Epigenome-Wide Analysis of DNA Methylation in Parkinson’s Disease Cortex
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Epigenome-Wide Methylation Analysis
2.3. Statistical Analysis of Normalised Methylation Data
2.4. Pyrosequencing of Cortex Samples
2.5. Pyrosequencing in the Cortex and Peripheral Blood with CYP2E1 Primer 2
2.6. Pyrosequencing in Human Peripheral Blood Mononuclear Cells (PBMCs)
2.7. Pyrosequencing in Human Peripheral Blood Mononuclear Cells (PBMCs) Exposed to l-DOPA
2.8. Pyrosequencing in Skin Fibroblasts
2.9. Western Blot Analysis
2.10. CYP2E1 Knock-Out Mice
3. Results
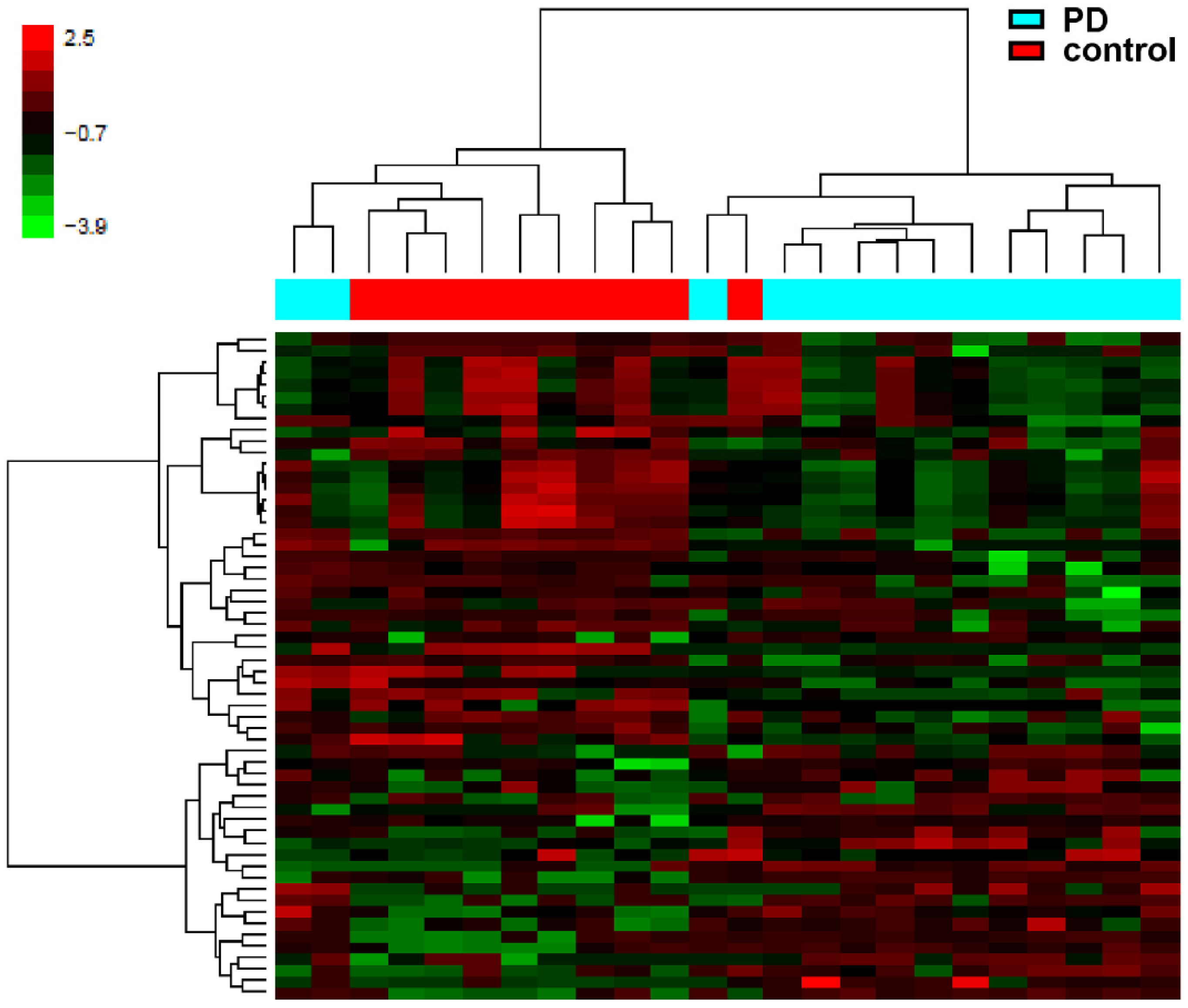
3.1. Epigenome-Wide DNA Methylation Analysis in Brain
3.2. Pyrosequencing of Candidate Genes in Brain
3.3. DNA Methylation of CYP2E1 in Skin Fibroblasts and Blood
3.4. Protein Expression of CYP2E1 in Cortex
3.5. Protein Expression of CYP2E1 in TCE Treated in Cultured Cells
3.6. SNCA Protein Levels in CYP2E1-Null Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urdinguio, R.G.; Sanchez-Mut, J.V.; Esteller, M. Epigenetic mechanisms in neurological diseases: Genes, syndromes, and therapies. Lancet Neurol. 2009, 8, 1056–1072. [Google Scholar] [CrossRef]
- Suzuki, M.M.; Bird, A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008, 9, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Dias, B.G.; Ressler, K.J. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 2014, 17, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Schulz, L.C. The Dutch Hunger Winter and the developmental origins of health and disease. Proc. Natl. Acad. Sci. USA 2010, 107, 16757–16758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaut, O.; Schmitt, I.; Wullner, U. Genome-scale methylation analysis of Parkinson’s disease patients’ brains reveals DNA hypomethylation and increased mRNA expression of cytochrome P450 2E1. Neurogenetics 2012, 13, 87–91. [Google Scholar] [CrossRef]
- Jakovcevski, M.; Akbarian, S. Epigenetic mechanisms in neurological disease. Nat. Med. 2012, 18, 1194–1204. [Google Scholar] [CrossRef] [Green Version]
- Barrachina, M.; Ferrer, I. DNA methylation of Alzheimer disease and tauopathy-related genes in postmortem brain. J. Neuropathol. Exp. Neurol. 2009, 68, 880–891. [Google Scholar] [CrossRef]
- De Mena, L.; Cardo, L.F.; Coto, E.; Alvarez, V.; Coto, E. No differential DNA methylation of PARK2 in brain of Parkinson’s disease patients and healthy controls. Mov. Disord. 2013, 28, 2032–2033. [Google Scholar] [CrossRef]
- Gu, J.; Barrera, J.; Yun, Y.; Murphy, S.K.; Beach, T.G.; Woltjer, R.L.; Serrano, G.E.; Kantor, B.; Chiba-Falek, O. Cell-Type Specific Changes in DNA Methylation of SNCA Intron 1 in Synucleinopathy Brains. Front. Neurosci. 2021, 15, 652226. [Google Scholar] [CrossRef]
- Matsumoto, L.; Takuma, H.; Tamaoka, A.; Kurisaki, H.; Date, H.; Tsuji, S.; Iwata, A. CpG demethylation enhances alpha-synuclein expression and affects the pathogenesis of Parkinson’s disease. PLoS ONE 2010, 5, e15522. [Google Scholar] [CrossRef] [Green Version]
- Jowaed, A.; Schmitt, I.; Kaut, O.; Wullner, U. Methylation regulates alpha-synuclein expression and is decreased in Parkinson’s disease patients’ brains. J. Neurosci. 2010, 30, 6355–6359. [Google Scholar] [CrossRef] [PubMed]
- de Boni, L.; Tierling, S.; Roeber, S.; Walter, J.; Giese, A.; Kretzschmar, H.A. Next-generation sequencing reveals regional differences of the alpha-synuclein methylation state independent of Lewy body disease. Neuromol. Med. 2011, 13, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Kibbe, W.A.; Lin, S.M. lumi: A pipeline for processing Illumina microarray. Bioinformatics 2008, 24, 1547–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Garza, O.; Baccarelli, A.A.; Byun, H.M.; Marquez-Gamino, S.; Barron-Vivanco, B.S.; Albores, A. CYP2E1 epigenetic regulation in chronic, low-level toluene exposure: Relationship with oxidative stress and smoking habit. Toxicol. Appl. Pharmacol. 2015, 286, 207–215. [Google Scholar] [CrossRef]
- Lee, S.S.; Buters, J.T.; Pineau, T.; Fernandez-Salguero, P.; Gonzalez, F.J. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J. Biol. Chem. 1996, 271, 12063–12067. [Google Scholar] [CrossRef] [Green Version]
- Watts, P.M.; Riedl, A.G.; Douek, D.C.; Edwards, R.J.; Boobis, A.R.; Jenner, P.; Marsden, C.D. Co-localization of P450 enzymes in the rat substantia nigra with tyrosine hydroxylase. Neuroscience 1998, 86, 511–519. [Google Scholar] [CrossRef]
- Vaglini, F.; Viaggi, C.; Piro, V.; Pardini, C.; Gerace, C.; Scarselli, M.; Corsini, G.U. Acetaldehyde and parkinsonism: Role of CYP450 2E1. Front. Behav. Neurosci. 2013, 7, 71. [Google Scholar] [CrossRef] [Green Version]
- Goldman, S.M.; Quinlan, P.J.; Ross, G.W.; Marras, C.; Meng, C.; Bhudhikanok, G.S.; Comyns, K.; Korell, M.; Chade, A.R.; Kasten, M.; et al. Solvent exposures and Parkinson disease risk in twins. Ann. Neurol. 2012, 71, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Gash, D.M.; Rutland, K.; Hudson, N.L.; Sullivan, P.G.; Bing, G.; Cass, W.A.; Pandya, J.D.; Liu, M.; Choi, D.Y.; Hunter, R.L.; et al. Trichloroethylene: Parkinsonism and complex 1 mitochondrial neurotoxicity. Ann. Neurol. 2008, 63, 184–192. [Google Scholar] [CrossRef]
- Gu, X.; Sun, J.; Li, S.; Wu, X.; Li, L. Oxidative stress induces DNA demethylation and histone acetylation in SH-SY5Y cells: Potential epigenetic mechanisms in gene transcription in Abeta production. Neurobiol. Aging 2013, 34, 1069–1079. [Google Scholar] [CrossRef]
- Caro, A.A.; Cederbaum, A.I. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, H.N.; Andersson, D.R.; Nissbrandt, H. Cytochrome P450 2E1 in the substantia nigra: Relevance for dopaminergic neurotransmission and free radical production. Synapse 2008, 62, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Viaggi, C.; Vaglini, F.; Pardini, C.; Caramelli, A.; Corsini, G.U. MPTP-induced model of Parkinson’s disease in cytochrome P450 2E1 knockout mice. Neuropharmacology 2009, 56, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Nissbrandt, H.; Bergquist, F.; Jonason, J.; Engberg, G. Inhibition of cytochrome P450 2E1 induces an increase in extracellular dopamine in rat substantia nigra: A new metabolic pathway? Synapse 2001, 40, 294–301. [Google Scholar] [CrossRef]
- Cao, Q.; Mak, K.M.; Lieber, C.S. Cytochrome P4502E1 primes macrophages to increase TNF-alpha production in response to lipopolysaccharide. Am. J. Physiol. Gastrointest Liver Physiol. 2005, 289, G95–G107. [Google Scholar] [CrossRef]
- Schmitt, I.; Kaut, O.; Khazneh, H.; deBoni, L.; Ahmad, A.; Berg, D.; Klein, C.; Frohlich, H.; Wullner, U. L-dopa increases alpha-synuclein DNA methylation in Parkinson’s disease patients in vivo and in vitro. Mov. Disord. 2015, 30, 1794–1801. [Google Scholar] [CrossRef]
- McDonnell, L.; Maginnis, C.; Lewis, S.; Pickering, N.; Antoniak, M.; Hubbard, R.; Lawson, I.; Britton, J. Occupational exposure to solvents and metals and Parkinson’s disease. Neurology 2003, 61, 716–717. [Google Scholar] [CrossRef]
- Guehl, D.; Bezard, E.; Dovero, S.; Boraud, T.; Bioulac, B.; Gross, C. Trichloroethylene and parkinsonism: A human and experimental observation. Eur. J. Neurol. 1999, 6, 609–611. [Google Scholar] [CrossRef]
- Liu, M.; Choi, D.Y.; Hunter, R.L.; Pandya, J.D.; Cass, W.A.; Sullivan, P.G.; Kim, H.C.; Gash, D.M.; Bing, G. Trichloroethylene induces dopaminergic neurodegeneration in Fisher 344 rats. J. Neurochem. 2010, 112, 773–783. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Takano, T.; Nakamura, K. Effect of ethanol on the metabolism of trichloroethylene in the perfused rat liver. J. Toxicol. Environ. Health A 1998, 55, 297–305. [Google Scholar] [CrossRef]
- Kaut, O.; Schmitt, I.; Tost, J.; Busato, F.; Liu, Y.; Hofmann, P.; Witt, S.H.; Rietschel, M.; Frohlich, H.; Wullner, U. Epigenome-wide DNA methylation analysis in siblings and monozygotic twins discordant for sporadic Parkinson’s disease revealed different epigenetic patterns in peripheral blood mononuclear cells. Neurogenetics 2017, 18, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, M.; Saiki, S.; Li, Y.; Kaga, N.; Taka, H.; Hatano, T.; Ishikawa, K.I.; Oji, Y.; Mori, A.; Okuzumi, A.; et al. Serum caffeine and metabolites are reliable biomarkers of early Parkinson disease. Neurology 2018, 90, e404–e411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson-Smith, A.; Fisch, K.M.; Hua, J.; Liu, G.; Ricciardelli, E.; Jepsen, K.; Huentelman, M.; Stalberg, G.; Edland, S.D.; Scherzer, C.R.; et al. DNA methylation changes associated with Parkinson’s disease progression: Outcomes from the first longitudinal genome-wide methylation analysis in blood. Epigenetics 2019, 14, 365–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, V. Epigenetics: The sins of the father. Nature 2014, 507, 22–24. [Google Scholar] [CrossRef]
- Liu, F.; Hindupur, J.; Nguyen, J.L.; Ruf, K.J.; Zhu, J.; Schieler, J.L.; Bonham, C.C.; Wood, K.V.; Davisson, V.J.; Rochet, J.C. Methionine sulfoxide reductase A protects dopaminergic cells from Parkinson’s disease-related insults. Free Radic. Biol. Med. 2008, 45, 242–255. [Google Scholar] [CrossRef] [Green Version]
- Maunakea, A.K.; Nagarajan, R.P.; Bilenky, M.; Ballinger, T.J.; D’Souza, C.; Fouse, S.D.; Johnson, B.E.; Hong, C.; Nielsen, C.; Zhao, Y.; et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature 2010, 466, 253–257. [Google Scholar] [CrossRef]
- Maas, A.M.; Bretz, A.C.; Mack, E.; Stiewe, T. Targeting p73 in cancer. Cancer Lett. 2013, 332, 229–236. [Google Scholar] [CrossRef]
- Tyrberg, B.; Miles, P.; Azizian, K.T.; Denzel, M.S.; Nieves, M.L.; Monosov, E.Z.; Levine, F.; Ranscht, B. T-cadherin (Cdh13) in association with pancreatic beta-cell granules contributes to second phase insulin secretion. Islets 2011, 3, 327–337. [Google Scholar] [CrossRef] [Green Version]


| Rank 1 | Target ID 2 | Gene 3 | Chr. 4 | Control 4 | PD | Difference 5 | p-Value | Adjusted p-Value |
|---|---|---|---|---|---|---|---|---|
| Genes hypomethylated in PD | ||||||||
| 1 | cg26077133 | MSRA | 8 | 1.375 | −0.5731 | −1.9481 | 0.0028 | 0.6990 |
| 2 | cg21388339 | TP73 | 1 | 1.4532 | −0.4532 | −1.9064 | 0.0006 | 0.6547 |
| 3 | cg00424152 | B3GNT7 | 2 | 1.9356 | 0.196 | −1.7396 | 0.0046 | 0.7104 |
| 4 | cg13573375 | PIAS4 | 19 | 1.3363 | −0.2018 | −1.5381 | 0.0038 | 0.7041 |
| 5 | cg13732083 | C21orf56 | 21 | −0.3452 | −1.7818 | −1.4366 | 0.0066 | 0.7352 |
| 6 | cg25708755 | PTPRN2 | 7 | 3.8666 | 2.4326 | −1.434 | 0.0002 | 0.6339 |
| 7 | cg05896524 | C21orf56 | 21 | 0.2687 | −1.0967 | −1.3654 | 0.0086 | 0.7445 |
| 8 | cg10296238 | C21orf56 | 21 | 1.6757 | 0.375 | −1.3007 | 0.0036 | 0.7028 |
| 9 | cg02898977 | TM9SF1 | 14 | 3.5181 | 2.2475 | −1.2706 | 0.0009 | 0.6608 |
| 10 | cg11445109 | CYP2E1 | 10 | −1.633 | −2.8688 | −1.2358 | 0.0042 | 0.7086 |
| 11 | cg04563766 | PRKACG | 9 | 1.6484 | 0.4168 | −1.2316 | 0.0033 | 0.7028 |
| 12 | cg23400446 | CYP2E1 | 10 | −0.7482 | −1.978 | −1.2298 | 0.0059 | 0.7226 |
| 13 | cg09672255 | ZNF879 | 5 | 1.9644 | 0.7365 | −1.2279 | 0.0056 | 0.7162 |
| 14 | cg17763566 | HLA-DPB2 | 6 | 2.9618 | 1.7579 | −1.2039 | 0.0013 | 0.6766 |
| 15 | cg25545878 | C21orf56 | 21 | 0.3828 | −0.8175 | −1.2003 | 0.0046 | 0.7105 |
| 16 | cg19299952 | MOBKL2A | 19 | 4.3039 | 3.1272 | −1.1767 | 0.0081 | 0.7393 |
| 17 | cg12016809 | C21orf56 | 21 | 0.3056 | −0.8639 | −1.1695 | 0.0061 | 0.7288 |
| 18 | cg08925606 | BPIL1 | 20 | 2.8527 | 1.6876 | −1.1651 | 0.0007 | 0.6608 |
| 19 | cg07747299 | C21orf56 | 21 | −0.0209 | −1.1486 | −1.1277 | 0.0043 | 0.7092 |
| 20 | cg13315147 | CYP2E1 | 10 | −0.5431 | −1.6485 | −1.1054 | 0.0034 | 0.7028 |
| 21 | cg23026554 | LOC441666 | 10 | −2.2328 | −3.3233 | −1.0905 | 0.0024 | 0.6948 |
| 22 | cg18093448 | WWC2 | 4 | 1.774 | 0.705 | −1.069 | 0.0004 | 0.6339 |
| 23 | cg20965743 | ASPG | 14 | −0.8006 | −1.8428 | −1.0422 | 0.0004 | 0.6065 |
| 24 | cg24753094 | THSD4 | 15 | 2.386 | 1.3613 | −1.0247 | 0.0038 | 0.7028 |
| 25 | cg24530264 | CYP2E1 | 10 | −1.3224 | −2.3267 | −1.0043 | 0.0096 | 0.7445 |
| 26 | cg10862468 | CYP2E1 | 10 | −0.8401 | −1.7957 | −0.9556 | 0.0025 | 0.6948 |
| 27 | cg24136292 | INSC | 11 | 1.2508 | 0.3108 | −0.94 | 0.0022 | 0.6948 |
| 28 | cg21787989 | LGALS8 | 1 | 2.5081 | 1.6097 | −0.8984 | 0.0037 | 0.7028 |
| 29 | cg04398451 | MYO15A | 17 | 3.0648 | 2.1705 | −0.8943 | 0.0095 | 0.7445 |
| 30 | cg19039925 | GVIN1 | 11 | 2.9298 | 2.1079 | −0.8219 | 0.0072 | 0.7377 |
| 31 | cg10309386 | BEND7 | 10 | 0.9543 | 0.1425 | −0.8118 | 0.0055 | 0.7153 |
| 32 | cg00631877 | PLXNC1 | 12 | 1.7795 | 1.0282 | −0.7513 | 0.0015 | 0.6796 |
| 33 | cg25094735 | NAPSB | 19 | 1.8552 | 1.1323 | −0.7229 | 0.0028 | 0.6990 |
| 34 | cg11133658 | SCARA5 | 8 | 3.6407 | 2.9677 | −0.673 | 0.0056 | 0.7165 |
| 35 | cg24705404 | DAB1 | 1 | 0.8954 | 0.3551 | −0.5403 | 0.0055 | 0.7157 |
| Genes hypermethylated in PD | ||||||||
| 1 | cg07179329 | CDH13 | 16 | −1.0161 | 3.2007 | 4.2168 | 2.41 × 10−6 | 0.2671 |
| 2 | cg21498547 | DLGAP2 | 19 | −2.498 | 0.2503 | 2.7483 | 0.0055 | 0.7157 |
| 3 | cg00713204 | BANP | 16 | −1.6172 | 0.2791 | 1.8963 | 0.0003 | 0.6339 |
| 4 | cg06051619 | DIP2C | 10 | −1.0376 | 0.8079 | 1.8455 | 0.0008 | 0.6608 |
| 5 | cg05385718 | D2HGDH | 2 | −0.1851 | 1.6527 | 1.8378 | 0.0092 | 0.7445 |
| 6 | cg22508145 | CPAMD8 | 19 | 0.2203 | 2.0561 | 1.8358 | 0.0055 | 0.7142 |
| 7 | cg27649396 | C11orf53 | 11 | 0.3793 | 2.1594 | 1.7801 | 0.0030 | 0.7000 |
| 8 | cg11716267 | LOC375190 | 2 | −0.9379 | 0.6719 | 1.6098 | 0.0025 | 0.6948 |
| 9 | cg24861399 | MGAT5 | 2 | −1.1351 | 0.3973 | 1.5324 | 0.0057 | 0.7197 |
| 10 | cg02938066 | NFKBIL2 | 8 | 1.4743 | 2.954 | 1.4797 | 0.0038 | 0.7028 |
| 11 | cg23633026 | RASL10B | 17 | 1.7681 | 3.223 | 1.4549 | 0.0018 | 0.6831 |
| 12 | cg19680693 | GPR83 | 11 | −0.5695 | 0.8113 | 1.3808 | 0.0034 | 0.7028 |
| 13 | cg10224537 | B3GALT1 | 2 | 2.1048 | 3.4393 | 1.3345 | 0.0004 | 0.6339 |
| 14 | cg25790212 | IGSF9B | 11 | −1.7987 | −0.4692 | 1.3295 | 0.0005 | 0.6489 |
| 15 | cg09746326 | DCUN1D2 | 13 | 1.559 | 2.7568 | 1.1978 | 0.0061 | 0.7288 |
| 16 | cg18662228 | AGAP1 | 6 | −1.0355 | 0.1227 | 1.1582 | 0.0066 | 0.7352 |
| 17 | cg12687426 | KCNMB3 | 3 | 2.2947 | 3.3274 | 1.0327 | 0.0001 | 0.6065 |
| 18 | cg04381873 | LOC284412 | 19 | 2.0913 | 2.9199 | 0.8286 | 0.0036 | 0.7028 |
| 19 | cg11342941 | AGPAT4 | 6 | 2.9545 | 3.7636 | 0.8091 | 0.0054 | 0.7142 |
| 20 | cg06979412 | RFC3 | 13 | 2.9372 | 3.6803 | 0.7431 | 0.0092 | 0.7445 |
| 21 | cg22402398 | FGR | 1 | 3.0874 | 3.8231 | 0.7357 | 0.0077 | 0.7377 |
| 22 | cg08955548 | PTPRN2 | 7 | 1.3085 | 1.9535 | 0.645 | 0.0019 | 0.6845 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaut, O.; Schmitt, I.; Stahl, F.; Fröhlich, H.; Hoffmann, P.; Gonzalez, F.J.; Wüllner, U. Epigenome-Wide Analysis of DNA Methylation in Parkinson’s Disease Cortex. Life 2022, 12, 502. https://doi.org/10.3390/life12040502
Kaut O, Schmitt I, Stahl F, Fröhlich H, Hoffmann P, Gonzalez FJ, Wüllner U. Epigenome-Wide Analysis of DNA Methylation in Parkinson’s Disease Cortex. Life. 2022; 12(4):502. https://doi.org/10.3390/life12040502
Chicago/Turabian StyleKaut, Oliver, Ina Schmitt, Fabian Stahl, Holger Fröhlich, Per Hoffmann, Frank J. Gonzalez, and Ullrich Wüllner. 2022. "Epigenome-Wide Analysis of DNA Methylation in Parkinson’s Disease Cortex" Life 12, no. 4: 502. https://doi.org/10.3390/life12040502
APA StyleKaut, O., Schmitt, I., Stahl, F., Fröhlich, H., Hoffmann, P., Gonzalez, F. J., & Wüllner, U. (2022). Epigenome-Wide Analysis of DNA Methylation in Parkinson’s Disease Cortex. Life, 12(4), 502. https://doi.org/10.3390/life12040502








