Impact of Phosphatic Nutrition on Growth Parameters and Artemisinin Production in Artemisia annua Plants Inoculated or Not with Funneliformis mosseae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mycorrhizal Inoculum
2.2. Experimental Design
2.3. Mycorrhizal Colonization in the Root System
2.4. Plant Parameters
2.5. Analysis of Leaf Photosynthetic Pigments
2.6. Leaf Extraction, HPLC Analysis, and MS Detection of Artemisinin
2.7. Element Concentrations by ICP-OES Analysis
2.8. Statistical Analysis
3. Results
3.1. Mycorrhizal Colonization
3.2. Biomass Production
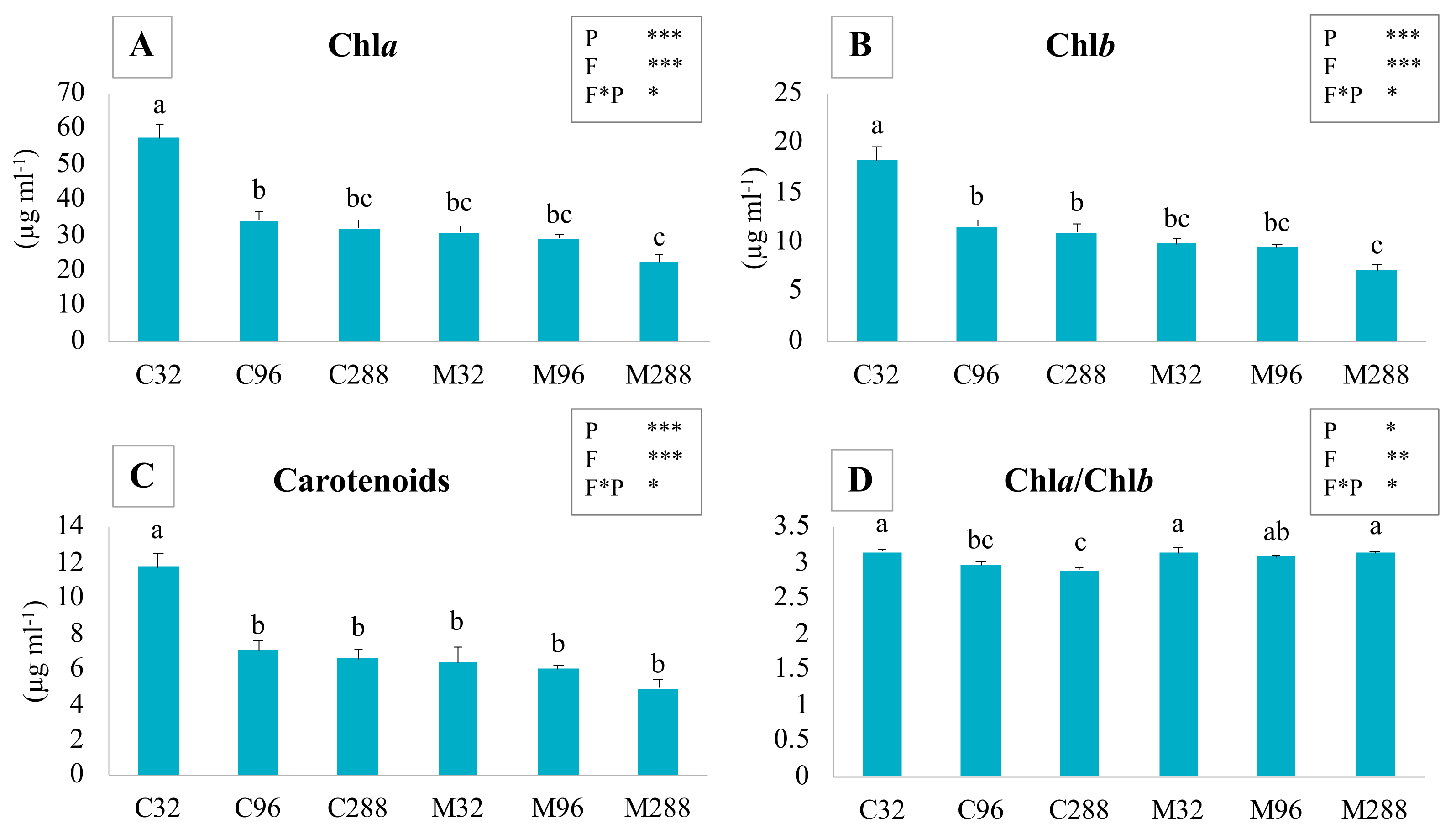
3.3. Photosynthetic Pigment Concentration
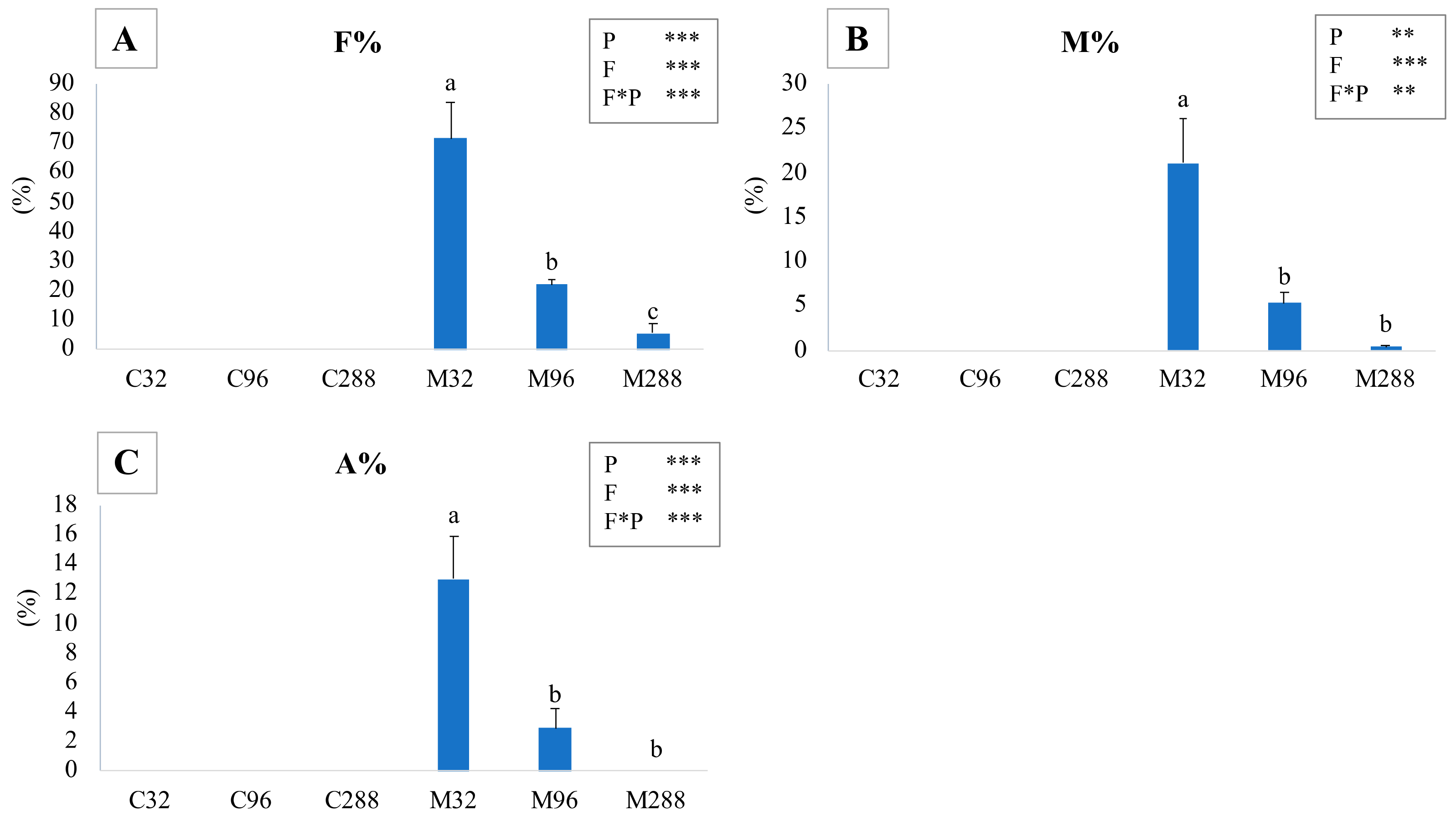
3.4. Artemisinin Concentration in Leaves
3.5. Nutrient Concentration in the Different Plant Organs
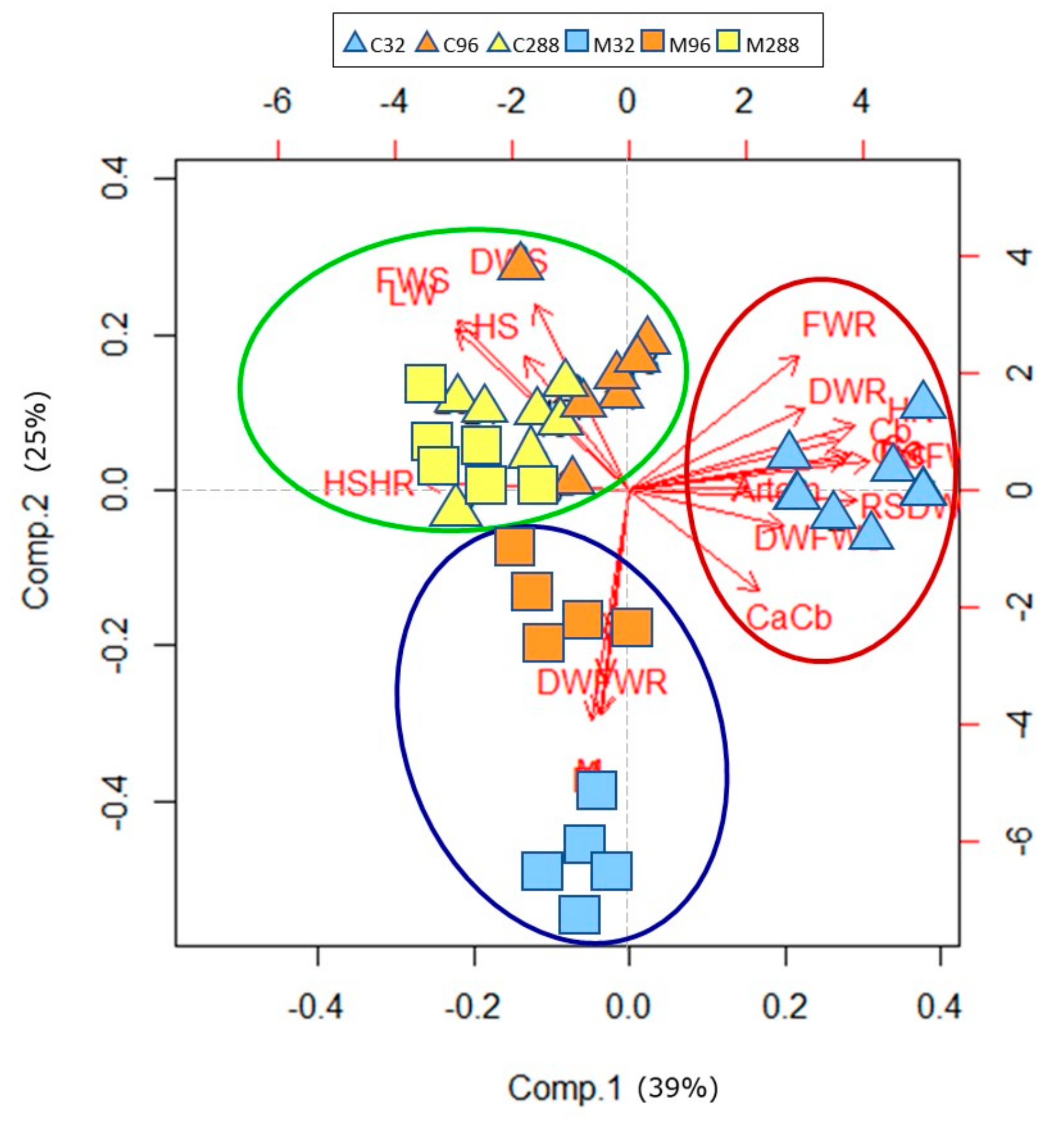
3.6. Principal Component Analysis—PCA
4. Discussion
4.1. Mycorrhization and Plant Growth Parameters
4.2. Photosynthetic Pigments
4.3. Artemisinin Production
4.4. Nutrient Uptake
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, L.E.; Bates, P.L.; Evans, T.M.; Unwin, M.M.; Estes, J.R. Molecular phylogeny of subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera. BMC Evol. Biol. 2002, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Taak, P.; Kumar, A.; Khatri, T.; Sanya, I. The artemisia genus: A review on traditional uses, phytochemical constituents, pharmacological properties and germplasm conservation. J. Glycom. Lipidom. 2017, 7, 1. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Zeng, F.L.; Xie, C.X. Spatial distribution and global potential suitability regions of Artemisia annua. J. Chin. Med. Mater. 2015, 38, 460–466. [Google Scholar]
- Muller, M.; Brandes, D. Growth and development of Artemisia annua L. on different soil types. Verh. Ges. Ökol. 1997, 27, 453–460. [Google Scholar]
- Aftab, T.; Masroor, M.; Khan, A.; Ferreira, J.F.S. Effect of mineral nutrition, growth regulators and environmental stresses on biomass production and artemisinin concentration of Artemisia annua L. In Artemisa annua—Pharmacology and Biotechnology; Aftab, T., Masroor, M., Ferreira, J.F.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 157–172. [Google Scholar] [CrossRef]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2020. Available online: https://www.who.int/publications/i/item/9789240015791 (accessed on 24 June 2021).
- Yakasai, A.M.; Hamza, M.; Dalhat, M.M. Adherence to artemisinin-based combination therapy for the treatment of uncomplicated malaria: A systematic review and meta-analysis. J. Trop Med. 2015, 2015, 189232. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y. Artemisinin—A gift from traditional chinese medicine to the world (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2016, 55, 10210–10226. [Google Scholar] [CrossRef]
- Olsson, M.E.; Olofsson, L.M.; Lindahl, A.L.; Lundgren, A.; Brodelius, M.; Brodelius, P.E. Localization of enzymes of artemisinin biosynthesis to the apical cells of glandular secretory trichomes of Artemisia annua L. Phytochemistry 2009, 70, 1123–1128. [Google Scholar] [CrossRef]
- Davies, M.J.; Atkinson, C.J.; Burns, C.; Arroo, R.; Woolley, J. Increases in leaf artemisinin concentration in Artemisia annua in response to the application of phosphorus and boron. Ind. Crops Prod. 2011, 34, 1465–1473. [Google Scholar] [CrossRef]
- Tan, H.; Xiao, L.; Gao, S.; Li, Q.; Chen, J.; Xiao, Y.; Ji, Q.; Chen, R.; Chen, W.; Zhang, L. TRICHOME AND ARTEMISININ REGULATOR 1 is required for trichome development and artemisinin biosynthesis in Artemisia annua. Mol. Plant 2015, 8, 1396–1411. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xuc, C.; Wong, Y.K.; Li, Y.; Liao, F.; Jiang, T.; Tu, Y. Artemisinin, the magic drug discovered from traditional chinese medicine. Engineering 2019, 5, 32–39. [Google Scholar] [CrossRef]
- Shen, Q.; Chen, Y.F.; Wang, T.; Wu, S.Y.; Lu, X.; Zhang, L.; Zhang, F.Y.; Jiang, W.M.; Wang, G.F.; Tang, K.X. Overexpression of the cytochrome P450 monooxygenase (cyp71av1) and cytochrome P450 reductase (cpr) genes increased artemisinin content in Artemisia annua (Asteraceae). Genet. Mol. Res. 2012, 11, 3298–3309. [Google Scholar] [CrossRef]
- Tang, K.; Shen, Q.; Yan, T.; Fu, X. Transgenic approach to increase artemisinin content in Artemisia annua L. Plant Cell Rep. 2014, 33, 605–615. [Google Scholar] [CrossRef]
- Schramek, N.; Wang, H.; Römisch-Margl, W.; Keil, B.; Radykewicz, T.; Winzenhörlein, B.; Beerhues, L.; Bacher, A.; Rohdich, F.; Gershenzon, J.; et al. Artemisinin biosynthesis in growing plants of Artemisia annua. A 13CO2 study. Phytochemistry 2010, 71, 179–187. [Google Scholar] [CrossRef]
- Wen, W.; Yu, R. Artemisinin biosynthesis and its regulatory enzymes: Progress and perspective. Pharm. Rev. 2011, 5, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Abdin, M.Z.; Israr, M.; Rehman, R.U.; Jain, S.K. Artemisinin, a novel antimalarial drug: Biochemical and molecular approaches for enhanced production. Planta Med. 2003, 69, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Efferth, T. Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Curr. Drug Targets 2006, 7, 407–421. [Google Scholar] [CrossRef]
- Kindermans, J.M.; Pilloy, J.; Olliaro, P.; Gomes, M. Ensuring sustained ACT production and reliable artemisinin supply. Malar J. 2007, 6, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, R.; Chaudhary, V.; Bhatnagar, A.K. Effects of arbuscular mycorrhiza and phosphorus application on artemisinin concentration in Artemisia annua L. Mycorrhiza 2007, 17, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Kapoor, R.; Bhatnagar, A.K. Effectiveness of two arbuscular mycorrhizal fungi on concentrations of essential oil and artemisinin in three accessions of Artemisia annua L. Appl. Soil Ecol. 2008, 40, 174–181. [Google Scholar] [CrossRef]
- Davies, M.J.; Atkinson, C.J.; Burns, C.; Woolley, J.G.; Hipps, N.A.; Arroo, R.R.J.; Dungey, N.; Robinson, T.; Brown, P.; Flockart, I.; et al. Enhancement of artemisinin concentration and yield in response to optimization of nitrogen and potassium supply to Artemisia annua. Ann. Bot. 2009, 104, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awasthi, A.; Bharti, N.; Nair, P.; Singh, R.; Shukla, A.K.; Gupta, M.M.; Darokar, M.P.; Kalra, A. Synergistic effect of Glomus mosseae and nitrogen fixing Bacillus subtilis strain Daz26 on artemisinin content in Artemisia annua L. Appl. Soil Ecol. 2011, 49, 125–130. [Google Scholar] [CrossRef]
- Mandal, S.; Upadhyay, S.; Wajid, S.; Ram, M.; Jain, D.C.; Singh, V.P.; Abdin, M.Z.; Kapoor, R. Arbuscular mycorrhiza increase artemisinin accumulation in Artemisia annua by higher expression of key biosynthesis genes via enhanced jasmonic acid levels. Mycorrhiza 2014, 25, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Saxena, P.; Choudhary, D.K.; Abdin, M.Z.; Varma, A. Dual symbiosis between Piriformospora indica and Azotobacter chroococcum enhances the artemisinin content in Artemisia annua L. World J. Microbiol. Biotechnol. 2016, 32, 19. [Google Scholar] [CrossRef]
- Gupta, R.; Singh, A.; Gupta, M.M.; Pandey, R. Cumulative role of bioinoculants on growth, antioxidant potential and artemisinin content in Artemisia annua L: Under organic field condition. World J. Microbiol. Biotechnol. 2016, 32, 167. [Google Scholar] [CrossRef]
- Domokos, E.; Jakab-Farkas, L.; Darko, B.; Biro-Janka, B.; Mara, G.; Albert, C.; Balog, A. Increase in Artemisia annua plant biomass artemisinin content and guaiacol peroxidase activity using the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front. Plant Sci. 2018, 9, 478. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.W.; Zhang, Z.; Tan, R.X. Stimulation of artemisinin production in Artemisia annua hairy roots by the elicitor from the endophytic Colletotrichum sp. Biotechnol. Lett. 2001, 23, 857–860. [Google Scholar] [CrossRef]
- Ikram, N.K.B.K.; Simonsen, H.T. A review of biotechnological artemisinin production in plants. Front. Plant Sci. 2017, 8, 1966. [Google Scholar] [CrossRef] [Green Version]
- Rapparini, F.; Llusià, J.; Penuelas, J. Effect of arbuscular mycorrhizal (AM) colonization on terpene emission and content of Artemisia annua L. Plant Biol. 2008, 10, 108–122. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Smith, F.A. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 2012, 104, 1–13. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bona, E.; Lingua, G.; Todeschini, V. Effect of bioinoculants on the quality of crops. In Bioformulations: For Sustainable Agriculture; Arora, N.K., Mehnaz, S., Balestrini, R., Eds.; Springer: New Delhi, India, 2016; pp. 93–124. ISBN 978-81-322-2777-9. [Google Scholar]
- Todeschini, V.; AitLahmidi, N.; Mazzucco, E.; Marsano, F.; Gosetti, F.; Robotti, E.; Bona, E.; Massa, N.; Bonneau, L.; Marengo, E.; et al. Impact of beneficial microorganisms on strawberry growth, fruit production, nutritional quality, and volatilome. Front. Plant Sci. 2018, 9, 1611. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Sharif, M.; Fahad, S.; Adnan, M.; Khan, I.A.; Aksoy, E.; Ali, A.; Sultan, T.; Alam, M.; Saeed, M.; et al. Arbuscular mycorrhizal fungi improve the growth and phosphorus uptake of mung bean plants fertilized with composted rock phosphate fed dung in alkaline soil environment. J. Plant Nutr. 2019, 42, 1760–1769. [Google Scholar] [CrossRef]
- Copetta, A.; Todeschini, V.; Massa, N.; Bona, E.; Berta, G.; Lingua, G. Inoculation with arbuscular mycorrhizal fungi improves melon (Cucumis melo) fruit quality under field conditions and plant performance in both field and greenhouse. Plant Biosyst. 2020, 155, 1063–1074. [Google Scholar] [CrossRef]
- Massa, N.; Cesaro, P.; Todeschini, V.; Capraro, J.; Scarafoni, A.; Cantamessa, S.; Copetta, A.; Anastasia, F.; Gamalero, E.; Lingua, G.; et al. Selected autochthonous rhizobia, applied in combination with am fungi, improve seed quality of common bean cultivated in reduced fertilization condition. Appl. Soil Ecol. 2020, 148, 103507. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Diversity, taxonomy and phylogeny of the Fungi. Biol. Rev. 2019, 94, 2101–2137. [Google Scholar] [CrossRef] [PubMed]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef]
- Atul-Nayyar, A.; Hamel, C.; Hanson, K.; Germida, J. The arbuscular mycorrhizal symbiosis links N mineralization to plant demand. Mycorrhiza 2009, 19, 239–246. [Google Scholar] [CrossRef]
- Nell, M.; Wawrosch, C.; Steinkellner, S.; Vierheilig, H.; Kopp, B.; Lössl, A.; Franz, C.; Novak, J.; Zitterl-Eglseer, K. Root colonization by symbiotic arbuscular mycorrhizal fungi increases sesquiterpenic acid concentrations in Valeriana officinalis L. Planta Med. 2010, 76, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.S.; Jakobsen, I.; Grønlund, M.; Smith, A.F. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; DePascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [Green Version]
- Koltai, H.; Kapulnik, Y. Arbuscular Mycorrhizas: Physiology and Function; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Lanfranco, L.; Bonfante, P.; Genre, A. The mutualistic interaction between plants and arbuscular mycorrhizal fungi. In The Fungal Kingdom; Heitman, J., Howlett, B.J., Crous, P.W., Stukenbrock, E.H., James, T.Y., Gow, N.A.R., Eds.; ASM Press: Washington, DC, USA, 2017; Volume 35, pp. 727–747. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Schröder, J.J.; Smit, A.L.; Cordell, D.; Rosemarin, A. Improved phosphorus use efficiency in agriculture: A key requirement for its sustainable use. Chemosphere 2011, 84, 822–831. [Google Scholar] [CrossRef]
- Bowles, T.M.; Barrios-Masias, F.H.; Carlisle, E.A.; Cavagnaro, T.R.; Jackson, L.E. Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci. Total Env. 2016, 566–567, 1223–1234. [Google Scholar] [CrossRef] [Green Version]
- Berdeni, D.; Cotton, T.E.A.; Daniell, T.J.; Bidartondo, M.I.; Cameron, D.D.; Evans, K.L. The effects of arbuscular mycorrhizal fungal colonisation on nutrient status, growth, productivity, and canker resistance of apple (Malus pumila). Front. Microbiol. 2018, 9, 1461. [Google Scholar] [CrossRef]
- De Lazzari Almeida, C.; Sawaya, A.C.H.F.; de Andrade, S.A.L. Mycorrhizal influence on the growth and bioactive compounds composition of two medicinal plants: Mikania glomerata Spreng. and Mikania laevigata Sch. Bip. ex Baker (Asteraceae). Rev. Bras. Bot. 2018, 41, 233–240. [Google Scholar] [CrossRef]
- Khaosaad, T.; Krenn, L.; Medjakovic, S.; Ranner, A.; Lossl, A.; Nell, M.; Jungbauer, A.; Vierheilig, H. Effect of mycorrhization on the isoflavone content and the phytoestrogen activity of red clover. J. Plant Physiol. 2007, 165, 1161–1167. [Google Scholar] [CrossRef]
- Toussaint, J.P.; Kraml, M.; Nell, S.E.; Smith, F.A.; Steinkellner, S.; Schmiderer, C.; Vierheilig, H.; Novak, J. Effect of Glomus mosseae on concentration of rosmarinic and caffeic acids and essential oil compounds in basil inoculated with Fusarium oxysporum f. sp. Basilica. Plant Pathol. 2008, 57, 1109–1116. [Google Scholar] [CrossRef]
- Ceccarelli, N.; Curadi, M.; Martelloni, L.; Sbrana, C.; Picciarelli, P.; Giovannetti, M. Mycorrhizal colonization impacts on phenolic content and antioxidant properties of artichoke leaves and flower heads two years after field transplant. Plant Soil 2010, 335, 311–323. [Google Scholar] [CrossRef]
- Zubek, S.; Stojakowska, A.; Anielska, T.; Turnau, K. Arbuscular mycorrhizal fungi alter thymol derivative contents of Inula ensifolia L. Mycorrhiza 2010, 20, 497–504. [Google Scholar] [CrossRef]
- Karagiannidis, T.; Thomidis, T.; Lazari, D.; Panou-Filotheou, E.; Karagiannidou, C. Effect of three Greek arbuscular mycorrhizal fungi in improving the growth, nutrient concentration, and production of essential oils of oregano and mint plants. Sci. Hortic. 2011, 129, 329–334. [Google Scholar] [CrossRef]
- Eftekhari, M.; Alizadeh, M.; Ebrahimi, P. Evaluation of the total phenolics and quercetin content of foliage in mycorrhizal grape (Vitis vinifera L.) varieties and effect of postharvest drying on quercetin yield. Ind. Crops Prod. 2012, 38, 160–165. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Campos, M.A.S.; Albuquerque, U.P.; Silva, F.B.S. Arbuscular mycorrhizal fungi (AMF) affect biomolecules content in Myracrodruon urundeuva seedlings. Ind. Crops Prod. 2013, 50, 244–247. [Google Scholar] [CrossRef]
- Zubek, S.; Rola, K.; Szewczyk, A.; Majewska, M.L.; Turnau, K. Enhanced concentrations of elements and secondary metabolites in Viola tricolor L. induced by arbuscular mycorrhizal fungi. Plant Soil 2015, 390, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Vance, C.; Uhde-Stone, C.; Allan, L.D. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to Plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; Volume 7, pp. 171–190. [Google Scholar] [CrossRef]
- Hisinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Seo, M.W.; Yang, D.S.; Kays, S.J.; Kim, J.H.; Wood, J.H.; Park, K.W. Effects of nutrient solution electrical conductivity and sulfur, magnesium, and phosphorus concentration on sesquiterpene lactones in hydroponically grown lettuce (Lactuca sativa L.). Sci. Hortic. 2009, 122, 369–374. [Google Scholar] [CrossRef]
- Namuli, A.; Bazira, J.; Casim, T.U.; Engeu, P.O. A review of various efforts to increase artemisinin and other antimalarial compounds in Artemisia annua L. Plant. Cogent Biol. 2018, 4, 1513312. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization va d′un système radiculaire: Recherche de méthodes d’estimation ayant une signification fonctionnelle. In Les Mycorhizes: Physiologie et Génétique; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA Press: Paris, France, 1986; pp. 217–221. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res. 2002, 73, 149–156. [Google Scholar] [CrossRef]
- Lapkin, A.; Walker, A.; Sullivan, N.; Khambay, B.; Mlambo, B.; Chemat, S. Development of HPLC analytical protocols for quantification of artemisinin in biomass and extracts. J. Pharm. Anal. 2009, 49, 908–915. [Google Scholar] [CrossRef]
- Ene-Obong, H.N.; Okudu, H.O.; Asumughab, U.V. Nutrient and phytochemical composition of two varieties of Monkey kola (Cola parchycarpa and Cola lepidota): An underutilised fruit. Food Chem. 2016, 193, 154–159. [Google Scholar] [CrossRef]
- Balzergue, C.; Chabaud, M.; Barker, D.G.; Becard, G.; Rochange, S.F. High phosphate reduces host ability to develop arbuscular mycorrhizal symbiosis without affecting root calcium spiking responses to the fungus. Front. Plant Sci. 2013, 4, 426. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.V.; Casaes, A.P.; Oliveira, R.; Jesus, R. Influence of arbuscular mycorrhizal fungi on growth, mineral composition and production of essential oil in Mentha × piperita L. var. citrata (Ehrh.) Briq. under two phosphorus levels. J. Med. Plant Res. 2014, 8, 1321–1332. [Google Scholar] [CrossRef]
- Tarraf, W.; Ruta, C.; Tagarelli, A.; De Cillis, F.; De Mastro, G. Influence of arbuscular mycorrhizae on plant growth, essential oil production and phosphorus uptake of Salvia officinalis L. Ind. Crops Prod. 2017, 102, 144–153. [Google Scholar] [CrossRef]
- Asensio, D.; Rapparini, F.; Peñuelas, J. AM fungi root colonization increases the production of essential isoprenoids vs nonessential isoprenoids especially under drought stress conditions or after jasmonic acid application. Phytochemistry 2012, 77, 149–161. [Google Scholar] [CrossRef]
- Tawaraya, K.; Hirose, R.; Wagatsuma, T. Inoculation of arbuscular mycorrhizal fungi can substantially reduce phosphate fertilizer application to Allium fistulosum L. and achieve marketable yield under field condition. Biol. Fertil. Soils 2012, 48, 839–843. [Google Scholar] [CrossRef] [Green Version]
- Berta, G.; Fusconi, A.; Hooker, J.E. Arbuscular mycorrhizal modifications to plant root systems: Scale, mechanisms and consequences. In Mycorrhizal Technology in Agriculture; Gianinazzi, S., Schüepp, H., Barea, J.M., Haselwandter, K., Eds.; Birkhäuser: Basel, Switzerland, 2002; pp. 71–85. [Google Scholar] [CrossRef]
- Fusconi, A. Regulation of root morphogenesis in arbuscular mycorrhizae: What role do fungal exudates, phosphate, sugars and hormones play in lateral root formation? Ann. Bot. 2013, 113, 19–33. [Google Scholar] [CrossRef]
- Kapoor, R.; Anand, G.; Gupta, P.; Mandal, S. Insight into the mechanisms of enhanced production of valuable terpenoids by arbuscular mycorrhiza. Phytochem. Rev. 2017, 16, 677–692. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Roldan, A.; Pascual, A.J. The interaction with arbuscular mycorrhizal fungi or Trichoderma harzianum alters the shoot hormonal profile in melon plants. Phytochemistry 2011, 72, 223–229. [Google Scholar] [CrossRef]
- Adolfsson, L.; Nziengui, H.; Abreu, I.N.; Šimura, J.; Beebo, A.; Herdean, A.; Aboalizadeh, J.; Široká, J.; Moritz, T.; Novák, O.; et al. Enhanced secondary- and hormone metabolism in leaves of arbuscular mycorrhizal Medicago truncatula. Plant Physiol. 2017, 175, 392–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anurada, M.; Narayanan, A. Promotion of root elongation by phosphorus deficiency. Plant Soil 1991, 136, 273–275. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Kim, H.I. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 2012, 235, 1197–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Shi, J.; Xie, Q.; Yu, N.; Wang, E. Nutrient exchange and regulation in arbuscular mycorrhizal symbiosis. Mol. Plant 2017, 10, 1147–1158. [Google Scholar] [CrossRef] [Green Version]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y.; Saifullah; Ahmad, M. Improving agricultural water use efficiency by nutrient management in crop plants. Acta Agric. Scand. B Soil Plant Sci. 2011, 61, 291–304. [Google Scholar] [CrossRef]
- Miransari, M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 2010, 12, 563–569. [Google Scholar] [CrossRef]
- Harris, R.W. Root-shoot ratios. J. Agric. 1992, 18, 39–42. [Google Scholar]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbrugen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Karthikeyan, A.S.; Varadarajan, V.K.; Jain, A.; Held, M.A.; Carpita, N.C.; Raghothama, K.G. Phosphate starvation responses are mediated by sugar signalling in Arabidopsis. Planta 2007, 225, 907–918. [Google Scholar] [CrossRef]
- Rao, I.M.; Terry, N. Leaf phosphate status, photosynthesis, and carbon partitioning in sugar beet: I. Changes in growth, gas exchange, and Calvin cycle enzymes. Plant Physiol. 1989, 90, 814–819. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y. Phosphate starvation and membrane lipid remodelling in seed plants. Prog. Lipid Res. 2013, 52, 43–50. [Google Scholar] [CrossRef]
- Marulanda, A.; Porcel, R.; Barea, J.M.; Azcon, R. Drought tolerance and antioxidant activities in lavander plants colonized by native drought-tolerant of drought-sensitive Glomus species. Microbiol. Ecol. 2007, 54, 543–552. [Google Scholar] [CrossRef]
- Toussaint, J.P.; Smith, F.A.; Smith, S.E. Arbuscular mycorrhizal fungi can induce the production of phytochemicals in sweet basil irrespective of phosphorus nutrition. Mycorrhiza 2007, 17, 291–297. [Google Scholar] [CrossRef]
- Domokos, E.; Bíró-Janka, B.; Bálint, J.; Molnár, K.; Fazakas, C.; Jakab-Farkas, L.; Domokos, J.; Albert, C.; Mara, G.; Balog, A. Arbuscular mycorrhizal fungus Rhizophagus irregularis influences Artemisia annua plant parameters and artemisinin content under different soil types and cultivation methods. Microorganisms 2020, 8, 899. [Google Scholar] [CrossRef]
- Sailo, G.L.; Bagyaraj, D.J. Influence of different AM-fungi on the growth, forskolin content of Coleus forskohlii. Mycol. Res. 2005, 109, 795–798. [Google Scholar] [CrossRef]
- Copetta, A.; Lingua, G.; Berta, G. Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 2006, 16, 485–494. [Google Scholar] [CrossRef]
- Sa, G.; Mi, M.; He-chun, Y.; Ben-ye, L.; Guo-feng, L.; Kang, C. Effects of ipt gene expression on the physiological and chemical characteristics of Artemisia annua L. Plant Sci. 2001, 160, 691–698. [Google Scholar] [CrossRef]
- Fester, T.; Hause, G. Accumulation of reactive oxigen species in arbuscular mycorrhizal roots. Mycorrhiza 2005, 15, 373–379. [Google Scholar] [CrossRef]
- Janos, D.P. Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza 2007, 17, 75–91. [Google Scholar] [CrossRef]
- Wang, D.; Lv, S.; Jiang, P.; Li, Y. Roles, regulation, and agricultural application of plant phosphate transporters. Front. Plant Sci. 2017, 8, 817. [Google Scholar] [CrossRef] [Green Version]
- Elbon, A.; Whalen, J.K. Phosphorus supply to vegetable crops from arbuscular mycorrhizal fungi: A review. Biol. Agric. Hortic. 2015, 31, 73–90. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Liu, A.; Zhao, P.; Wang, M.; et al. Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front. Microbiol. 2017, 8, 2516. [Google Scholar] [CrossRef] [PubMed]
- Mensah, A.J.; Alexander; Koch, M.A.; Antunes, M.P.; Kiers, T.E.; Hart, M.; Bücking, H. High functional diversity within species of arbuscular mycorrhizal fungi is associated with differences in phosphate and nitrogen uptake and fungal phosphate metabolism. Mycorrhiza 2015, 25, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, I.; Fontaine, J.; Lounès-Hadji Sahraoui, A. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef]
- Versaw, W.K.; Garcia, L.R. Intracellular transport and compartmentation of phosphate in plants. Curr. Opin. Plant Biol. 2017, 39, 25–30. [Google Scholar] [CrossRef]
- Azcon, R.; Ambrosano, E.; Charest, C. Nutrient acquisition in mycorrhizal lettuce plants under different phosphorus and nitrogen concentration. Plant Sci. 2003, 165, 1137–1145. [Google Scholar] [CrossRef]
- Santander, C.; Sanhueza, M.; Olave, J.; Borie, F.; Valentine, C.; Cornejo, P. Arbuscular mycorrhizal colonization promotes the tolerance to salt stress in lettuce plants through an efficient modification of ionic balance. J. Soil Sci. Plant Nutr. 2019, 19, 321–331. [Google Scholar] [CrossRef]

| Fungal Treatment | P Concentration in the LA Solution | Plant Treatment Abbreviation |
|---|---|---|
| No fungus | 32 µM | C32 |
| No fungus | 96 µM | C96 |
| No fungus | 288 µM | C288 |
| Funneliformis mosseae BEG12 | 32 µM | M32 |
| Funneliformis mosseae BEG12 | 96 µM | M96 |
| Funneliformis mosseae BEG12 | 288 µM | M288 |
| Plant Treatments | Two-Way ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | C32 | C96 | C288 | M32 | M96 | M288 | P | F | F * P |
| Shoot FW (g) | 7.063 ± 0.339 c | 12.489 ± 0.675 a | 12.647 ± 0.449 a | 6.723 ± 0.606 c | 8.900 ± 0.565 b | 12.587 ± 0.081 a | *** | * | * |
| Shoot DW (g) | 1.441 ± 0.063 cd | 2.100 ± 0.093 a | 1.877 ± 0.124 ab | 1.213 ± 0.159 d | 1.503 ± 0.235 bcd | 1.687 ± 0.054 bc | ** | * | ns |
| Shoot DW/FW | 0.205 ± 0.006 a | 0.169 ± 0.006 b | 0.148 ± 0.006 cd | 0.148 ± 0.007 b | 0.168 ± 0.020 bc | 0.134 ± 0.004 d | *** | ns | ns |
| Stem height (cm) | 14.571 ± 0.369 c | 20.214 ± 1.430 a | 17.914 ± 0.582 ab | 15.100 ± 0.551 bc | 14.833 ± 2.987 bc | 15.100 ± 0.208 bc | ns | * | ns |
| Leaf FW (g) | 4.531 ± 0.231 c | 8.464 ± 0.467 a | 8.463 ± 0.538 a | 4.447 ± 0.401 bc | 6.147 ± 0.471 b | 9.237 ± 0.052 a | *** | ns | ns |
| Leaf DW (g) | 0.946 ± 0.052 b | 1.400 ± 0.078 a | 1.239 ± 0.100 a | 0.850 ± 0.122 b | 1.163 ± 0.16 ab | 1.293 ± 0.003 a | ** | ns | ns |
| Root FW (g) | 7.949 ± 0.694 a | 7.090 ± 0.892 a | 4.189 ± 0.525 b | 3.103 ± 0.820 b | 3.843 ± 0.334 b | 4.217 ± 0.105 b | ns | *** | * |
| Root DW (g) | 0.787 ± 0.085 a | 0.660 ± 0.077 ab | 0.414 ± 0.047 c | 0.423 ± 0.128 bc | 0.473 ± 0.068 bc | 0.410 ± 0.097 bc | ns | * | ns |
| Root DW/FW (g) | 0.099 ± 0.004 bc | 0.095 ± 0.005 c | 0.100 ± 0.001 bc | 0.134 ± 0.005 a | 0.123 ± 0.014 ab | 0.096 ± 0.022 bc | ns | ** | ns |
| Root length (cm) | 51.857 ± 2.567 a | 36.957 ± 2.652 b | 24.671 ± 2.694 c | 23.933 ± 1.126 c | 27.767 ± 4.436 bc | 26.200 ± 2.914 c | ** | *** | *** |
| Root/shoot FW | 1.124 ± 0.082 a | 0.563 ± 0.061 b | 0.329 ± 0.038 c | 0.450 ± 0.081 bc | 0.433 ± 0.020 bc | 0.333 ± 0.009 c | *** | *** | *** |
| Root/shoot DW | 0.544 ± 0.048 a | 0.311 ± 0.033 b | 0.217 ± 0.021 b | 0.333 ± 0.060 b | 0.317 ± 0.032 b | 0.240 ± 0.055 b | *** | ns | * |
| Stem height/root length | 0.286 ± 0.026 c | 0.571 ± 0.061 b | 0.771 ± 0.081 a | 0.633 ± 0.033 ab | 0.600 ± 0.200 ab | 0.600 ± 0.058 ab | * | ns | * |
| Plant Treatments | Two-Way ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Shoot | C32 | C96 | C288 | M32 | M96 | M288 | P | F | F * P |
| P (mg Kg−1) | 221.124 ± 20.662 c | 339.754 ± 55.78 bc | 779.161 ± 82.347 a | 293.395 ± 53.127 bc | 415.841 ± 78.893 b | 839.561 ± 62.995 a | *** | ns | ns |
| K (mg Kg−1) | 14,803.52 ± 967.717 a | 16,564.5 ± 1917.294 a | 18,652.62 ± 1038.739 a | 15,342.78 ± 798.021 a | 18,258.80 ± 1189.825 a | 21,823.20 ± 1997.428 a | ns | ns | ns |
| S (mg Kg−1) | 846.989 ± 85.015 a | 767.614 ± 91.817 a | 717.273 ± 35.069 a | 640.817 ± 54.926 a | 788.680 ± 59.105 a | 924.677 ± 64.822 a | ns | ns | ns |
| Mg (mg Kg−1) | 1035.383 ± 100.02 a | 15,342.78 ± 798.021 a | 640.817 ± 54.926 a | 853.243 ± 168.847 a | 1019.653 ± 172.100 a | 1235.59 ± 308.338 a | ns | ns | ns |
| Na (mg Kg−1) | 133.679 ± 16.216 b | 153.279 ± 12.011 b | 247.156 ± 52.729 ac | 143.207 ± 18.522 bc | 233.837 ± 50.435 ab | 293.107 ± 40.678 a | ** | ns | ns |
| Root | C32 | C96 | C288 | M32 | M96 | M288 | |||
| P (mg Kg−1) | 275.757 ± 20.662 b | 414.767 ± 55.78 b | 744.550 ± 82.347 a | 416.122 ± 53.127 b | 444.173 ± 78.893 b | 863.473 ± 62.995 a | *** | ns | ns |
| K (mg Kg−1) | 17,357.52 ± 1110.445 a | 12,532.82 ± 1671.586 a | 13,450.90 ± 1754.138 a | 14,105.12 ± 396.816 a | 14,160.66 ± 1561.905 a | 1226.813 ± 243.734 a | ns | ns | ns |
| S (mg Kg−1) | 921.494 ± 88.324 a | 1349.837 ± 125.182 a | 1203.774 ± 132.662 a | 897.983 ± 90.698 a | 829.893 ± 115.171 a | 1226.813 ± 243.734 a | ns | ns | ns |
| Mg (mg Kg−1) | 1801.303 ± 293.776 ab | 2288.069 ± 366.821 a | 1190.891 ± 171.064 bc | 797.967 ± 6.885 c | 1032.543 ± 281.134 bc | 1005.577 ± 262.912 bc | ns | ** | ns |
| Na (mg Kg−1) | 1005.599 ± 132.433 bc | 2368.611 ± 456.079 a | 1754.737 ± 317.798 ab | 351.343 ± 75.64 c | 629.317 ± 109.468 c | 1339.047 ± 106.568 abc | ns | * | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todeschini, V.; Anastasia, F.; Massa, N.; Marsano, F.; Cesaro, P.; Bona, E.; Gamalero, E.; Oddi, L.; Lingua, G. Impact of Phosphatic Nutrition on Growth Parameters and Artemisinin Production in Artemisia annua Plants Inoculated or Not with Funneliformis mosseae. Life 2022, 12, 497. https://doi.org/10.3390/life12040497
Todeschini V, Anastasia F, Massa N, Marsano F, Cesaro P, Bona E, Gamalero E, Oddi L, Lingua G. Impact of Phosphatic Nutrition on Growth Parameters and Artemisinin Production in Artemisia annua Plants Inoculated or Not with Funneliformis mosseae. Life. 2022; 12(4):497. https://doi.org/10.3390/life12040497
Chicago/Turabian StyleTodeschini, Valeria, Flavio Anastasia, Nadia Massa, Francesco Marsano, Patrizia Cesaro, Elisa Bona, Elisa Gamalero, Ludovica Oddi, and Guido Lingua. 2022. "Impact of Phosphatic Nutrition on Growth Parameters and Artemisinin Production in Artemisia annua Plants Inoculated or Not with Funneliformis mosseae" Life 12, no. 4: 497. https://doi.org/10.3390/life12040497
APA StyleTodeschini, V., Anastasia, F., Massa, N., Marsano, F., Cesaro, P., Bona, E., Gamalero, E., Oddi, L., & Lingua, G. (2022). Impact of Phosphatic Nutrition on Growth Parameters and Artemisinin Production in Artemisia annua Plants Inoculated or Not with Funneliformis mosseae. Life, 12(4), 497. https://doi.org/10.3390/life12040497










