The Green Microalga Coelastrella thermophila var. globulina (Scenedesmaceae, Chlorophyta) Isolated from an Algerian Hot Spring as a Potential Source of Fatty Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site and Physico-Chemical Analysis
2.2. Isolation and Enrichment Cultures
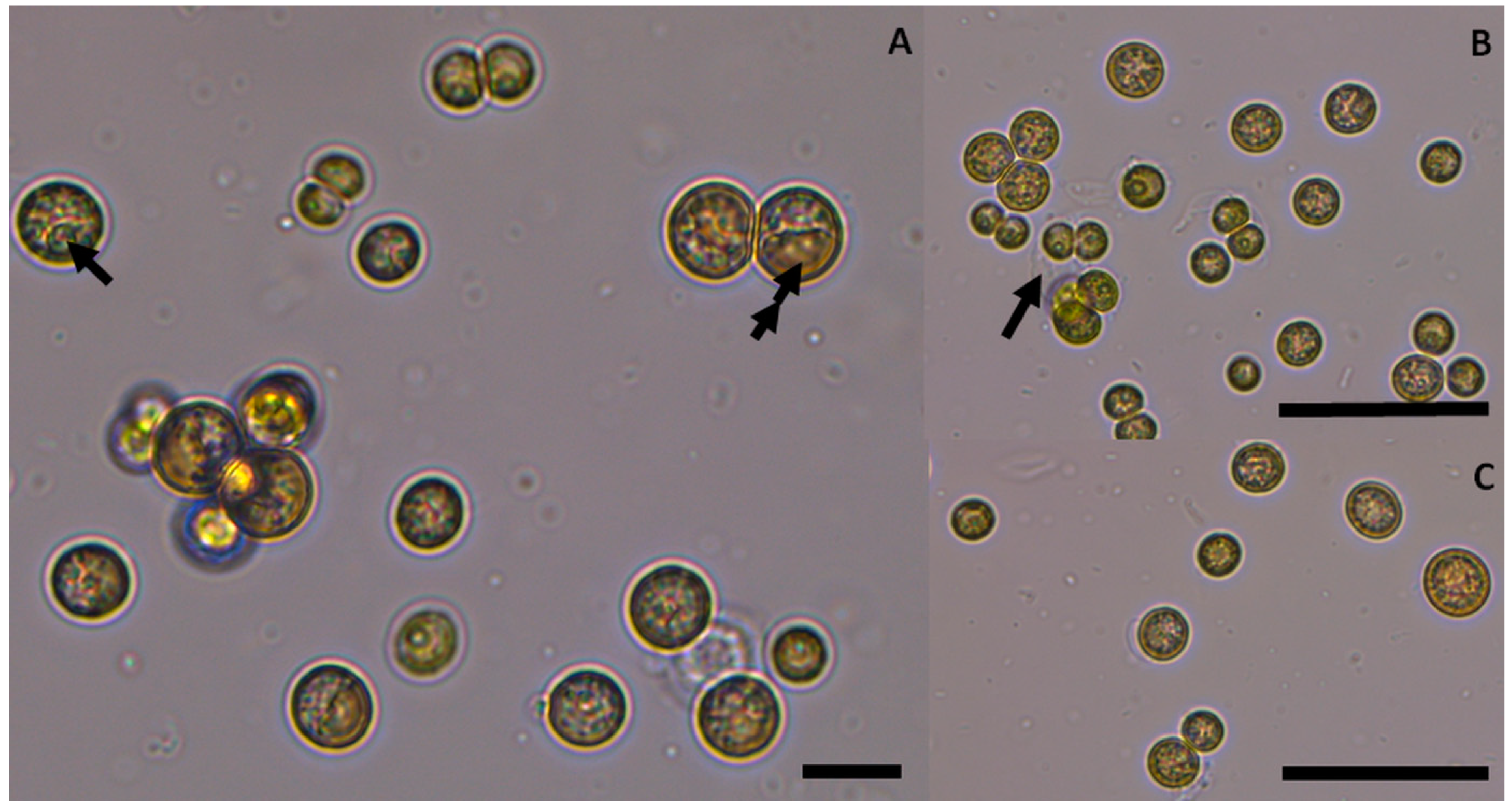
2.3. Morphological Identification
2.4. Molecular Identification and Phylogenetic Analysis
2.5. Lipid Extraction and Fatty Acids Quantification
3. Results
3.1. Physico-Chemical Characteristics of the Algerian Hot Spring
3.2. Species Description
3.3. Phylogenetic Identification
3.4. Fatty Acid Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abou-Shanab, R.A.I.; Hwang, J.-H.; Yunchul, C.; Booki, M.; Jeon, B.-H. Characterization of microalgal species isolated from fresh water bodies as a potential source for biodiesel production. Appl. Energy 2011, 88, 3300–3306. [Google Scholar] [CrossRef]
- De la Vega, M.; Díaz, E.; Vila, M.; León, R. Isolation of a new strain of Picochlorum sp. and characterization of its potential biotechnological applications. Biotechnol. Prog. 2011, 27, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.-P.; Morant-Manceau, A.; Schoefs, B. The Potential of Microalgae for the Production of Bioactive Molecules of Pharmaceutical Interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Wu, K.-C.; Chan, S.C.-Y.; Yau, Y.-H.; Chan, K.-K.; Lee, F.W.-F. Investigation of Growth, Lipid Productivity, and Fatty Acid Profiles in Marine Bloom-Forming Dinoflagellates as Potential Feedstock for Biodiesel. J. Mar. Sci. Eng. 2020, 8, 381. [Google Scholar] [CrossRef]
- Aguilera, A.; Souza-Egipsy, V.; Amils, R. Photosynthesis in Extreme Environments. In Artificial Photosynthesis, 1st ed.; Najafpour, M.M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 271–288. [Google Scholar]
- Jonker, C.Z.; Van Ginkel, C.; Olivier, J. Association between physical and geochemical characteristics of thermal springs and algal diversity in Limpopo Province, South Africa. Water SA 2013, 39, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. A perspective on biotechnological applications of thermophilic microalgae and cyanobacteria. Bioresour. Technol. 2019, 278, 424–434. [Google Scholar] [CrossRef]
- Aboal, M.; González-Silvera, D.; Roldán, M.; Hernández-Mariné, M.; López-Jiménez, J.Á.; Whitton, B.A. The freshwater alga Chroothece richteriana (Rhodophyta) as a potential source of lipids. Food Chem. 2014, 162, 143–148. [Google Scholar] [CrossRef]
- Liu, J.; Vanormelingen, P.; Vyverman, W. Fatty acid profiles of four filamentous green algae under varying culture conditions. Bioresour. Technol. 2016, 200, 1080–1084. [Google Scholar] [CrossRef]
- Thao, T.Y.; Linh, D.T.N.; Si, V.C.; Carter, T.W.; Hill, R.T. Isolation and selection of microalgal strains from natural water sources in Viet Nam with potential for edible oil production. Mar. Drugs 2017, 15, 194. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lim, S.R.; Jeong, D.G.; Kim, J.H. Characterization of an Oleaginous Unicellular Green Microalga, Lobosphaera incisa (Reisigl, 1964) Strain K-1, Isolated From a Tidal Flat in the Yellow Sea, Republic of Korea. Front. Microbiol. 2018, 9, 2159. [Google Scholar] [CrossRef]
- Metzger, P.; Largeau, C. Botryococcus braunii: A rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol. 2005, 66, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Bux, F. Biotechnological applications of microalgae. IeJSME 2013, 6, S24–S37. [Google Scholar]
- Hu, C.-W.; Chuang, L.-T.; Yu, P.-C.; Chen, C.-N.N. Pigment production by a new thermotolerant microalga Coelastrella sp. F50. Food Chem. 2013, 138, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- De Morais, M.G.; Vaz, B.D.S.; Morais, E.G.D.; Costa, J.A.V. Biologically active metabolites synthesized by microalgae. Biomed. Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaignarda, C.; Larochea, C.; Pierrea, G.; Dubessaya, P.; Delattrea, C.; Gardarina, C.; Gourvil, P.; Probertb, I.; Dubuffetc, A.; Michaud, P. Screening of marine microalgae: Investigation of new exopolysaccharide producers. Algal. Res. 2019, 44, 101711. [Google Scholar] [CrossRef]
- Maltsev, Y.; Krivova, Z.; Maltseva, S.; Maltseva, K.; Gorshkova, E.; Kulikovskiy, M. Lipid accumulation by Coelastrella multistriata (Scenedesmaceae, Sphaeropleales) during nitrogen and phosphorus starvation. Sci. Rep. 2021, 11, 19818. [Google Scholar] [CrossRef]
- Abe, K.; Hattori, H.; Hirano, M. Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chem. 2007, 100, 656–661. [Google Scholar] [CrossRef]
- Minyuk, G.; Chelebieva, E.; Chubchikova, I.; Dantsyuk, N.; Drobetskaya, I.; Sakhon, E.; Chekanov, K.; Solovchenko, A. Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids. Algae 2017, 32, 1–15. [Google Scholar] [CrossRef]
- Dimitrova, P.; Marinova, G.; Alexandrov, S.; Iliev, I.; Pilarski, P. Biochemical characteristics of a newly isolated strain Coelastrella sp. BGV cultivated at different temperatures and light intensities. Annu. L Univ. Sofia St. Kliment Ohridski Fac. Biol. 2017, 102, 139–146. [Google Scholar]
- Chodat, R. Matériaux pour l’histoire des algues de la Suisse. Bull. Soc. Bot Geneve. Sér. 1922, 2, 66–114. (In French) [Google Scholar]
- John, D.M. Filamentous and plantlike green algae. In Freshwater Algae of North America, Ecology and Classification, 1st ed.; Wehr, J., Sheath, R., Kociolek, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2002; pp. 311–352. [Google Scholar]
- Wang, Q.; Song, H.; Liu, X.; Liu, B.; Hu., Z.; Liu, G. Morphology and molecular phylogeny of coccoid green algae Coelastrella sensu lato (Scenedesmaceae, Sphaeropeales), including the description of three new species and two new varieties. J. Phycol. 2019, 55, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Goecke, F.; Noda, J.; Paliocha, M.; Gislerød, H.R. Revision of Coelastrella (Scenedesmaceae, Chlorophyta) and first register of this green coccoid microalga for continental Norway. World J. Microbiol. Biotechnol. 2020, 36, 149. [Google Scholar] [CrossRef] [PubMed]
- Ancona-Canché, K.; López-Adrián, S.; Espinosa-Aguilar, M.; Garduño-Solórzano, G.; Toledano-Thompson, T.; Narváez-Zapata, J.; ValdezOjeda, R. Molecular phylogeny and morphologic data of strains of the genus Coelastrella (Chlorophyta, Scenedesmaceae) from a tropical region in North America (Yucatán Peninsula). Bot. Sci. 2017, 95, 527–537. [Google Scholar] [CrossRef] [Green Version]
- MubarakAli, D.; Arunkumar, J.; Nag, K.H.; SheikSyedIshack, K.A.; Baldev, E.; Pandiaraj, D.; Thajuddin, N. Gold nanoparticles from Pro and eukaryotic photosynthetic microorganisms−Comparative studies on synthesis and its application on biolabelling. Colloids Surf. B Biointerfaces 2013, 103, 166–173. [Google Scholar] [CrossRef]
- Luo, L.; He, H.; Yang, C.; Wen, S.; Zeng, G.; Wu, M.; Zhou, Z.; Lou, W. Nutrient removal and lipid production by Coelastrella sp. in anaerobically and aerobically treated swine wastewater. Bioresour. Technol. 2016, 216, 135–141. [Google Scholar] [CrossRef]
- Hindák, F.; Kvíderová, J.; Lukavský, J. Growth characteristics of selected thermophilic strains of cyanobacteria using crossed gradients of temperature and light. Biologia 2013, 68, 830–837. [Google Scholar] [CrossRef]
- Strunecký, O.; Kopejtka, K.; Goecke, F.; Tomasch, J.; Lukavský, J.; Neori, A.; Kahl, S.; Pieper, D.H.; Pilarski, P.; Kaftan, D.; et al. High diversity of thermophilic cyanobacteria in Rupite hot spring identified by microscopy, cultivation, single-cell PCR and amplicon sequencing. Extremophiles 2019, 23, 35–48. [Google Scholar] [CrossRef]
- Adjeroud, M.; Escuder-Rodríguez, J.-J.; González-Siso, M.-I.; Kecha, M. Metagenomic Investigation of Bacterial and Archaeal Diversity of Hammam Essalihine Hot Spring from Khenchela, Algeria. Geomicrobiol. J. 2020, 37, 804–817. [Google Scholar] [CrossRef]
- Bauman, A.J.; Simmonds, P.G. Fatty Acids and Polar Lipids of Extremely Thermophilic Filamentous Bacterial Masses from Two Yellowstone Hot Springs. J. Bacteriol. 1969, 98, 528–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covarrubias, Y.; Cantoral-Uriza, E.A.; Casas-Flores, J.S.; García-Meza, J.V. Thermophile mats of microalgae growing on the woody structure of a cooling tower of a thermoelectric power plant in Central Mexico. Rev. Mex. Biodivers. 2016, 87, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Bouaicha, F.; Dib, H.; Bouteraa, O.; Manchar, N.; Boufaa, K.; Chabour, N.; Demdoum, A. Geochemical assessment, mixing behavior and environmental impact of thermal waters in the Guelma geothermal system, Algeria. Acta. Geochim. 2019, 38, 683–702. [Google Scholar] [CrossRef]
- Foued, B.; Hénia, D.; Lazhar, B.; Nabil, M.; Nabil, C. Hydrogeochemistry and geothermometry of thermal springs from the Guelma region, Algeria. J. Geol. Soc. India 2017, 90, 226–232. [Google Scholar] [CrossRef]
- Michalski, R. Ion Chromatography as a Reference Method for Determination of Inorganic Ions in Water and Wastewater. Crit. Rev. Anal. Chem. 2006, 36, 107–127. [Google Scholar] [CrossRef]
- García, R.; Belmont, R.; Padilla, H.; Torres, M.D.C.; Baez, A.P. Determination of inorganic ions and trace elements in total suspended particles at three urban zones in the Mexico City Metropolitan Area and one rural site. Atmos. Res. 2009, 94, 313–319. [Google Scholar] [CrossRef]
- Muhammad, G.; Alam, M.A.; Xiong, W.; Lv, Y.; Xu, J.L. Microalgae Biomass Production: An Overview of Dynamic Operational Methods. In Microalgae Biotechnology for Food, Health and High Value Products, 1st ed.; Alam, M., Xu, J.L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 415–432. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Alverson, A.J.; Jansen, R.K.; Theriot, E.C. Bridging the Rubicon: Phylogenetic analysis reveals repeated colonizations of marine and fresh waters by thalassiosiroid diatoms. Mol. Phylogenetics Evol. 2007, 45, 193–210. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lee, M.; Stanley, G.A. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Christie, W.W. Lipid Analysis in Isolation, Separation, Identification and Structural Analysis of Lipids, 3rd ed.; The Oily Press: Bridgewater, UK, 2003; pp. 373–387. [Google Scholar]
- Gonzalez-Silvera, D.; Pérez, S.; Korbee, N.; Figueroa, F.L.; Asencio, A.D.; Aboal, M.; López-Jiménez, J.A. Effects of global change factors on fatty acids and mycosporine-like amino acid production in Chroothece richteriana (Rhodophyta). J. Phycol. 2017, 53, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.S.; Seepana, S.; Elankovan, R.; Arumugan, S.; Premalatha, M. Isolation, identification and outdoor cultivation of thermophilic freshwater microalgae Coelastrella sp. FI69 in bubble column reactor for the application of biofuel production. Biocatal. Agric. Biotechnol. 2018, 14, 357–365. [Google Scholar] [CrossRef]
- Sadvakasova, A.K.; Akmukhanova, N.R.; Bolatkhan, K.; Zayadan, B.K.; Usserbayeva, A.A.; Bauenova, M.O.; Akhmetkaliyeva, A.E.; Allakhverdiev, S.I. Search for new strains of microalgae-producers of lipids from natural sources for biodiesel production. Int. J. Hydrogen Energy 2019, 44, 5844–5853. [Google Scholar] [CrossRef]
- Bahri, F.; Saibi, H.; Cherchali, M.E.H. Characterization, classification, and determination of drinkability of some Algerian thermal waters. Arab. J. Geosci. 2011, 4, 207–219. [Google Scholar] [CrossRef]
- Najar, I.N.; Sherpa, M.T.; Das, S.; Das, S.; Thakur, N. Microbial ecology of two hot springs of Sikkim: Predominate population and geochemistry. Sci. Total Env. 2018, 637–638, 730–745. [Google Scholar] [CrossRef]
- Alshareef, M.; Elbeshehy, E.K.F.; Alshehrif, W.A.; Omar, H.H. Identification and Growth Characterization of Native Microalgae Isolated from Different Environments of Saudi Arabia. Biosci. Biotechnol. Res. Commun. 2021, 14, 1065–1075. [Google Scholar] [CrossRef]
- D’Alessandroa, E.B.; Soaresa, A.T.; da Costaa, D.C.; Netoa, H.d.A.S.; Fernandesb, V.d.O.; Filho, N.R.A. A thermal water microalga: Eutetramorus planctonicus as a promising source of fatty acids and lutein. J. Environ. Chem. Eng. 2018, 6, 6707–6713. [Google Scholar] [CrossRef]
- Mutanda, T.; Ramesh, D.; Karthikeyan, S.; Kumari, S.; Anandraj, A.; Bux, F. Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour. Technol. 2011, 102, 57–70. [Google Scholar] [CrossRef]
- Oliveira, D.D.; Vasconcelos, C.T.; Feitosa, A.M.T.; Aboim, J.B.; Oliveira, A.N.; Xavier, L.P.; Santos, A.S.; Gonçalves, E.C.; Filho, G.N.R.; Nascimento, L.A.S. Lipid profile analysis of three new Amazonian cyanobacteria as potential sources of biodiesel. Fuel 2018, 234, 785–788. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Kaiwan-arporn, P.; Hai, P.D.; Thu, N.T.; Annachhatre, A.P. Cultivation of cyanobacteria for extraction of lipids. Biomass Bioenergy 2012, 44, 142–149. [Google Scholar] [CrossRef]
- Vargas, M.A.; Rodrıguez, H.; Moreno, J.; Olivares, H.; Del campo, J.A.; Rivas, J.; Guerrero, M.G. Biochemical composition and fatty acid content of filamentous nitrogen-fixing cyanobacteria. J. Phycol. 1998, 34, 812–817. [Google Scholar] [CrossRef]
- Zili, F.; Mezhoud, N.; Trabelsi, L.; Chreif, I.; Ben Ouada, H. Fatty acid composition of the thermophilic Gloeocapsa gelatinosa under different combinations of temperature, light intensity, and NaNO3 concentration. J. Appl. Phycol. 2015, 27, 97–107. [Google Scholar] [CrossRef]

| Meskhoutine Hot Spring | Meskhoutine |
|---|---|
| Locality | Guelma |
| Coordinates | 36°36′0″ N 7°24′0″ E |
| Temperature °C | 63.00 |
| pH | 7.08 |
| Conductivity mS/m | 0.23 |
| Dissolved oxygen mg/L | 0.63 |
| Ca2+ mg/L | 220.00 |
| Mg2+ mg/L | 33.56 |
| Na+ mg/L | 219.00 |
| K+ mg/L | 25.00 |
| HCO3− mg/L | 366.00 |
| F− mg/L | 2.70 |
| Cl− mg/L | 318.00 |
| Br2 mg/L | 2.17 |
| SO42− mg/L | 367.00 |
| NO3− mg/L | 0.36 |
| SiO2 mg/L | 54.90 |
| Li+ mg/L | 1.16 |
| Fe mg/L | 0.13 |
| Fatty Acids | Coelastrella thermophila var. globulina | Coelastrella sp. L3 | Coelastrella sp. F50 | Coelastrella multistriata MZ–Ch23 | Coelastrella sp. FGS-001 | Coelastrella sp. FI69 | Coelastrella striolata var. multistriata | Coelastrella Rubescens V 195 | Coelastrella sp. BGV |
|---|---|---|---|---|---|---|---|---|---|
| This study | [10] | [16] | [19] | [26] | [47] | [20] | [21] | [22] | |
| 14:0 | 0.40 | 2.46 | - | 0.25 | - | 0.35 | - | - | 2.60 |
| 15:0 | 1.39 | 0.60 | - | - | 0.15 | - | - | - | - |
| 16:0 | 21.45 | 35.98 | 22.23 | 18.61 | 17.70 | 19.88 | 17.90 | 20.05 | 20.30 |
| 18:0 | 2.88 | 14.54 | 3.55 | 1.49 | 0.24 | 3.85 | 1.30 | 0.80 | - |
| 20:0 | 0.44 | 1.26 | 0.47 | - | - | 0.87 | - | - | - |
| 22:0 | 0.27 | 3.80 | - | 0.27 | - | 0.87 | - | - | - |
| 24:0 | 0.19 | ||||||||
| Total SFA | 27.01 | 62.97 | 26.25 | 21.16 | 18.68 | 34.00 | 18.90 | 22.00 | 22.90 |
| 15:1n−5 | 0.12 | - | - | - | - | - | - | - | - |
| 16:1n−9 | 4.67 | - | - | - | - | - | - | - | - |
| 16:1n−7 | 0.71 | 2.64 | 3.34 | 1.63 | 11.18 | 2.70 | 1.00 | 1.00 | - |
| 18:1n−9 | 35.95 | 19.53 | 36.47 | 8.05 | 22.60 | 31.43 | 13.10 | 6.80 | 26.00 |
| 18:1n−7 | 3.04 | - | 1.53 | 2.63 | - | - | 2.20 | - | - |
| 20:1n−9 | 6.45 | 1.21 | 0.43 | - | 0.21 | - | - | - | - |
| 22:1n−9 | 0.08 | ||||||||
| 24:1n−9 | 0.10 | ||||||||
| Total MUFA | 51.12 | 24.52 | 41.47 | 12.04 | 35.43 | 37.00 | 15.30 | 11.60 | 26.00 |
| 18:2n−6 | 14.38 | 6.24 | 13.57 | 7.32 | 7.12 | 14.99 | 22.70 | 20.80 | 15.60 |
| 18:3n−6 | 0.96 | 0.32 | 1.07 | - | 0.89 | - | - | - | - |
| 20:3n−6 | 0.27 | - | - | - | - | - | - | - | - |
| 20:4n−6 | 0.13 | - | - | - | - | - | - | - | - |
| Totaln−6 PUFA | 15.80 | ||||||||
| 18:3n−3 | 4.22 | 3.82 | 8.63 | 38.12 | 32.40 | 6.92 | 28.30 | 30.40 | 35.50 |
| 18:4n−3 | 1.16 | - | 1.33 | - | - | - | - | - | - |
| 22:5n−3 | 0.29 | - | - | - | - | - | - | - | - |
| Totaln−3 PUFA | 5.76 | ||||||||
| Total PUFA | 21.87 | 10.38 | 30.87 | 66.80 | 42.71 | 27.00 | 51.00 | 61.20 | 51.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutarfa, S.; Senoussi, M.M.; Gonzalez-Silvera, D.; López-Jiménez, J.Á.; Aboal, M. The Green Microalga Coelastrella thermophila var. globulina (Scenedesmaceae, Chlorophyta) Isolated from an Algerian Hot Spring as a Potential Source of Fatty Acids. Life 2022, 12, 560. https://doi.org/10.3390/life12040560
Boutarfa S, Senoussi MM, Gonzalez-Silvera D, López-Jiménez JÁ, Aboal M. The Green Microalga Coelastrella thermophila var. globulina (Scenedesmaceae, Chlorophyta) Isolated from an Algerian Hot Spring as a Potential Source of Fatty Acids. Life. 2022; 12(4):560. https://doi.org/10.3390/life12040560
Chicago/Turabian StyleBoutarfa, Soumia, Mohammed Mourad Senoussi, Daniel Gonzalez-Silvera, José Ángel López-Jiménez, and Marina Aboal. 2022. "The Green Microalga Coelastrella thermophila var. globulina (Scenedesmaceae, Chlorophyta) Isolated from an Algerian Hot Spring as a Potential Source of Fatty Acids" Life 12, no. 4: 560. https://doi.org/10.3390/life12040560







