The Palliative Care in the Metastatic Spinal Tumors. A Systematic Review on the Radiotherapy and Surgical Perspective
Abstract
1. Introduction
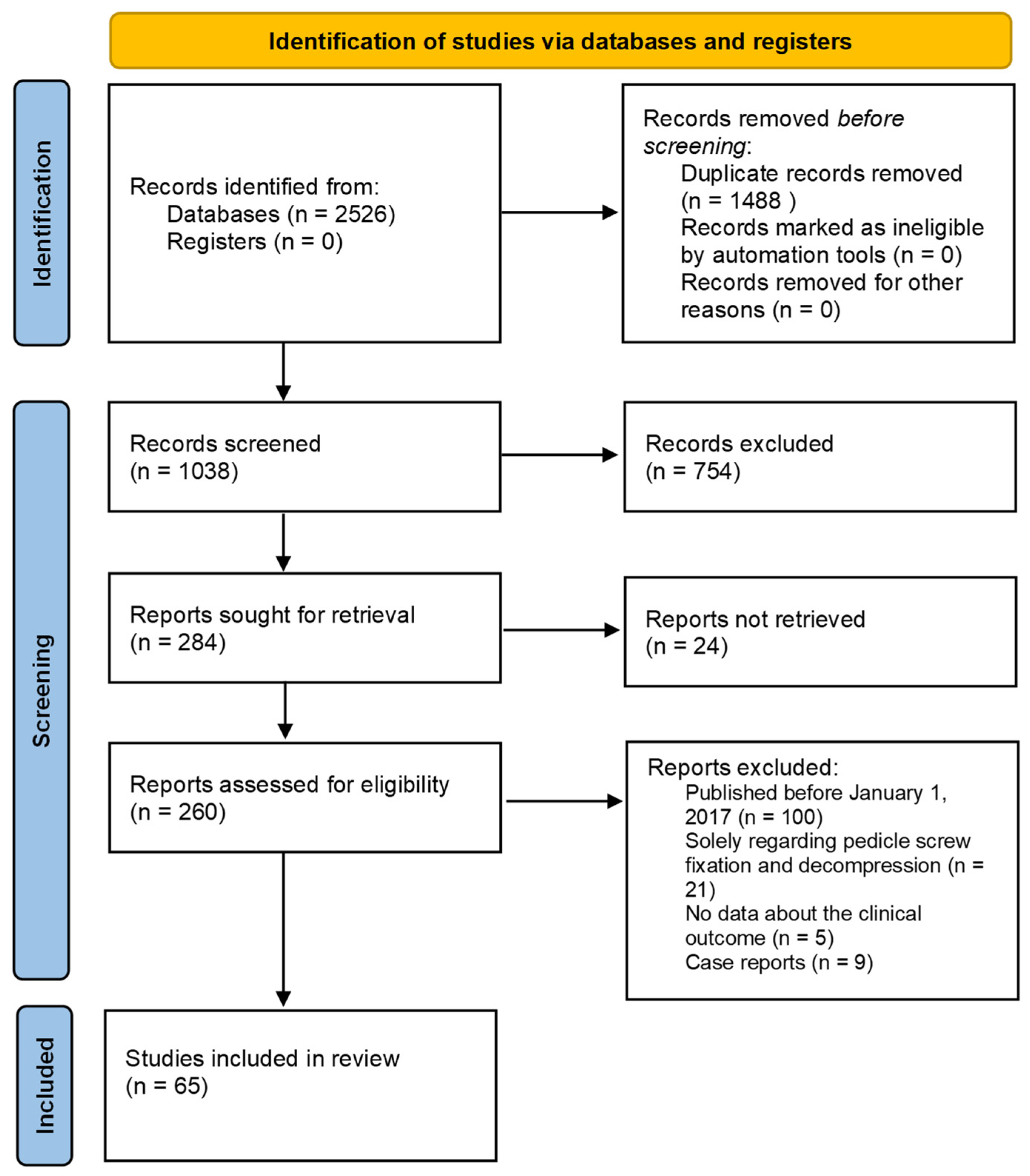
2. Materials and Methods
3. Results
4. Discussion
4.1. Prognostic Scoring Systems for Spinal Metastases
4.2. Radiotherapy
4.2.1. Radiotherapy Protocols and Tolerance
4.2.2. Combination of Radiotherapy and Other Treatments
4.3. Ablative Surgery
4.3.1. Radiofrequency Thermal Ablation (RFA)
4.3.2. Combination of Radiofrequency Thermal Ablation and Other Treatments
4.3.3. Other Ablative Techniques
4.4. Augmentation Surgery
4.4.1. Percutaneous Vertebroplasty (PVP)
4.4.2. Balloon Kyphoplasty (BKP)
4.4.3. Open Kyphoplasty
4.5. Spinal Cord Stimulation (SCS)
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Tsukamoto, S.; Kido, A.; Tanaka, Y.; Facchini, G.; Peta, G.; Rossi, G.; Mavrogenis, A.F. Current Overview of Treatment for Metastatic Bone Disease. Curr. Oncol. 2021, 28, 3347–3372. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, R.; Giammalva, G.R.; Cicero, G.; de Luca, R.; Gulì, C.; Graziano, F.; Basile, L.; Giugno, A.; Iacopino, D.G. Unusual Case of Dorsal Vertebral Metastases from a Male Breast Cancer. Acta Med. Mediterr. 2017, 2017, 1157–1161. [Google Scholar] [CrossRef]
- Kastler, A.; Barbé, D.A.; Alemann, G.; Hadjidekov, G.; Cornelis, F.H.; Kastler, B. Bipolar Radiofrequency Ablation of Painful Spinal Bone Metastases Performed under Local Anesthesia: Feasibility Regarding Patient’s Experience and Pain Outcome. Medicina 2021, 57, 966. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, R.; Giugno, A.; Giammalva, R.G.; Gulì, C.; Basile, L.; Graziano, F.; Iacopino, D.G.G. A Thoracic Vertebral Localization of a Metastasized Cutaneous Merkel Cell Carcinoma: Case Report and Review of Literature. Surg. Neurol. Int. 2017, 8, 190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van der Velden, J.M.; Versteeg, A.L.; Verkooijen, H.M.; Fisher, C.G.; Chow, E.; Oner, F.C.; van Vulpen, M.; Weir, L.; Verlaan, J.-J. Prospective Evaluation of the Relationship Between Mechanical Stability and Response to Palliative Radiotherapy for Symptomatic Spinal Metastases. Oncologist 2017, 22, 972–978. [Google Scholar] [CrossRef]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A Novel Classification System for Spinal Instability in Neoplastic Disease. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef]
- Salvo, N.; Christakis, M.; Rubenstein, J.; de Sa, E.; Napolskikh, J.; Sinclair, E.; Ford, M.; Goh, P.; Chow, E. The Role of Plain Radiographs in Management of Bone Metastases. J. Palliat. Med. 2009, 12, 195–198. [Google Scholar] [CrossRef]
- Bernard, F.; Lemée, J.M.M.; Lucas, O.; Menei, P. Postoperative Quality-of-Life Assessment in Patients with Spine Metastases Treated with Long-Segment Pedicle-Screw Fixation. J. Neurosurg. Spine 2017, 26, 725–735. [Google Scholar] [CrossRef]
- Abrahm, J.L.; Banffy, M.B.; Harris, M.B. Spinal Cord Compression in Patients with Advanced Metastatic Cancer: “All I Care about Is Walking and Living My Life”. JAMA 2008, 299, 937–946. [Google Scholar] [CrossRef]
- Huynh, M.A.; Roldan, C.; Nunes, P.; Kelly, A.; Taylor, A.; Richards, C.; Fareed, M.M.M.; Gorman, D.; Groff, M.; Ferrone, M.; et al. Characteristics of Patients and Treatment Recommendations from a Multidisciplinary Spinal Tumor Program. Palliat. Med. Rep. 2020, 1, 143–148. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents; World Health Organ: Geneva, Switzerland, 2018; ISBN 13: 978-92-4-155039-0. [Google Scholar]
- Paice, J.A.; Portenoy, R.; Lacchetti, C.; Campbell, T.; Cheville, A.; Citron, M.; Constine, L.S.; Cooper, A.; Glare, P.; Keefe, F.; et al. Management of Chronic Pain in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 3325–3345. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, A.; Hanks, G.; Kaasa, S.; Bennett, M.I.; Brunelli, C.; Cherny, N.; Dale, O.; de Conno, F.; Fallon, M.; Hanna, M.; et al. Use of Opioid Analgesics in the Treatment of Cancer Pain: Evidence-Based Recommendations from the EAPC. Lancet. Oncol. 2012, 13, e58–e68. [Google Scholar] [CrossRef]
- Rosenberg, J.; Fabi, A.; Candido, K.; Knezevic, N.; Creamer, M.; Carayannopoulos, A.; Ghodsi, A.; Nelson, C.; Bennett, M. Spinal Cord Stimulation Provides Pain Relief with Improved Psychosocial Function: Results from EMP3OWER. Pain Med. 2016, 17, 2311–2325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vallejo, R.; Gupta, A.; Cedeno, D.L.; Vallejo, A.; Smith, W.J.; Thomas, S.M.; Benyamin, R.; Kaye, A.D.; Manchikanti, L. Clinical Effectiveness and Mechanism of Action of Spinal Cord Stimulation for Treating Chronic Low Back and Lower Extremity Pain: A Systematic Review. Curr. Pain Headache Rep. 2020, 24, 70. [Google Scholar] [CrossRef]
- Smeijers, S.; Depreitere, B. Prognostic Scores for Survival as Decisional Support for Surgery in Spinal Metastases: A Performance Assessment Systematic Review. Eur. Spine J. 2021, 30, 2800–2824. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Uei, H.; Oshima, M.; Ajiro, Y. Scoring System for Prediction of Metastatic Spine Tumor Prognosis. World J. Orthop. 2014, 5, 262–271. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Kawano, H.; Ohsaka, S.; Matsuzaki, H.; Toriyama, S. A Scoring System for Preoperative Evaluation of the Prognosis of Metastatic Spine Tumor (a Preliminary Report). J. Jpn. Orthop. Assoc. 1989, 63, 482–489. [Google Scholar]
- Tokuhashi, Y.; Matsuzaki, H.; Toriyama, S.; Kawano, H.; Ohsaka, S. Scoring System for the Preoperative Evaluation of Metastatic Spine Tumor Prognosis. Spine 1990, 15, 1110–1113. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A Revised Scoring System for Preoperative Evaluation of Metastatic Spine Tumor Prognosis. Spine 2005, 30, 2186–2191. [Google Scholar] [CrossRef]
- Tomita, K.; Kawahara, N.; Kobayashi, T.; Yoshida, A.; Murakami, H.; Akamaru, T. Surgical Strategy for Spinal Metastases. Spine 2001, 26, 298–306. [Google Scholar] [CrossRef]
- Bauer, H.C.F.; Wedin, R. Survival after Surgery for Spinal and Extremity Metastases: Prognostication in 241 Patients. Acta Orthop. 1995, 66, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, Y.M.; Dijkstra, S.P.D.S.; Vonk, E.J.A.; Marijnen, C.A.M.; Leer, J.W.H. Prediction of Survival in Patients with Metastases in the Spinal Column: Results Based on a Randomized Trial of Radiotherapy. Cancer 2005, 103, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, H.; Takahashi, M.; Wakai, K.; Sugiura, H.; Kataoka, T.; Nakanishi, K. Prognostic Factors and a Scoring System for Patients with Skeletal Metastasis. J. Bone Jt. Surg.—Ser. B 2005, 87, 698–703. [Google Scholar] [CrossRef]
- Balain, B.; Jaiswal, A.; Trivedi, J.M.; Eisenstein, S.M.; Kuiper, J.H.; Jaffray, D.C. The Oswestry Risk Index: An Aid in the Treatment of Metastatic Disease of the Spine. J. Bone Jt. Surg.—Ser. B 2013, 95, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Dunst, J.; Schild, S.E. The First Score Predicting Overall Survival in Patients with Metastatic Spinal Cord Compression. Cancer 2008, 112, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Paulino Pereira, N.R.; Mclaughlin, L.; Janssen, S.J.; van Dijk, C.N.; Bramer, J.A.M.; Laufer, I.; Bilsky, M.H.; Schwab, J.H. The SORG Nomogram Accurately Predicts 3- and 12-Months Survival for Operable Spine Metastatic Disease: External Validation. J. Surg. Oncol. 2017, 115, 1019–1027. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Ferrone, M.L.; Schwab, J.H.; Blucher, J.A.; Barton, L.B.; Tobert, D.G.; Chi, J.H.; Shin, J.H.; Kang, J.D.; Harris, M.B. Prospective Validation of a Clinical Prediction Score for Survival in Patients with Spinal Metastases: The New England Spinal Metastasis Score. Spine J. 2021, 21, 28–36. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Le, H.V.; Marjoua, Y.; Leonard, D.A.; Belmont, P.J.; Bono, C.M.; Harris, M.B. Assessing the Utility of a Clinical Prediction Score Regarding 30-Day Morbidity and Mortality Following Metastatic Spinal Surgery: The New England Spinal Metastasis Score (NESMS). Spine J. 2016, 16, 482–490. [Google Scholar] [CrossRef]
- Nakata, E.; Sugihara, S.; Kataoka, M.; Yamashita, N.; Furumatsu, T.; Takigawa, T.; Tetsunaga, T.; Ozaki, T. Early Response Assessment of Re-Ossification after Palliative Conventional Radiotherapy for Vertebral Bone Metastases. J. Orthop. Sci. 2019, 24, 332–336. [Google Scholar] [CrossRef]
- Nakata, E.; Sugihara, S.; Kataoka, M.; Yamashita, N.; Furumatsu, T.; Takigawa, T.; Tetsunaga, T.; Ozaki, T. Early Response Assessment of Palliative Conventional Radiotherapy for Painful Uncomplicated Vertebral Bone Metastases. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2018, 23, 912–917. [Google Scholar] [CrossRef]
- Barzilai, O.; Versteeg, A.L.; Sahgal, A.; Rhines, L.D.; Bilsky, M.H.; Sciubba, D.M.; Schuster, J.M.; Weber, M.H.; Pal Varga, P.; Boriani, S.; et al. Survival, Local Control, and Health-Related Quality of Life in Patients with Oligometastatic and Polymetastatic Spinal Tumors: A Multicenter, International Study. Cancer 2019, 125, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Gallizia, E.; Apicella, G.; Cena, T.; di Genesio Pagliuca, M.; Deantonio, L.; Krengli, M. The Spine Instability Neoplastic Score (SINS) in the Assessment of Response to Radiotherapy for Bone Metastases. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2017, 19, 1382–1387. [Google Scholar] [CrossRef]
- Olson, R.; Schlijper, R.; Chng, N.; Matthews, Q.; Arimare, M.; Mathews, L.; Hsu, F.; Berrang, T.; Louie, A.; Mou, B.; et al. SUPR-3D: A Randomized Phase Iii Trial Comparing Simple Unplanned Palliative Radiotherapy versus 3d Conformal Radiotherapy for Patients with Bone Metastases: Study Protocol. BMC Cancer 2019, 19, 1011. [Google Scholar] [CrossRef]
- Parisi, S.; Napoli, I.; Lillo, S.; Cacciola, A.; Ferini, G.; Iatì, G.; Pontoriero, A.; Tamburella, C.; Davì, V.; Pergolizzi, S. Spine Eburnation in a Metastatic Lung Cancer Patient Treated with Immunotherapy and Radiotherapy. The First Case Report of Bystander Effect on Bone. J. Oncol. Pharm. Pract. 2022, 28, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Furlan, C.; Trovo, M.; Drigo, A.; Capra, E.; Trovo, M.G. Half-Body Irradiation with Tomotherapy for Pain Palliation in Metastatic Breast Cancer. J. Pain Symptom Manag. 2014, 47, 174–180. [Google Scholar] [CrossRef]
- Sahgal, A.; Myrehaug, S.D.; Siva, S.; Masucci, G.L.; Maralani, P.J.; Brundage, M.; Butler, J.; Chow, E.; Fehlings, M.G.; Foote, M.; et al. Stereotactic Body Radiotherapy versus Conventional External Beam Radiotherapy in Patients with Painful Spinal Metastases: An Open-Label, Multicentre, Randomised, Controlled, Phase 2/3 Trial. Lancet Oncol. 2021, 22, 1023–1033. [Google Scholar] [CrossRef]
- Sasamura, K.; Suzuki, R.; Kozuka, T.; Yoshimura, R.; Yoshioka, Y.; Oguchi, M. Outcomes after Reirradiation of Spinal Metastasis with Stereotactic Body Radiation Therapy (SBRT): A Retrospective Single Institutional Study. J. Radiat. Res. 2020, 61, 929–934. [Google Scholar] [CrossRef]
- Ferini, G.; Pergolizzi, S. A Ten-Year-Long Update on Radiation Proctitis among Prostate Cancer Patients Treated with Curative External Beam Radiotherapy. In Vivo 2021, 35, 1379–1391. [Google Scholar] [CrossRef]
- Ferini, G.; Tripoli, A.; Molino, L.; Cacciola, A.; Lillo, S.; Parisi, S.; Umina, V.; Illari, S.I.; Marchese, V.A.; Cravagno, I.R.; et al. How Much Daily Image-Guided Volumetric Modulated Arc Therapy Is Useful for Proctitis Prevention with Respect to Static Intensity Modulated Radiotherapy Supported by Topical Medications among Localized Prostate Cancer Patients? Anticancer. Res. 2021, 41, 2101–2110. [Google Scholar] [CrossRef]
- Vadalà, R.E.; Santacaterina, A.; Sindoni, A.; Platania, A.; Arcudi, A.; Ferini, G.; Mazzei, M.M.; Marletta, D.; Rifatto, C.; Risoleti, E.V.I.; et al. Stereotactic Body Radiotherapy in Non-Operable Lung Cancer Patients. Clin. Transl. Oncol. 2016, 18, 1158–1159. [Google Scholar] [CrossRef]
- Rades, D.; Cacicedo, J.; Conde-Moreno, A.J.; Segedin, B.; But-Hadzic, J.; Groselj, B.; Kevlishvili, G.; Lomidze, D.; Raquel, C.J.; Rubio, C.; et al. Precision Radiation Therapy for Metastatic Spinal Cord Compression: Final Results of the PRE-MODE Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.D.; Trifiletti, D.M.; Bauer-Nilsen, K.; Wages, N.A.; Watkins, W.T.; Read, P.W.; Showalter, T.N. Clinical Outcomes of Helical Conformal versus Nonconformal Palliative Radiation Therapy for Axial Skeletal Metastases. Pract. Radiat. Oncol. 2017, 7, e479–e487. [Google Scholar] [CrossRef] [PubMed]
- Sprave, T.; Verma, V.; Förster, R.; Schlampp, I.; Hees, K.; Bruckner, T.; Bostel, T.; el Shafie, R.A.; Welzel, T.; Nicolay, N.H.; et al. Bone Density and Pain Response Following Intensity-Modulated Radiotherapy versus Three-Dimensional Conformal Radiotherapy for Vertebral Metastases—Secondary Results of a Randomized Trial. Radiat. Oncol. 2018, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Rozanec, N.; Allibhai, Z.; Bhatti, M.; Chan, E.; McIntosh, M.; Moseley, D.; Taremi, M.; Abbas, A. Palliation of Vertebral Metastases with Radiotherapy: Exploration of Volumetric-Modulated Arc Therapy From Development to Implementation in Routine Clinical Practice. J. Med. Imaging Radiat. Sci. 2019, 50, 68–73. [Google Scholar] [CrossRef]
- Pontoriero, A.; Iatì, G.; Cacciola, A.; Conti, A.; Brogna, A.; Siragusa, C.; Ferini, G.; Davì, V.; Tamburella, C.; Molino, L.; et al. Stereotactic Body Radiation Therapy With Simultaneous Integrated Boost in Patients With Spinal Metastases. Technol. Cancer Res. Treat. 2020, 19, 1533033820904447. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Andratschke, N.; Alheit, H.; Holy, R.; Moustakis, C.; Nestle, U.; Sauer, O. Definition of Stereotactic Body Radiotherapy: Principles and Practice for the Treatment of Stage I Non-Small Cell Lung Cancer. Strahlenther. Und Onkol. 2014, 190, 26–33. [Google Scholar] [CrossRef]
- Ferini, G.; Viola, A.; Valenti, V.; Tripoli, A.; Molino, L.; Marchese, V.A.; Illari, S.I.; Rita Borzì, G.; Prestifilippo, A.; Umana, G.E.; et al. Whole Brain Irradiation or Stereotactic RadioSurgery for Five or More Brain Metastases (WHOBI-STER): A Prospective Comparative Study of Neurocognitive Outcomes, Level of Autonomy in Daily Activities and Quality of Life. Clin. Transl. Radiat. Oncol. 2022, 32, 52–58. [Google Scholar] [CrossRef]
- Parisi, S.; Ferini, G.; Cacciola, A.; Lillo, S.; Tamburella, C.; Santacaterina, A.; Bottari, A.; Brogna, A.; Ferrantelli, G.; Pontoriero, A.; et al. A Non-Surgical COMBO-Therapy Approach for Locally Advanced Unresectable Pancreatic Adenocarcinoma: Preliminary Results of a Prospective Study. La Radiol. Med. 2022, 127, 214–219. [Google Scholar] [CrossRef]
- Ito, K.; Ogawa, H.; Nakajima, Y. Efficacy and Toxicity of Re-Irradiation Spine Stereotactic Body Radiotherapy with Respect to Irradiation Dose History. Jpn. J. Clin. Oncol. 2021, 51, 264–270. [Google Scholar] [CrossRef]
- Doi, H.; Tamari, K.; Oh, R.J.; Nieder, C. New Clinical Data on Human Spinal Cord Re-Irradiation Tolerance. Strahlenther. Und Onkol. Organ Der Dtsch. Rontgenges. 2021, 197, 463–473. [Google Scholar] [CrossRef]
- Sprave, T.; Verma, V.; Förster, R.; Schlampp, I.; Bruckner, T.; Bostel, T.; el Shafie, R.A.; Nicolay, N.H.; Debus, J.; Rief, H. Quality of Life Following Stereotactic Body Radiotherapy Versus Three-Dimensional Conformal Radiotherapy for Vertebral Metastases: Secondary Analysis of an Exploratory Phase II Randomized Trial. Anticancer Res. 2018, 38, 4961–4968. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Tey, J.; Cheo, T.; Lee, C.H.; Wong, A.; Kumar, N.; Vellayappan, B. Outcomes of Patients With Spinal Metastases From Prostate Cancer Treated With Conventionally-Fractionated External Beam Radiation Therapy. Glob. Spine J. 2021, 2192568221. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Sullivan, A.J.; Shi, D.D.; Krishnan, M.S.; Hertan, L.M.; Roldan, C.S.; Huynh, M.A.; Spektor, A.; Fareed, M.M.; Lam, T.C.; et al. Characteristics and Predictors of Radiographic Local Failure in Patients With Spinal Metastases Treated With Palliative Conventional Radiation Therapy. Adv. Radiat. Oncol. 2021, 6, 100665. [Google Scholar] [CrossRef] [PubMed]
- Abbouchie, H.; Chao, M.; Tacey, M.; Joon, D.L.; Ho, H.; Guerrieri, M.; Ng, M.; Foroudi, F. Vertebral Fractures Following Stereotactic Body Radiotherapy for Spine Oligometastases: A Multi-Institutional Analysis of Patient Outcomes. Clin. Oncol. 2020, 32, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.N.; Robinson, C.G.; Meyer, J.; Tran, N.D.; Gangi, A.; Callstrom, M.R.; Chao, S.T.; van Tine, B.A.; Morris, J.M.; Bruel, B.M.; et al. The Metastatic Spine Disease Multidisciplinary Working Group Algorithms. Oncologist 2019, 24, 424. [Google Scholar] [CrossRef] [PubMed]
- Gerszten, P.C.; Quader, M.; Novotny, J.; Flickinger, J.C. Prospective Evaluation of Spinal Cord and Cauda Equina Dose Constraints Using Cone Beam Computed Tomography (CBCT) Image Guidance for Spine Radiosurgery. J. Radiosurgery SBRT 2011, 1, 197. [Google Scholar]
- Pontoriero, A.; Lillo, S.; Caravatta, L.; Bellafiore, F.; Longo, S.; Lattanzi, E.; Parisi, S.; Fiorica, F.; Massaccesi, M. Cumulative Dose, Toxicity, and Outcomes of Spinal Metastases Re-Irradiation: Systematic Review on Behalf of the Re-Irradiation Working Group of the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Strahlenther. Und Onkol. 2021, 197, 369–384. [Google Scholar] [CrossRef]
- Snider, J.W.; Molitoris, J.; Shyu, S.; Diwanji, T.; Rice, S.; Kowalski, E.; Decesaris, C.; Remick, J.S.; Yi, B.; Zhang, B.; et al. Spatially Fractionated Radiotherapy (GRID) Prior to Standard Neoadjuvant Conventionally Fractionated Radiotherapy for Bulky, High-Risk Soft Tissue and Osteosarcomas: Feasibility, Safety, and Promising Pathologic Response Rates. Radiat. Res. 2020, 194, 707–714. [Google Scholar] [CrossRef]
- Ferini, G.; Castorina, P.; Valenti, V.; Illari, S.I.; Sachpazidis, I.; Castorina, L.; Marrale, M.; Pergolizzi, S. A Novel Radiotherapeutic Approach to Treat Bulky Metastases Even From Cutaneous Squamous Cell Carcinoma: Its Rationale and a Look at the Reliability of the Linear-Quadratic Model to Explain Its Radiobiological Effects. Front. Oncol. 2022, 12, 809279. [Google Scholar] [CrossRef]
- Rades, D.; Cacicedo, J.; Conde-Moreno, A.J.; Segedin, B.; Lomidze, D.; Ciervide, R.; Hollaender, N.H.; Schild, S.E. Comparison of 5 × 5 Gy and 10 × 3 Gy for Metastatic Spinal Cord Compression Using Data from Three Prospective Trials. Radiat. Oncol. 2021, 16, 7. [Google Scholar] [CrossRef]
- Bostel, T.; Rühle, A.; Rackwitz, T.; Mayer, A.; Klodt, T.; Oebel, L.; Förster, R.; Schlampp, I.; Wollschläger, D.; Rief, H.; et al. The Role of Palliative Radiotherapy in the Treatment of Spinal Bone Metastases from Head and Neck Tumors-A Multicenter Analysis of a Rare Event. Cancers 2020, 12, 1950. [Google Scholar] [CrossRef] [PubMed]
- Shuja, M.; Elghazaly, A.A.; Iqbal, A.; Mohamed, R.; Marie, A.; Tunio, M.A.; Aly, M.M.; Balbaid, A.; Asiri, M. Efficacy of 8 Gy Single Fraction Palliative Radiation Therapy in Painful Bone Metastases: A Single Institution Experience. Cureus 2018, 10, e2036. [Google Scholar] [CrossRef] [PubMed]
- Kachnic, L.; Berk, L. Palliative Single-Fraction Radiation Therapy: How Much More Evidence Is Needed? J. Natl. Cancer Inst. 2005, 97, 786–788. [Google Scholar] [CrossRef]
- Ignat, P.; Todor, N.; Ignat, R.M.; Șuteu, O. Prognostic Factors Influencing Survival and a Treatment Pattern Analysis of Conventional Palliative Radiotherapy for Patients with Bone Metastases. Curr. Oncol. 2021, 28, 3876–3890. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; Marta, G.N.; Lisboa, F.P.C.; Watte, G.; Trippa, F.; Maranzano, E.; da Motta, N.W.; Popovic, M.; Ha, T.; Burmeister, B.; et al. Hypofractionated Radiotherapy for Complicated Bone Metastases in Patients with Poor Performance Status: A Phase II International Trial. Tumori 2019, 105, 181–187. [Google Scholar] [CrossRef]
- Capuccini, J.; Macchia, G.; Farina, E.; Buwenge, M.; Genovesi, D.; Caravatta, L.; Nguyen, N.P.; Cammelli, S.; Cilla, S.; Wondemagegnhu, T.; et al. Short-Course Regimen of Palliative Radiotherapy in Complicated Bone Metastases: A Phase i-Ii Study (SHARON Project). Clin. Exp. Metastasis 2018, 35, 605–611. [Google Scholar] [CrossRef]
- Wegner, R.E.; Matani, H.; Colonias, A.; Price, F.; Fuhrer, R.; Abel, S. Trends in Radiation Fractionation for Bone Metastases: A Contemporary Nationwide Analysis. Pract. Radiat. Oncol. 2020, 10, 402–408. [Google Scholar] [CrossRef]
- Fischer-Valuck, B.W.; Baumann, B.C.; Apicelli, A.; Rao, Y.J.; Roach, M.; Daly, M.; Dans, M.C.; White, P.; Contreras, J.; Henke, L.; et al. Palliative Radiation Therapy (RT) for Prostate Cancer Patients with Bone Metastases at Diagnosis: A Hospital-Based Analysis of Patterns of Care, RT Fractionation Scheme, and Overall Survival. Cancer Med. 2018, 7, 4240–4250. [Google Scholar] [CrossRef]
- Bostel, T.; Förster, R.; Schlampp, I.; Sprave, T.; Bruckner, T.; Nicolay, N.H.; Welte, S.E.; Debus, J.; Rief, H. Spinal Bone Metastases in Colorectal Cancer: A Retrospective Analysis of Stability, Prognostic Factors and Survival after Palliative Radiotherapy. Radiat. Oncol. 2017, 12, 115. [Google Scholar] [CrossRef]
- Westhoff, P.G.; de Graeff, A.; Monninkhof, E.M.; de Pree, I.; van Vulpen, M.; Leer, J.W.H.; Marijnen, C.A.M.; van der Linden, Y.M. Effectiveness and Toxicity of Conventional Radiotherapy Treatment for Painful Spinal Metastases: A Detailed Course of Side Effects after Opposing Fields versus a Single Posterior Field Technique. J. Radiat. Oncol. 2018, 7, 17–26. [Google Scholar] [CrossRef]
- Harada, H.; Shikama, N.; Wada, H.; Uchida, N.; Nozaki, M.; Hayakawa, K.; Yamada, K.; Nagakura, H.; Ogawa, H.; Miyazawa, K.; et al. A Phase II Study of Palliative Radiotherapy Combined with Zoledronic Acid Hydrate for Metastatic Bone Tumour from Renal Cell Carcinoma. Jpn. J. Clin. Oncol. 2021, 51, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Fareed, M.M.; Pike, L.R.G.; Bang, A.; Huynh, M.A.; Taylor, A.; Spektor, A.; Awad, M.M.; Ott, P.A.; Krishnan, M.; Balboni, T.A.; et al. Palliative Radiation Therapy for Vertebral Metastases and Metastatic Cord Compression in Patients Treated With Anti-PD-1 Therapy. Front. Oncol. 2019, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Pessina, F.; Navarria, P.; Carta, G.A.; D’Agostino, G.R.; Clerici, E.; Nibali, M.C.; Costa, F.; Fornari, M.; Scorsetti, M. Long-Term Follow-Up of Patients with Metastatic Epidural Spinal Cord Compression from Solid Tumors Submitted for Surgery Followed by Radiation Therapy. World Neurosurg. 2018, 115, e681–e687. [Google Scholar] [CrossRef] [PubMed]
- Prezzano, K.M.; Prasad, D.; Hermann, G.M.; Belal, A.N.; Alberico, R.A. Radiofrequency Ablation and Radiation Therapy Improve Local Control in Spinal Metastases Compared to Radiofrequency Ablation Alone. Am. J. Hosp. Palliat. Care 2019, 36, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sprave, T.; Rosenberger, F.; Verma, V.; Förster, R.; Bruckner, T.; Schlampp, I.; Bostel, T.; Welzel, T.; Akbaba, S.; Rackwitz, T.; et al. Paravertebral Muscle Training in Patients with Unstable Spinal Metastases Receiving Palliative Radiotherapy: An Exploratory Randomized Feasibility Trial. Cancers 2019, 11, 1771. [Google Scholar] [CrossRef]
- Cacicedo, J.; Ciria, J.P.; Morillo, V.; Martinez-Indart, L.; Gómez-Iturriaga, A.; del Hoyo, O.; Büchser, D.; Frias, A.; San Miguel, I.; Suarez, F.; et al. Pain Response and Quality of Life Assessment in Patients with Moderate/Severe Neuropathic Pain Due to Bone Metastasis Undergoing Treatment with Palliative Radiotherapy and Tapentadol: A Prospective Multicentre Pilot Study. J. Med. Imaging Radiat. Oncol. 2020, 64, 859–865. [Google Scholar] [CrossRef]
- Zhang, C.; Han, X.; Li, L.; Zhang, C.; Ma, Y.; Wang, G. Posterior Decompression Surgery and Radiofrequency Ablation Followed by Vertebroplasty in Spinal Metastases from Lung Cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e965169. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, H.; Hu, J.H.; Peng, Z.H.; Chen, J.Z.; Huang, J.Q.; Jiang, Y.N.; Luo, G.; Yi, G.F.; Shen, J.; et al. Palliative Pain Relief and Safety of Percutaneous Radiofrequency Ablation Combined with Cement Injection for Bone Metastasis. Jpn. J. Clin. Oncol. 2018, 48, 753–759. [Google Scholar] [CrossRef]
- Zhou, X.; Li, H.; Qiao, Q.; Pan, H.; Fang, Y. CT-Guided Percutaneous Minimally Invasive Radiofrequency Ablation for the Relief of Cancer Related Pain from Metastatic Non-Small Cell Lung Cancer Patients: A Retrospective Study. Ann. Palliat. Med. 2021, 10, 1494–1502. [Google Scholar] [CrossRef]
- Levy, J.; Hopkins, T.; Morris, J.; Tran, N.D.; David, E.; Massari, F.; Farid, H.; Vogel, A.; O’Connell, W.G.; Sunenshine, P.; et al. Radiofrequency Ablation for the Palliative Treatment of Bone Metastases: Outcomes from the Multicenter OsteoCool Tumor Ablation Post-Market Study (OPuS One Study) in 100 Patients. J. Vasc. Interv. Radiol. JVIR 2020, 31, 1745–1752. [Google Scholar] [CrossRef]
- Shawky Abdelgawaad, A.; Ezzati, A.; Krajnovic, B.; Seyed-Emadaldin, S.; Abdelrahman, H. Radiofrequency Ablation and Balloon Kyphoplasty for Palliation of Painful Spinal Metastases. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2021, 30, 2874–2880. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, R.; Graziano, F.; Basile, L.; Gulì, C.; Giugno, A.; Giammalva, G.R.; Visocchi, M.; Iacopino, D.G. Reconstruction of Vertebral Body after Radiofrequency Ablation and Augmentation in Dorsolumbar Metastatic Vertebral Fracture: Analysis of Clinical and Radiological Outcome in a Clinical Series of 18 Patients. Trends Reconstr. Neurosurg. 2017, 124, 81–86. [Google Scholar]
- Zhang, X.; Ye, X.; Zhang, K.; Qiu, Y.; Fan, W.; Yuan, Q.; Fan, J.; Wu, L.; Yang, S.; Hu, M.; et al. Computed Tomography—Guided Microwave Ablation Combined with Osteoplasty for the Treatment of Bone Metastases: A Multicenter Clinical Study. J. Vasc. Interv. Radiol. JVIR 2021, 32, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Autrusseau, P.A.; Cazzato, R.L.; de Marini, P.; Auloge, P.; Koch, G.; Dalili, D.; Weiss, J.; Mayer, T.; Garnon, J.; Gangi, A. Pain Relief and Local Tumour Control Following Percutaneous Image-Guided Cryoablation for Spine Metastasis: A 12-Year Single-Centre Experience. Clin. Radiol. 2021, 76, 674–680. [Google Scholar] [CrossRef]
- Yang, L.; Wang, F.; Zhang, H.; Yang, X.G.; Zhang, H.R.; Li, J.K.; Qiao, R.Q.; Zhang, G.C.; Hu, Y.C. Patient Characteristics Following Surgery for Spinal Metastases: A Multicenter Retrospective Study. Orthop. Surg. 2019, 11, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Sailhan, F.; Prost, S.; Zairi, F.; Gille, O.; Pascal-Mousselard, H.; Bennis, S.; Charles, Y.P.; Blondel, B.; Fuentes, S. Retrospective Multicenter Study by the French Spine Society of Surgical Treatment for Spinal Metastasis in France. Orthop. Traumatol. Surg. Res. OTSR 2018, 104, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Ptashnikov, D.; Zaborovskii, N.; Kostrickii, S.; Mikaylov, D.; Masevnin, S.; Smekalenkov, O.; Kuparadze, I. Metastasectomy and Targeted Therapy for Patients With Spinal Metastases of Renal Cell Carcinoma. Int. J. Spine Surg. 2020, 14, 982–988. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Sun, Z.; Qian, Z. Safety of Cement Distribution Patterns in Metastatic Vertebral Tumors: A Retrospective Study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 7228–7234. [Google Scholar] [CrossRef]
- Stangenberg, M.; Viezens, L.; Eicker, S.O.; Mohme, M.; Mende, K.C.; Dreimann, M. Cervical Vertebroplasty for Osteolytic Metastases as a Minimally Invasive Therapeutic Option in Oncological Surgery: Outcome in 14 Cases. Neurosurg. Focus 2017, 43, E3. [Google Scholar] [CrossRef]
- Sebaaly, A.; Najjar, A.; Wang, Z.; Boubez, G.; Masucci, L.; Shedid, D. Anterolateral Cervical Kyphoplasty for Metastatic Cervical Spine Lesions. Asian Spine J. 2018, 12, 823–829. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, H.; Sun, W.; Lu, Z.; Pan, S. Risk Factors for Local Bone Destruction Progression in Palliative Percutaneous Vertebroplasty for Vertebral Metastases and the Significance of Bone Cement Filling Rates. Pain Physician 2021, 24, E101–E109. [Google Scholar] [CrossRef] [PubMed]
- Telera, S.; Pompili, A.; Crispo, F.; Giovannetti, M.; Pace, A.; Villani, V.; Fabi, A.; Sperduti, I.; Raus, L. Kyphoplasty with Purified Silicone VK100 (Elastoplasty) to Treat Spinal Lytic Lesions in Cancer Patients: A Retrospective Evaluation of 41 Cases. Clin. Neurol. Neurosurg. 2018, 171, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Telera, S.; Gorgoglione, N.; Raus, L.; Vidiri, A.; Villani, V.; Pace, A.; Fabi, A.; Crispo, F.; Castiglione, M.; Sperduti, I.; et al. Open Kyphoplasty for Metastatic Spine Disease: A Retrospective Clinical Series. World Neurosurg. 2019, 127, e751–e760. [Google Scholar] [CrossRef] [PubMed]
- Giugno, A.; Gulì, C.; Basile, L.; Graziano, F.; Maugeri, R.; Visocchi, M.; Iacopino, D.G. Spinal Cord Stimulation: An Alternative Concept of Rehabilitation? Acta Neurochir. Suppl. 2017, 124, 15–18. [Google Scholar]
- Graziano, F.; Gerardi, R.M.; lo Bue, E.; Basile, L.; Brunasso, L.; Somma, T.; Maugeri, R.; Nicoletti, G.; Iacopino, D.G. Surgical Back Risk Syndrome and Spinal Cord Stimulation: Better Safe Than Sorry. World Neurosurg. 2020, 133, e658–e665. [Google Scholar] [CrossRef]
- Hagedorn, J.M.; Pittelkow, T.P.; Hunt, C.L.; D’souza, R.S.; Lamer, T.J. Current Perspectives on Spinal Cord Stimulation for the Treatment of Cancer Pain. J. Pain Res. 2020, 13, 3295–3305. [Google Scholar] [CrossRef]
- Aman, M.M.; Mahmoud, A.; Deer, T.; Sayed, D.; Hagedorn, J.M.; Brogan, S.E.; Singh, V.; Gulati, A.; Strand, N.; Weisbein, J.; et al. The American Society of Pain and Neuroscience (ASPN) Best Practices and Guidelines for the Interventional Management of Cancer-Associated Pain. J. Pain Res. 2021, 14, 2139–2164. [Google Scholar] [CrossRef]
- Yakovlev, A.E.; Resch, B.E. Treatment of Multifocal Pain with Spinal Cord Stimulation. Neuromodulation 2012, 15, 210–213. [Google Scholar] [CrossRef]
- Skolasky, R.L.; Wegener, S.T.; Maggard, A.M.; Riley, L.H. The Impact of Reduction of Pain after Lumbar Spine Surgery: The Relationship between Changes in Pain and Physical Function and Disability. Spine 2014, 39, 1426–1432. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giammalva, G.R.; Ferini, G.; Torregrossa, F.; Brunasso, L.; Musso, S.; Benigno, U.E.; Gerardi, R.M.; Bonosi, L.; Costanzo, R.; Paolini, F.; et al. The Palliative Care in the Metastatic Spinal Tumors. A Systematic Review on the Radiotherapy and Surgical Perspective. Life 2022, 12, 571. https://doi.org/10.3390/life12040571
Giammalva GR, Ferini G, Torregrossa F, Brunasso L, Musso S, Benigno UE, Gerardi RM, Bonosi L, Costanzo R, Paolini F, et al. The Palliative Care in the Metastatic Spinal Tumors. A Systematic Review on the Radiotherapy and Surgical Perspective. Life. 2022; 12(4):571. https://doi.org/10.3390/life12040571
Chicago/Turabian StyleGiammalva, Giuseppe Roberto, Gianluca Ferini, Fabio Torregrossa, Lara Brunasso, Sofia Musso, Umberto Emanuele Benigno, Rosa Maria Gerardi, Lapo Bonosi, Roberta Costanzo, Federica Paolini, and et al. 2022. "The Palliative Care in the Metastatic Spinal Tumors. A Systematic Review on the Radiotherapy and Surgical Perspective" Life 12, no. 4: 571. https://doi.org/10.3390/life12040571
APA StyleGiammalva, G. R., Ferini, G., Torregrossa, F., Brunasso, L., Musso, S., Benigno, U. E., Gerardi, R. M., Bonosi, L., Costanzo, R., Paolini, F., Palmisciano, P., Umana, G. E., Di Bonaventura, R., Sturiale, C. L., Iacopino, D. G., & Maugeri, R. (2022). The Palliative Care in the Metastatic Spinal Tumors. A Systematic Review on the Radiotherapy and Surgical Perspective. Life, 12(4), 571. https://doi.org/10.3390/life12040571











