Enhanced Effects of Chronic Restraint-Induced Psychological Stress on Total Body Fe-Irradiation-Induced Hematopoietic Toxicity in Trp53-Heterozygous Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Groups
2.2. Mouse Model for Chronic-Restraint-Induced Psychological Stress
2.3. Irradiation
2.4. Evaluation of Physiological Conditions
2.5. Assessment of Peripheral Hemogram
2.6. Micronucleus Assay
2.7. Statistical Analysis
2.8. Ethics Approval and Disclosure
3. Results
3.1. General Physiological Conditions
3.2. Verification of the CRIPS Model
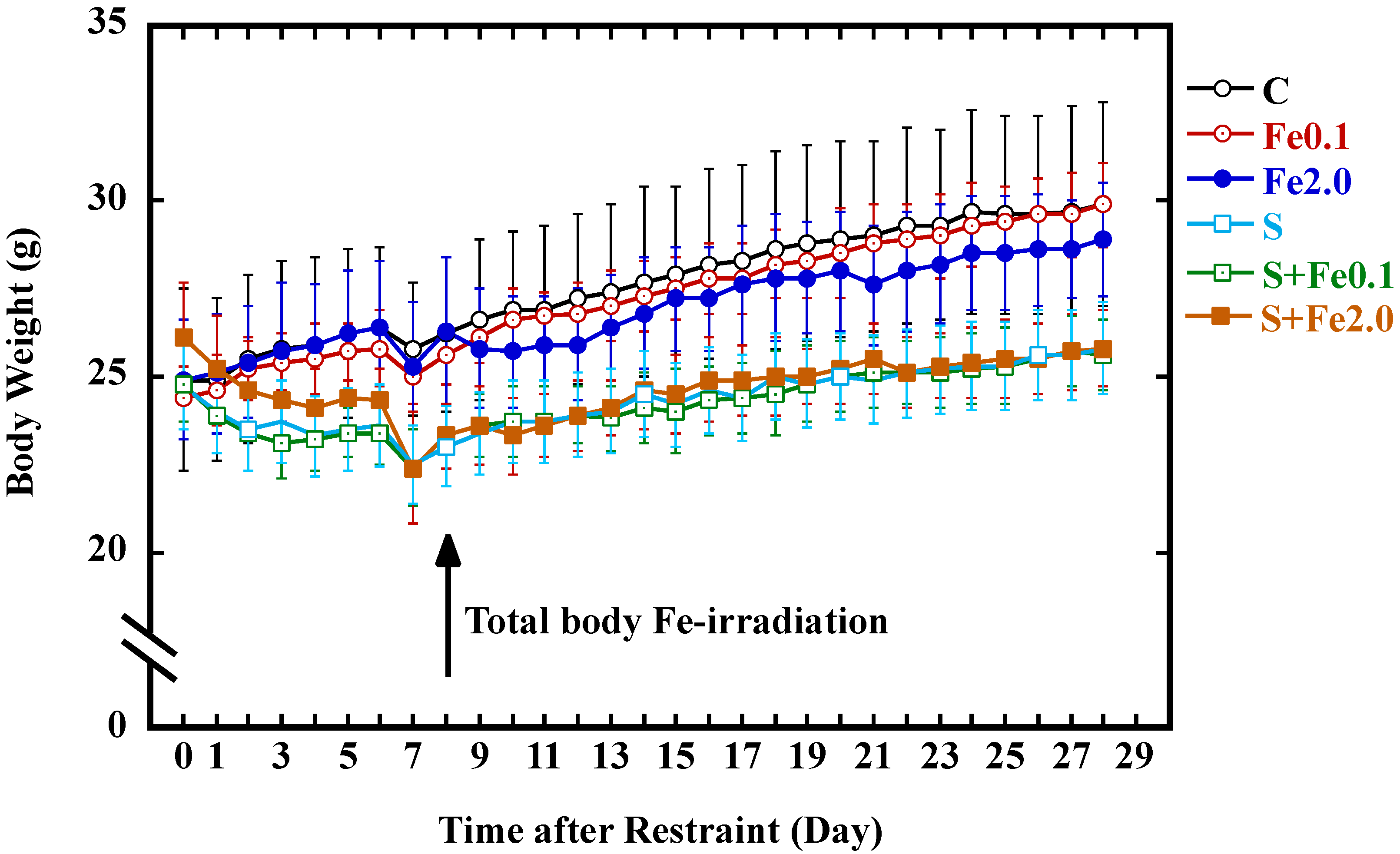
3.3. Change in Body Weight Gain
3.4. Hematological Abnormality in the Peripheral Hemogram
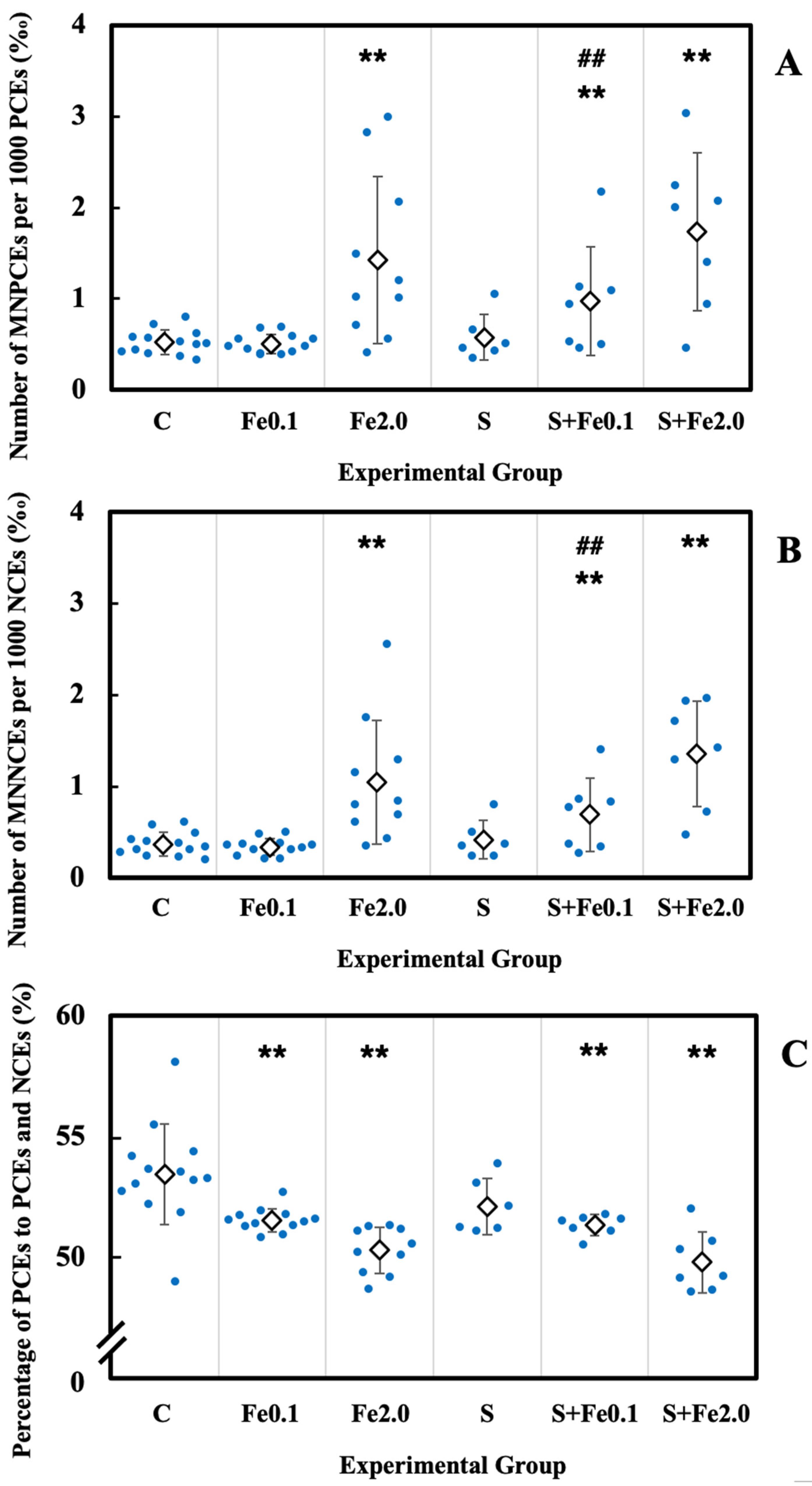
3.5. Residual Damage in Bone Marrow Erythrocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furukawa, S.; Nagamatsu, A.; Nenoi, M.; Fujimori, A.; Kakinuma, S.; Katsube, T.; Wang, B.; Tsuruoka, C.; Shirai, T.; Nakamura, A.J.; et al. Space radiation biology for “Living in Space”. Biomed. Res. Int. 2020, 2020, 4703286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobias, C.A.; Lyman, J.T.; Chatterjee, A.; Howard, J.; Maccabee, H.D.; Raju, M.R.; Smith, A.R.; Sperinde, J.M.; Welch, G.P. Radiological physics characteristics of the extracted heavy ion beams of the bevatron. Science 1971, 174, 1131–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourdarie, S.; Xapsos, M. The near-Earth space radiation environment. IEEE Trans. Nucl. Sci. 2008, 55, 1810–1832. [Google Scholar] [CrossRef] [Green Version]
- Durante, M.; Cucinotta, F.A. Physical basis of radiation protection in space travel. Rev. Mod. Phys. 2011, 83, 1245–1281. [Google Scholar] [CrossRef]
- Durante, M.; Cucinotta, F.A. Heavy ion carcinogenesis and human space exploration. Nat. Rev. Cancer 2008, 8, 465–472. [Google Scholar] [CrossRef]
- Nelson, G.A. Space radiation and human exposures, a primer. Radiat. Res. 2016, 185, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Böttcher, S.; Böhm, E.; et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef] [Green Version]
- Mewaldt, R.A. Galactic cosmic ray composition and energy spectra. Adv. Space Res. 1994, 14, 737–747. [Google Scholar] [CrossRef]
- Yan, X.; Sasi, S.P.; Gee, H.; Lee, J.; Yang, Y.; Mehrzad, R.; Onufrak, J.; Song, J.; Enderling, H.; Agarwal, A.; et al. Cardiovascular risks associated with low dose ionizing particle radiation. PLoS ONE 2014, 9, e110269. [Google Scholar]
- Uri, J. Space Station 20th: Long-duration missions. In NASA History; Mars, K., Ed.; National Aeronautics and Space Administration: Washington, DC, USA, 2020. Available online: https://www.nasa.gov/feature/space-station-20th-long-duration-missions (accessed on 1 February 2022).
- Mars Architecture Steering Group. Human Exploration of Mars: Design Reference Architecture 5.0; Report No. SP-2009-566; NASA Johnson Space Center: Houston, TX, USA, 2009. Available online: https://go.nasa.gov/3uoyT4k (accessed on 1 February 2022).
- Cohen, S.; Janicki-Deverts, D.; Miller, G.E. Psychological stress and disease. JAMA 2007, 298, 1685–1687. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, N.; Kusano, T.; Kinoshita, S.; Nakamoto, H. Influence of perceived stress and stress coping adequacy on multiple health-related lifestyle behaviors. Int. J. Environ. Res. Public Health 2021, 19, 284. [Google Scholar] [CrossRef] [PubMed]
- Biddle, S.J.; Asare, M. Physical activity and mental health in children and adolescents: A review of reviews. Br. J. Sports Med. 2011, 45, 886–895. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, D.B.; Thayer, J.F.; Vedhara, K. Stress and health: A review of psychobiological processes. Annu. Rev. Psychol. 2021, 72, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, M.J.; Latimer, J.J.; Baum, A. The effects of tress on DNA repair capacity. Psychol. Health 2000, 15, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Van der Ham, A.J.; van der Aa, H.P.; Brunes, A.; Heir, T.; de Vries, R.; van Rens, G.H.; van Nispen, R.M. The development of posttraumatic stress disorder in individuals with visual impairment: A systematic search and review. Ophthalmic Physiol. Opt. 2021, 41, 331–341. [Google Scholar]
- Fishta, A.; Backé, E.M. Psychosocial stress at work and cardiovascular diseases: An overview of systematic reviews. Int. Arch. Occup. Environ. Health 2015, 88, 997–1014. [Google Scholar] [CrossRef] [Green Version]
- Hackett, R.A.; Steptoe, A. Psychosocial factors in diabetes and cardiovascular risk. Curr. Cardiol. Rep. 2016, 18, 95. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, K.; Schuler, B.R.; Kobulsky, J.M.; Sarwer, D.B. The association between adverse childhood experiences and childhood obesity: A systematic review. Obes. Rev. 2021, 22, e13204. [Google Scholar] [CrossRef]
- Bougea, A.; Anagnostouli, M.; Angelopoulou, E.; Spanou, I.; Chrousos, G. Psychosocial and trauma-related stress and risk of dementia: A meta-analytic systematic review of longitudinal studies. J. Geriatr. Psychiatry Neurol. 2022, 35, 24–37. [Google Scholar] [CrossRef]
- Niedhammer, I.; Bertrais, S.; Witt, K. Psychosocial work exposures and health outcomes: A meta-review of 72 literature reviews with meta-analysis. Scand. J. Work Environ. Health 2021, 47, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.D.; Tarr, A.J.; Sheridan, J.F. Psychosocial stress and inflammation in cancer. Brain Behav. Immun. 2013, 30, S41–S47. [Google Scholar] [CrossRef] [PubMed]
- Cordova, M.J.; Riba, M.B.; Spiegel, D. Post-traumatic stress disorder and cancer. Lancet Psychiatry 2017, 4, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Ports, K.A.; Holman, D.M.; Guinn, A.S.; Pampati, S.; Dyer, K.E.; Merrick, M.T.; Lunsford, N.B.; Metzler, M. Adverse childhood experiences and the presence of cancer risk factors in adulthood: A scoping review of the literature from 2005 to 2015. J. Pediatr. Nurs. 2019, 44, 81–96. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Bernstein, J.; Gronostaj, M. Psychological stress and cellular aging in cancer: A meta-analysis. Oxid. Med. Cell. Longev. 2019, 2019, 1270397. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.M.; Son, C.G. The risk of psychological stress on cancer recurrence: A systematic review. Cancers 2021, 13, 5816. [Google Scholar] [CrossRef]
- Wang, B.; Katsube, T.; Begum, N.; Nenoi, M. Revisiting the health effects of psychological stress-its influence on susceptibility to ionizing radiation: A mini-review. J. Radiat. Res. 2016, 57, 325–335. [Google Scholar] [CrossRef]
- Antoniuk, S.; Bijata, M.; Ponimaskin, E.; Wlodarczyk, J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci. Biobehav. Rev. 2019, 99, 101–116. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Diamond, D.M. Predator-based psychosocial stress animal model of PTSD: Preclinical assessment of traumatic stress at cognitive, hormonal, pharmacological, cardiovascular and epigenetic levels of analysis. Exp. Neurol. 2016, 284, 211–219. [Google Scholar] [CrossRef]
- Golbidi, S.; Frisbee, J.C.; Laher, I. Chronic stress impacts the cardiovascular system: Animal models and clinical outcomes. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1476–H1498. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, J.; Toth, M.; Der-Avakian, A.; Risbrough, V.B. Current status of animal models of posttraumatic stress disorder: Behavioral and biological phenotypes, and future challenges in improving translation. Biol. Psychiatry 2018, 83, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Verbitsky, A.; Dopfel, D.; Zhang, N. Rodent models of post-traumatic stress disorder: Behavioral assessment. Transl. Psychiatry 2020, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tanaka, K.; Katsube, T.; Ninomiya, Y.; Varès, G.; Liu, Q.; Morita, A.; Nakajima, T.; Nenoi, M. Chronic restraint-induced stress has little modifying effect on radiation hematopoietic toxicity in mice. J. Radiat. Res. 2015, 56, 760–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsube, T.; Wang, B.; Tanaka, K.; Ninomiya, Y.; Varès, G.; Kawagoshi, T.; Shiomi, N.; Kubota, Y.; Liu, Q.; Morita, A.; et al. Effects of chronic restraint-induced stress on radiation-induced chromosomal aberrations in mouse splenocytes. Mutat. Res. 2017, 813, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Liu, L.; Zhang, C.; Zheng, T.; Wang, J.; Lin, M.; Zhao, Y.; Wang, X.; Levine, A.J.; Hu, W. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 7013–7018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsube, T.; Wang, B.; Tanaka, K.; Ninomiya, Y.; Hirakawa, H.; Liu, C.; Maruyama, K.; Varès, G.; Liu, Q.; Kito, S.; et al. Synergistic effects of chronic restraint-induced stress and low-dose 56Fe particle irradiation on induction of chromosomal aberrations in Trp53-heterozygous mice. Radiat. Res. 2021, 196, 100–112. [Google Scholar] [CrossRef]
- Tsukada, T.; Tomooka, Y.; Takai, S.; Ueda, Y.; Nishikawa, S.; Yagi, T.; Tokunaga, T.; Takeda, N.; Suda, Y.; Abe, S.; et al. Enhanced proliferative potential in culture of cells from p53-deficient mice. Oncogene 1993, 8, 3313–3322. [Google Scholar]
- Janssen, A.; van der Burg, M.; Szuhai, K.; Kops, G.J.; Medema, R.H. Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations. Science 2011, 333, 1895–1898. [Google Scholar] [CrossRef]
- Hayashi, M.T.; Karlseder, J. DNA damage associated with mitosis and cytokinesis failure. Oncogene 2013, 32, 4593–4601. [Google Scholar] [CrossRef] [Green Version]
- Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The state of art, and future directions. Int. J. Mol. Sci. 2020, 21, 1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawagoshi, T.; Shiomi, N.; Takahashi, H.; Watanabe, Y.; Fuma, S.; Doi, K.; Kawaguchi, I.; Aoki, M.; Kubota, M.; Furuhata, Y.; et al. Chromosomal aberrations in large Japanese field mice (Apodemus speciosus) captured near Fukushima Dai-ichi Nuclear Power Plant. Environ. Sci. Technol. 2017, 51, 4632–4641. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nagae, Y.; Li, J.; Sakaba, H.; Mozawa, K.; Takahashi, A.; Shimizu, H. The micronucleus test and erythropoiesis. Effects of erythropoietin and a mutagen on the ratio of polychromatic to normochromatic erythrocytes (P/N ratio). Mutagenesis 1989, 4, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; McGuire, L.; Robles, T.F.; Glaser, R. Psychoneuroimmunology: Psychological influences on immune function and health. J. Consult. Clin. Psychol. 2002, 70, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Vitlic, A.; Lord, J.M.; Phillips, A.C. Stress, ageing and their influence on functional, cellular and molecular aspects of the immune system. Age 2014, 36, 9631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragoş, D.; Tănăsescu, M.D. The effect of stress on the defense systems. J. Med. Life 2010, 3, 10–18. [Google Scholar] [PubMed]
- Nishitani, N.; Sakakibara, H. Association of psychological stress response of fatigue with white blood cell count in male daytime workers. Ind. Health 2014, 52, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Nwaigwe, C.U.; Ihedioha, J.I.; Shoyinka, S.V.; Nwaigwe, C.O. Evaluation of the hematological and clinical biochemical markers of stress in broiler chickens. Vet. World 2020, 13, 2294–2300. [Google Scholar] [CrossRef]
- Morita, K.; Saito, T.; Ohta, M.; Ohmori, T.; Kawai, K.; Teshima-Kondo, S.; Rokutan, K. Expression analysis of psychological stress-associated genes in peripheral blood leukocytes. Neurosci. Lett. 2005, 381, 57–62. [Google Scholar] [CrossRef]
- Maes, M.; Van Bockstaele, D.R.; Gastel, A.; Song, C.; Schotte, C.; Neels, H.; DeMeester, I.; Scharpe, S.; Janca, A. The effects of psychological stress on leukocyte subset distribution in humans: Evidence of immune activation. Neuropsychobiology 1999, 39, 1–9. [Google Scholar] [CrossRef]
- Guan, S.Z.; Liu, J.W.; Fang, E.F.; Ng, T.B.; Lian, Y.L.; Ge, H. Chronic unpredictable mild stress impairs erythrocyte immune function and changes T-lymphocyte subsets in a rat model of stress-induced depression. Environ. Toxicol. Pharmacol. 2014, 37, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Frick, L.R.; Arcos, M.L.; Rapanelli, M.; Zappia, M.P.; Brocco, M.; Mongini, C.; Genaro, A.M.; Cremaschi, G.A. Chronic restraint stress impairs T-cell immunity and promotes tumor progression in mice. Stress 2009, 12, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Frick, L.R.; Rapanelli, M.; Bussmann, U.A.; Klecha, A.J.; Arcos, M.L.; Genaro, A.M.; Cremaschi, G.A. Involvement of thyroid hormones in the alterations of T-cell immunity and tumor progression induced by chronic stress. Biol. Psychiatry 2009, 65, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Reiche, E.M.; Morimoto, H.K.; Nunes, S.M. Stress and depression-induced immune dysfunction: Implications for the development and progression of cancer. Int. Rev. Psychiatry 2005, 17, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pan, J.; Chen, W.; Jiang, J.; Huang, J. Chronic stress-induced immune dysregulation in cancer: Implications for initiation, progression, metastasis, and treatment. Am. J. Cancer Res. 2020, 10, 1294–1307. [Google Scholar]
- Hong, H.; Ji, M.; Lai, D. Chronic stress effects on tumor: Pathway and mechanism. Front. Oncol. 2021, 11, 738252. [Google Scholar] [CrossRef]
- Tian, W.; Liu, Y.; Cao, C.; Zeng, Y.; Pan, Y.; Liu, X.; Peng, Y.; Wu, F. Chronic stress: Impacts on tumor microenvironment and implications for anti-cancer treatments. Front. Cell Dev. Biol. 2021, 9, 777018. [Google Scholar] [CrossRef]
- Tang, F.R.; Loke, W.K. Molecular mechanisms of low dose ionizing radiation-induced hormesis, adaptive responses, radioresistance, bystander effects, and genomic instability. Int. J. Radiat. Biol. 2015, 91, 13–27. [Google Scholar] [CrossRef]
- Tang, F.R.; Loke, W.K.; Khoo, B.C. Low-dose or low-dose-rate ionizing radiation-induced bioeffects in animal models. J. Radiat. Res. 2017, 58, 165–182. [Google Scholar] [CrossRef]
- Tang, F.R.; Loganovsky, K. Low dose or low dose rate ionizing radiation-induced health effect in the human. J. Environ. Radioact. 2018, 192, 32–47. [Google Scholar] [CrossRef]
- Fournier, C.; Taucher-Scholz, G. Radiation induced cell cycle arrest: An overview of specific effects following high-LET exposure. Radiother. Oncol. 2004, 73, S119–S122. [Google Scholar] [CrossRef]
- Niemantsverdriet, M.; van Goethem, M.J.; Bron, R.; Hogewerf, W.; Brandenburg, S.; Langendijk, J.A.; van Luijk, P.; Coppes, R.P. High and low LET radiation differentially induce normal tissue damage signals. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1291–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, K.J.; Coates, P.J.; Lorimore, S.A.; Wright, E.G. The genetic basis of tissue responses to ionizing radiation. Br. J. Radiol. 2007, 80, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.P. Cancer. p53, guardian of the genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Blagih, J.; Buck, M.D.; Vousden, K.H. p53, cancer and the immune response. J. Cell Sci. 2020, 133, jcs237453. [Google Scholar] [CrossRef] [Green Version]
- The TP53 WEB SITE. Available online: http://p53.fr/tp53-information (accessed on 1 February 2022).
- Branca, J.A.; Low, B.E.; Saxl, R.L.; Sargent, J.K.; Doty, R.A.; Wiles, M.V.; Dumont, B.L.; Hasham, M.G. Loss of TRP53 (p53) accelerates tumorigenesis and changes the tumor spectrum of SJL/J mice. Genes Cancer 2020, 11, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Klimovich, B.; Merle, N.; Neumann, M.; Elmshäuser, S.; Nist, A.; Mernberger, M.; Kazdal, D.; Stenzinger, A.; Timofeev, O.; Stiewe, T. p53 partial loss-of-function mutations sensitize to chemotherapy. Oncogene 2021, 41, 1011–1023. [Google Scholar] [CrossRef]
- Pant, V.; Quintás-Cardama, A.; Lozano, G. The p53 pathway in hematopoiesis: Lessons from mouse models, implications for humans. Blood 2012, 120, 5118–5127. [Google Scholar] [CrossRef] [Green Version]
- Mori, E.; Takahashi, A.; Yamakawa, N.; Kirita, T.; Ohnishi, T. High LET heavy ion radiation induces p53-independent apoptosis. J. Radiat. Res. 2009, 50, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Morita, A.; Wang, B.; Tanaka, K.; Katsube, T.; Murakami, M.; Shimokawa, T.; Nishiyama, Y.; Ochi, S.; Satoh, H.; Nenoi, M.; et al. Protective effects of p53 regulatory agents against high-LET radiation-induced injury in mice. Front. Public Health 2020, 8, 601124. [Google Scholar] [CrossRef]
- Shao, L.; Luo, Y.; Zhou, D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid. Redox Signal. 2014, 20, 1447–1462. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.F.; Zhou, P.K.; Woolford, L.B.; Lord, B.I.; Hendry, J.H.; Wang, D.W. Apoptosis in bone marrow cells of mice with different p53 genotypes after gamma-rays irradiation in vitro. J. Environ. Pathol. Toxicol. Oncol. 1995, 14, 159–163. [Google Scholar]
- Engelbrecht, M.; Ndimba, R.; de Kock, M.; Miles, X.; Nair, S.; Fisher, R.; du Plessis, P.; Bolcaen, J.; Botha, M.H.; Zwanepoel, E.; et al. DNA damage response of haematopoietic stem and progenitor cells to high-LET neutron irradiation. Sci. Rep. 2021, 11, 20854. [Google Scholar] [CrossRef]
- French, J.; Storer, R.D.; Donehower, L.A. The nature of the heterozygous Trp53 knockout model for identification of mutagenic carcinogens. Toxicol. Pathol. 2001, 29, 24–29. [Google Scholar] [CrossRef]
- Ranaldi, R.; Palma, S.; Tanzarella, C.; Lascialfari, A.; Cinelli, S.; Pacchierotti, F. Effect of p53 haploinsufficiency on melphalan-induced genotoxic effects in mouse bone marrow and peripheral blood. Mutat. Res. 2007, 615, 57–65. [Google Scholar] [CrossRef]
- Maes, M.; Van Der Planken, M.; Van Gastel, A.; Bruyland, K.; Van Hunsel, F.; Neels, H.; Hendriks, D.; Wauters, A.; Demedts, P.; Janca, A.; et al. Influence of academic examination stress on hematological measurements in subjectively healthy volunteers. Psychiatry Res. 1998, 80, 201–212. [Google Scholar] [CrossRef]
- Qureshi, F.; Alam, J.; Khan, M.A.; Sheraz, G. Effect of examination stress on blood cell parameters of students in a Pakistani medical college. J. Ayub. Med. Coll. Abbottabad. 2002, 14, 20–22. [Google Scholar]
- Crucian, B.; Stowe, R.P.; Pierson, D.L.; Sams, C.F. Immune system dysregulation following short- vs long-duration spaceflight. Aviat. Space Environ. Med. 2008, 79, 835–843. [Google Scholar] [CrossRef]
- Crucian, B.; Stowe, R.P.; Mehta, S.; Quiriarte, H.; Pierson, D.; Sams, C. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity 2015, 1, 15013. [Google Scholar] [CrossRef]
- Rooney, B.V.; Laughlin, M.S.; Mehta, S.K.; Pierson, D.L.; Crucian, B.E.; Simpson, R.J. NK cell function is impaired during long-duration spaceflight. J. Appl. Physiol. 2019, 126, 842–853. [Google Scholar]
- Krieger, S.S.; Zwart, S.R.; Mehta, S.; Wu, H.; Simpson, R.J.; Smith, S.M.; Crucian, B. Alterations in saliva and plasma cytokine concentrations during long-duration spaceflight. Front. Immunol. 2021, 12, 725748. [Google Scholar] [CrossRef] [PubMed]
- Buchheim, J.I.; Matzel, S.; Rykova, M.; Vassilieva, G.; Ponomarev, S.; Nichiporuk, I.; Hörl, M.; Moser, D.; Biere, K.; Feuerecker, M.; et al. Stress related shift toward inflammaging in cosmonauts after long-duration space flight. Front. Physiol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, J.; Ma, W.; Lago, C.U.; Hwang, P.M. Metabolic regulation of oxygen and redox homeostasis by p53: Lessons from evolutionary biology? Free Radic. Biol. Med. 2012, 53, 1279–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humpton, T.J.; Vousden, K.H. Regulation of cellular metabolism and hypoxia by p53. Cold Spring Harb. Perspect. Med. 2016, 6, a026146. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.T.; Patterson, S.M. Hemoconcentration and stress: A review of physiological mechanisms and relevance for cardiovascular disease risk. Biol. Psychol. 1995, 41, 1–27. [Google Scholar] [CrossRef]
- Li, H.; Wang, B.; Zhang, H.; Katsube, T.; Xie, Y.; Gan, L. Apoptosis induction by iron radiation via inhibition of autophagy in Trp53+/- mouse testes: Is chronic restraint-induced stress a modifying factor? Int. J. Biol. Sci. 2018, 14, 1109–1121. [Google Scholar] [CrossRef] [Green Version]
- Beckerman, R.; Prives, C. Transcriptional regulation by p53. Cold Spring Harb. Perspect. Biol. 2010, 2, a000935. [Google Scholar] [CrossRef] [Green Version]
- Speidel, D. The role of DNA damage responses in p53 biology. Arch. Toxicol. 2015, 89, 501–517. [Google Scholar] [CrossRef]
- Stewart-Ornstein, J.; Iwamoto, Y.; Miller, M.A.; Prytyskach, M.A.; Ferretti, S.; Holzer, P.; Kallen, J.; Furet, P.; Jambhekar, A.; Forrester, W.C.; et al. p53 dynamics vary between tissues and are linked with radiation sensitivity. Nat. Commun. 2021, 12, 898. [Google Scholar] [CrossRef]
- Rizzotto, D.; Englmaier, L.; Villunger, A. At a crossroads to cancer: How p53-induced cell fate decisions secure genome integrity. Int. J. Mol. Sci. 2021, 22, 10883. [Google Scholar] [CrossRef]
- Makedonas, G.; Mehta, S.; Chouker, A.; Simpson, R.J.; Marshall, G.; Orange, J.S.; Aunon-Chancellor, S.; Smith, S.M.; Zwart, S.R.; Stowe, R.P.; et al. Specific immunologic countermeasure protocol for deep-space exploration missions. Front. Immunol. 2019, 10, 2407. [Google Scholar] [CrossRef] [Green Version]
- Pavez Loriè, E.; Baatout, S.; Choukér, A.; Buchheim, J.I.; Baselet, B.; Dello Russo, C.; Wotring, V.; Monici, M.; Morbidelli, L.; Gagliardi, D.; et al. The future of personalized medicine in space: From observations to countermeasures. Front. Bioeng. Biotechnol. 2021, 9, 739747. [Google Scholar] [CrossRef]


| Abbreviation | Treatment |
|---|---|
| C | The control group, receiving neither chronic restraint nor total body irradiation (TBI) with Fe-particle radiation (Fe) |
| Fe0.1 | The irradiation group exposed to 0.1 Gy Fe-TBI, receiving only Fe-TBI at 0.1 Gy |
| Fe2.0 | The irradiation group exposed to 2.0 Gy Fe-TBI, receiving only 2.0 Gy Fe-TBI |
| S | The chronic restraint-induced psychological stress (CRIPS) group, receiving only chronic restraint |
| S + Fe0.1 | The chronic restraint and irradiation group exposed to 0.1 Gy Fe-TBI, receiving both chronic restraint and 0.1 Gy Fe-TBI |
| S + Fe2.0 | The chronic restraint and irradiation group exposed to 2.0 Gy Fe-TBI, receiving both chronic restraint and 2.0 Gy Fe-TBI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Katsube, T.; Tanaka, K.; Ninomiya, Y.; Hirakawa, H.; Liu, C.; Maruyama, K.; Varès, G.; Kito, S.; Nakajima, T.; et al. Enhanced Effects of Chronic Restraint-Induced Psychological Stress on Total Body Fe-Irradiation-Induced Hematopoietic Toxicity in Trp53-Heterozygous Mice. Life 2022, 12, 565. https://doi.org/10.3390/life12040565
Wang B, Katsube T, Tanaka K, Ninomiya Y, Hirakawa H, Liu C, Maruyama K, Varès G, Kito S, Nakajima T, et al. Enhanced Effects of Chronic Restraint-Induced Psychological Stress on Total Body Fe-Irradiation-Induced Hematopoietic Toxicity in Trp53-Heterozygous Mice. Life. 2022; 12(4):565. https://doi.org/10.3390/life12040565
Chicago/Turabian StyleWang, Bing, Takanori Katsube, Kaoru Tanaka, Yasuharu Ninomiya, Hirokazu Hirakawa, Cuihua Liu, Kouichi Maruyama, Guillaume Varès, Seiji Kito, Tetsuo Nakajima, and et al. 2022. "Enhanced Effects of Chronic Restraint-Induced Psychological Stress on Total Body Fe-Irradiation-Induced Hematopoietic Toxicity in Trp53-Heterozygous Mice" Life 12, no. 4: 565. https://doi.org/10.3390/life12040565
APA StyleWang, B., Katsube, T., Tanaka, K., Ninomiya, Y., Hirakawa, H., Liu, C., Maruyama, K., Varès, G., Kito, S., Nakajima, T., Fujimori, A., & Nenoi, M. (2022). Enhanced Effects of Chronic Restraint-Induced Psychological Stress on Total Body Fe-Irradiation-Induced Hematopoietic Toxicity in Trp53-Heterozygous Mice. Life, 12(4), 565. https://doi.org/10.3390/life12040565







