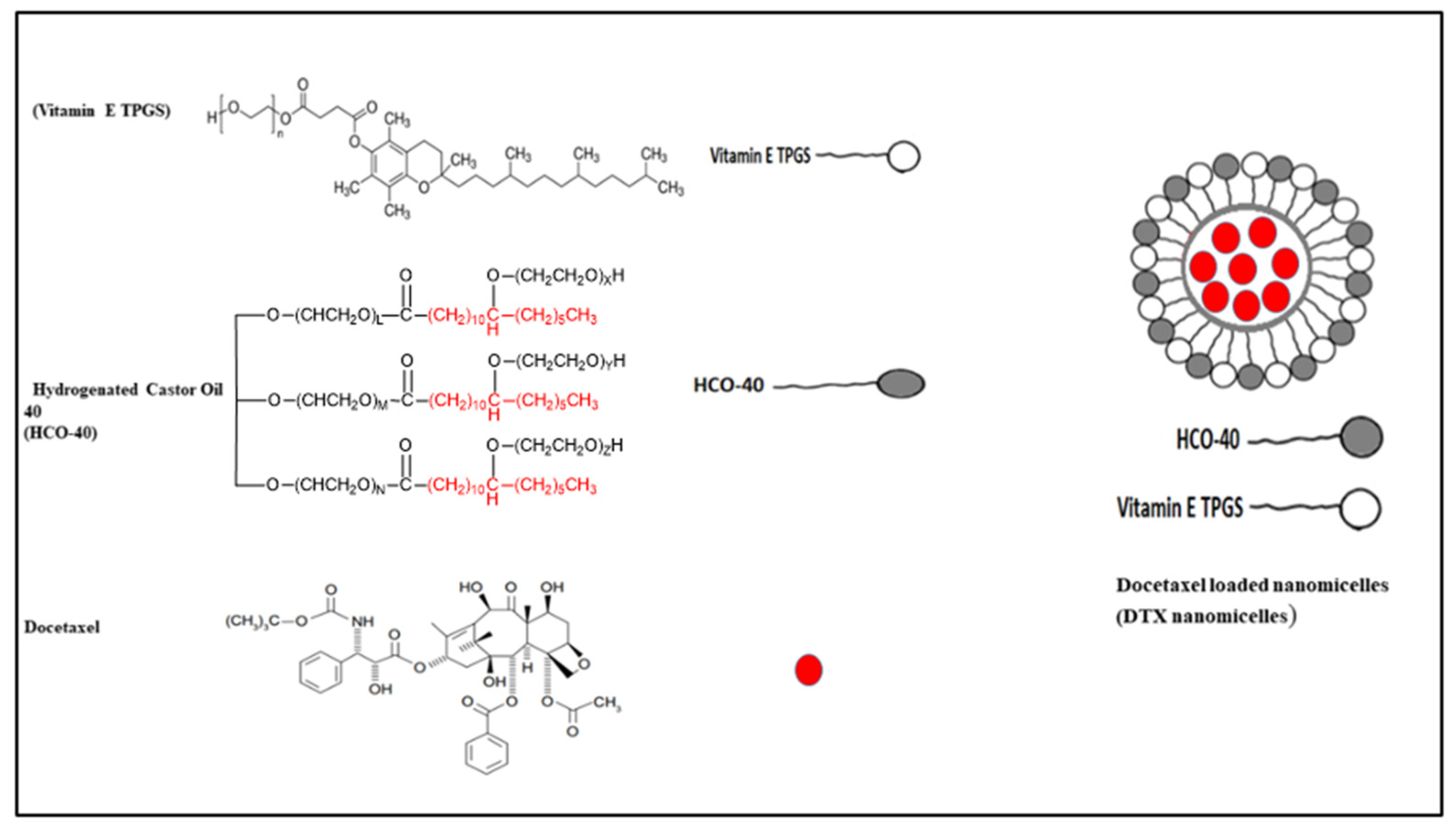
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Preparation of DTX Loaded Nanomicelles
2.3. Characterization of the Synthesized DTX Loaded Nanomicellar Formulations with 1H NMR, FT–IR, and XRD
2.4. Size, PDI, and Zeta Potential
2.5. Entrapment Efficiency and Drug Loading
2.6. In Vitro Release of Docetaxel from the DTX Nanomicelles
2.7. Dilution Stability of DTX Nanomicellar Formulation
2.8. In Vitro Cytotoxicity: MTS Assay
2.9. Liquid Chromatography (LC)–Tandem Mass Spectrometry (LC–MS/MS)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Synthesized Nanomicelles Loaded with DTX with 1H NMR, FT–IR, and XRD
3.2. Size, PDI, Zeta Potential, and Surface Morphology
3.3. Entrapment Efficiency and Drug Loading
3.4. Dilution Stability of DTX Nanomicellar Formulation
3.5. In Vitro Release of Docetaxel from the Nanomicellar Formulation
3.6. In Vitro Cell Viability Assay against MCF-7 Cell Line
3.7. LC–MS/MS Analysis for Chemical Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiang, L.; Song, Z.; Rong, G. Taxotere-induced WNT16 Expression in Carcinoma-Associated Fibroblasts Might Associate with Progression and Chemoresistance of Breast Cancer. Ann. Clin. Lab. Sci. 2020, 50, 205–212. [Google Scholar]
- Xu, M.; Yao, C.; Zhang, W.; Gao, S.; Zou, H.; Gao, J. Erratum: Anti-Cancer Activity Based on the High Docetaxel Loaded Poly(2-Oxazoline)s Micelles [Corrigendum]. Int. J. Nanomed. 2021, 16, 4675. [Google Scholar] [CrossRef] [PubMed]
- Babasaki, T.; Sentani, K.; Sekino, Y.; Kobayashi, G.; Thang Pham, Q.; Katsuya, N.; Akabane, S.; Taniyama, D.; Hayashi, T.; Shiota, M.; et al. Overexpression of claspin promotes docetaxel resistance and is associated with prostate-specific antigen recurrence in prostate cancer. Cancer Med. 2021, 10, 5574–5588. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zhang, Q.; Fang, S.; Han, X.; Wang, Z. Anti-tumor activity of high-dose EGFR tyrosine kinase inhibitor and sequential docetaxel in wild type EGFR non-small cell lung cancer cell nude mouse xenografts. Oncotarget 2017, 8, 9134–9143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nader, R.; El Amm, J.; Aragon-Ching, J.B. Role of chemotherapy in prostate cancer. Asian J. Androl. 2018, 20, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Varnai, R.; Koskinen, L.M.; Mäntylä, L.E.; Szabo, I.; FitzGerald, L.M.; Sipeky, C. Pharmacogenomic Biomarkers in Docetaxel Treatment of Prostate Cancer: From Discovery to Implementation. Genes 2019, 10, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuweboer, A.J.; de Morrée, E.S.; de Graan, A.J.; Sparreboom, A.; de Wit, R.; Mathijssen, R.H. Inter-patient variability in docetaxel pharmacokinetics: A review. Cancer Treat. Rev. 2015, 41, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Gligorov, J.; Lotz, J.P. Preclinical pharmacology of the taxanes: Implications of the differences. Oncologist 2004, 9 (Suppl. 2), 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreis, K.; Horenkamp-Sonntag, D.; Schneider, U.; Zeidler, J.; Glaeske, G.; Weissbach, L. Safety and survival of docetaxel and cabazitaxel in metastatic castration-resistant prostate cancer. BJU Int. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.Y.; Mackey, J.R. Presentation and management of docetaxel-related adverse effects in patients with breast cancer. Cancer Manag. Res. 2014, 6, 253–259. [Google Scholar] [CrossRef]
- Alsalhi, A.; Ayon, N.J.; Coulibaly, F.; Alshamrani, M.; Al-Nafisah, A.; Youan, B.-B.C. Enhancing Etoposide Aqueous Solubility and Anticancer Activity with L-Arginine. ASSAY Drug Dev. Technol. 2021, 19, 508–525. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Navari, R.M. Safety of Polysorbate 80 in the Oncology Setting. Adv. Ther. 2018, 35, 754–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bibera, M.A.T.; Lo, K.M.K.; Steele, A. Potential cross-reactivity of polysorbate 80 and cremophor: A case report. J. Oncol. Pharm. Pract. 2020, 26, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Chi, S.-C.; Lee, W.S.; Lee, W.M.; Koo, Y.B.; Yong, C.S.; Choi, H.G.; Woo, J.S. Toxicity studies of cremophor-free paclitaxel solid dispersion formulated by a supercritical antisolvent process. Arch. Pharm. Res. 2009, 32, 139–148. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci. Rep. 2018, 8, 967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollazadeh, S.; Sahebkar, A.; Hadizadeh, F.; Behravan, J.; Arabzadeh, S. Structural and functional aspects of P-glycoprotein and its inhibitors. Life Sci. 2018, 214, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Razak, S.A.; Wahab, H.A.; Fisol, F.; Abdulbaqi, I.M.; Parumasivam, T.; Mohtar, N.; Gazzali, A.M. Advances in Nanocarriers for Effective Delivery of Docetaxel in the Treatment of Lung Cancer: An Overview. Cancers 2021, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, M.C.O.; da Silva, P.B.; Radicchi, M.A.; Andrade, B.Y.G.; de Oliveira, J.V.; Venus, T.; Merker, C.; Estrela-Lopis, I.; Longo, J.P.F.; Báo, S.N. Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J. Nanobiotechnol. 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ni, S.; Chen, W.; Liu, M.; Feng, J.; Hu, K. Improved Anti-Triple Negative Breast Cancer Effects of Docetaxel by RGD-Modified Lipid-Core Micelles. Int. J. Nanomed. 2021, 16, 5265–5279. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pukale, S.; Sahel, D.K.; Singh, P.; Mittal, A.; Chitkara, D. Folate targeted hybrid lipo-polymeric nanoplexes containing docetaxel and miRNA-34a for breast cancer treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112305. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarmpi, P.; Flanagan, T.; Meehan, E.; Mann, J.; Fotaki, N. Biopharmaceutical Understanding of Excipient Variability on Drug Apparent Solubility Based on Drug Physicochemical Properties. Case Study: Superdisintegrants. AAPS J. 2020, 22, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsalhi, A.; Ayon, N.J.; Sikder, S.; Youan, B.-B.C. Self-Assembled Nanomicelles to Enhance Solubility and Anticancer Activity of Etoposide. ASSAY Drug Dev. Technol. 2021, 19, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, S.M.; Azizov, S.; Elmasry, M.R.; Sharipov, M.; Lee, Y.-I. Recent Advances in Nanomicelles Delivery Systems. Nanomaterials 2020, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Dian, L.-H.; Hu, Y.-J.; Lin, J.-Y.; Zhang, J.-Y.; Yan, Y.; Cui, Y.-N.; Su, Z.-B.; Lu, W.-L. Fabrication of paclitaxel hybrid nanomicelles to treat resistant breast cancer via oral administration. Int. J. Nanomed. 2018, 13, 719–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent Advances in the Application of Vitamin E TPGS for Drug Delivery. Theranostics 2018, 8, 464–485. [Google Scholar] [CrossRef]
- Collnot, E.M.; Baldes, C.; Schaefer, U.F.; Edgar, K.J.; Wempe, M.F.; Lehr, C.M. Vitamin E TPGS P-glycoprotein inhibition mechanism: Influence on conformational flexibility, intracellular ATP levels, and role of time and site of access. Mol. Pharm. 2010, 7, 642–651. [Google Scholar] [CrossRef]
- Leopoldo, M.; Nardulli, P.; Contino, M.; Leonetti, F.; Luurtsema, G.; Colabufo, N.A. An updated patent review on P-glycoprotein inhibitors (2011–2018). Expert Opin. Ther. Pat. 2019, 29, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.W.; Rodriguez, B.L.; Li, X.; Hursting, S.D.; Williams, R.O.; Cui, Z. Solid Lipid Nanoparticle Formulations of Docetaxel Prepared with High Melting Point Triglycerides: In Vitro and in Vivo Evaluation. Mol. Pharm. 2014, 11, 1239–1249. [Google Scholar] [CrossRef]
- Evans, W.C.; Evans, D. Chapter 19—Hydrocarbons and derivatives. In Trease and Evans’ Pharmacognosy (Sixteenth Edition); Evans, W.C., Evans, D., Eds.; W.B. Saunders: Edinburgh, UK, 2009; pp. 173–193. [Google Scholar] [CrossRef]
- Araya, H.; Tomita, M.; Hayashi, M. The novel formulation design of O/W microemulsion for improving the gastrointestinal absorption of poorly water soluble compounds. Int. J. Pharm. 2005, 305, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Gote, V.; Mandal, A.; Alshamrani, M.; Pal, D. Self-Assembling Tacrolimus Nanomicelles for Retinal Drug Delivery. Pharmaceutics 2020, 12, 1072. [Google Scholar] [CrossRef]
- Alshamrani, M.; Sikder, S.; Coulibaly, F.; Mandal, A.; Pal, D.; Mitra, A.K. Self-Assembling Topical Nanomicellar Formulation to Improve Curcumin Absorption Across Ocular Tissues. AAPS PharmSciTech 2019, 20, 254. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, F.S.; Ezoulin, M.J.M.; Purohit, S.S.; Ayon, N.J.; Oyler, N.A.; Youan, B.-B.C. Layer-by-Layer Engineered Microbicide Drug Delivery System Targeting HIV-1 gp120: Physicochemical and Biological Properties. Mol. Pharm. 2017, 14, 3512–3527. [Google Scholar] [CrossRef]
- Ngo, A.N.; Thomas, D.; Murowchick, J.; Ayon, N.J.; Jaiswal, A.; Youan, B.C. Engineering fast dissolving sodium acetate mediated crystalline solid dispersion of docetaxel. Int. J. Pharm. 2018, 545, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Cholkar, K.; Gilger, B.C.; Mitra, A.K. Topical, Aqueous, Clear Cyclosporine Formulation Design for Anterior and Posterior Ocular Delivery. Transl. Vis. Sci. Technol. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khare, V.; Sakarchi, W.A.; Gupta, P.N.; Curtis, A.D.M.; Hoskins, C. Synthesis and characterization of TPGS–gemcitabine prodrug micelles for pancreatic cancer therapy. RSC Adv. 2016, 6, 60126–60137. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Chen, H.; Zheng, Y.; Song, X.; Liu, R.; Liu, K.; Zeng, X.; Mei, L. Nanoformulation of d-α-tocopheryl polyethylene glycol 1000 succinate-b-poly(ε-caprolactone-ran-glycolide) diblock copolymer for breast cancer therapy. Integr. Biol. 2011, 3, 993–1002. [Google Scholar] [CrossRef]
- Guo, Y.; Niu, B.; Song, Q.; Zhao, Y.; Bao, Y.; Tan, S.; Si, L.; Zhang, Z. RGD-decorated redox-responsive d-α-tocopherol polyethylene glycol succinate–poly(lactide) nanoparticles for targeted drug delivery. J. Mater. Chem. B 2016, 4, 2338–2350. [Google Scholar] [CrossRef] [PubMed]
- Huyan, Z.; Ding, S.; Yu, X.; Liu, X. Preparation and Characterization of Hydrogenated Castor Oil-Based Coating Wax. European J. Lipid Sci. Technol. 2018, 120, 1700444. [Google Scholar] [CrossRef]
- Xue, B.; Zhao, J.; Fan, Y.; Chen, S.; Li, W.; Chen, J.; Li, Z.; Wang, H.; Kong, H. Synthesis of Taxol and Docetaxel by Using 10-Deacetyl-7-xylosyltaxanes. Chem. Biodivers. 2020, 17, e1900631. [Google Scholar] [CrossRef] [PubMed]
- Huynh, L.; Leroux, J.-C.; Allen, C. Enhancement of docetaxel solubility via conjugation of formulation-compatible moieties. Org. Biomol. Chem. 2009, 7, 3437–3446. [Google Scholar] [CrossRef] [PubMed]
- Youm, I.; Bazzil, J.D.; Otto, J.W.; Caruso, A.N.; Murowchick, J.B.; Youan, B.B. Influence of surface chemistry on cytotoxicity and cellular uptake of nanocapsules in breast cancer and phagocytic cells. AAPS J. 2014, 16, 550–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, H.M.; Cholkar, K.; Joseph, M.; Yang, X.; Mitra, A.K. Clear, Aqueous Topical Drop of Triamcinolone Acetonide. AAPS PharmSciTech 2017, 18, 2466–2478. [Google Scholar] [CrossRef]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar] [CrossRef]
- Chang, T.; Gosain, P.; Stenzel, M.H.; Lord, M.S. Drug-loading of poly(ethylene glycol methyl ether methacrylate) (PEGMEMA)-based micelles and mechanisms of uptake in colon carcinoma cells. Colloids Surf. B Biointerfaces 2016, 144, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Yamazaki, M. Role of P-glycoprotein in pharmacokinetics: Clinical implications. Clin. Pharmacokinet. 2003, 42, 59–98. [Google Scholar] [CrossRef]
- Bittner, B.; Bravo González, R.C.; Bohrmann, B.; Kuentz, M.; Huwyler, J. Drug-excipient interactions by Vitamin E-TPGS: In vitro studies on inhibition of P-glycoprotein and colonic drug absorption. J. Drug Deliv. Sci. Technol. 2008, 18, 145–148. [Google Scholar] [CrossRef]
- Tavares Luiz, M.; Delello Di Filippo, L.; Carolina Alves, R.; Sousa Araújo, V.H.; Lobato Duarte, J.; Maldonado Marchetti, J.; Chorilli, M. The use of TPGS in drug delivery systems to overcome biological barriers. Eur. Polym. J. 2021, 142, 110129. [Google Scholar] [CrossRef]








| Formulation | DTX (wt %) | Polymer Ratio (wt %) (HCO-40: VIT E TPGS) | Size (nm) | Entrapment Efficiency % | Drug Loading % |
|---|---|---|---|---|---|
| * F-1 | 0.2 | 0.1:0.1 | 24.23 ± 2.20 | 18.32 ± 2.32 | 1.12 ± 0.03 |
| F-2 | 0.2 | 2.5:1 | 13.42 ± 0.62 | 99.30 ± 1.96 | 3.62 ± 0.11 |
| F-3 | 0.2 | 0.5:0.01 | 28.23 ± 0.92 | 35.73 ± 2.23 | 2.10 ± 0.23 |
| F-4 | 0.2 | 2.5:0.01 | 14.40 ± 0.52 | 90.12 ± 1.92 | 3.53 ± 0.21 |
| Parent Ion (Q1) | Fragment Ion (Q3) | Collision Energy (V) | |
|---|---|---|---|
| Docetaxel | 830.26 | 304.2 | 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshamrani, M.; Ayon, N.J.; Alsalhi, A.; Akinjole, O. Self-Assembled Nanomicellar Formulation of Docetaxel as a Potential Breast Cancer Chemotherapeutic System. Life 2022, 12, 485. https://doi.org/10.3390/life12040485
Alshamrani M, Ayon NJ, Alsalhi A, Akinjole O. Self-Assembled Nanomicellar Formulation of Docetaxel as a Potential Breast Cancer Chemotherapeutic System. Life. 2022; 12(4):485. https://doi.org/10.3390/life12040485
Chicago/Turabian StyleAlshamrani, Meshal, Navid J. Ayon, Abdullah Alsalhi, and Omowumi Akinjole. 2022. "Self-Assembled Nanomicellar Formulation of Docetaxel as a Potential Breast Cancer Chemotherapeutic System" Life 12, no. 4: 485. https://doi.org/10.3390/life12040485
APA StyleAlshamrani, M., Ayon, N. J., Alsalhi, A., & Akinjole, O. (2022). Self-Assembled Nanomicellar Formulation of Docetaxel as a Potential Breast Cancer Chemotherapeutic System. Life, 12(4), 485. https://doi.org/10.3390/life12040485







