Epigenetic Effect of Maternal Methyl-Group Donor Intake on Offspring’s Health and Disease
Abstract
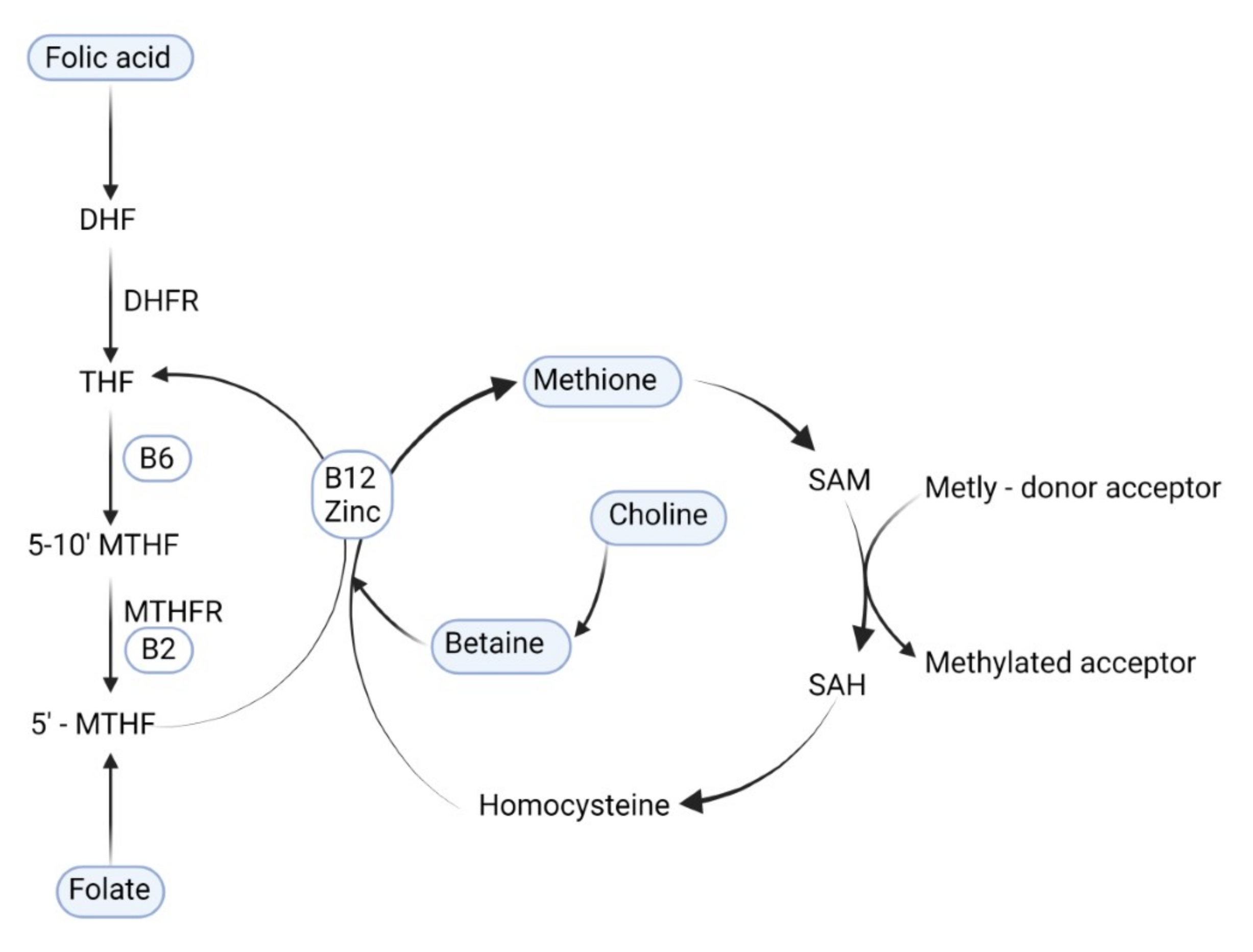
:1. Introduction
2. Methods
3. Epigenetic Effect of Maternal Methyl-Group Donor Intake on Reproductivity
The Epigenetic Role of Maternal Methyl-Group Donor Status in the Perinatal Outcomes of Assisted Reproduction Technology
4. Epigenetic Effect of Maternal Methyl-Group Donor Intake on Perinatal Outcome
4.1. Low Birth Weight
4.2. Cleft Lip and Cleft Palate
4.3. Congenital Heart Defects
4.4. Neural Tube Defects
4.5. Brain Development and Cognitive Functions
5. Epigenetic Effect of Maternal One-Carbon Metabolism Nutrients on Offspring Obesity and Associated Non-Communicable Diseases
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubini, E.; Baijens, I.M.M.; Horánszky, A.; Schoenmakers, S.; Sinclair, K.D.; Zana, M.; Dinnyés, A.; Steegers-Theunissen, R.P.M.; Rousian, M. Maternal One-Carbon Metabolism during the Periconceptional Period and Human Foetal Brain Growth: A Systematic Review. Genes 2021, 12, 1634. [Google Scholar] [CrossRef] [PubMed]
- Moster, D.; Lie, R.T.; Markestad, T. Long-Term Medical and Social Consequences of Preterm Birth. N. Engl. J. Med. 2008, 359, 262–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lillycrop, K.A.; Burdge, G.C. Maternal Diet as a Modifier of Offspring Epigenetics. J. Dev. Orig. Health Dis. 2015, 6, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGee, M.; Bainbridge, S.; Fontaine-Bisson, B. A Crucial Role for Maternal Dietary Methyl Donor Intake in Epigenetic Programming and Fetal Growth Outcomes. Nutr. Rev. 2018, 76, 469–478. [Google Scholar] [CrossRef]
- Faulk, C.; Dolinoy, D.C. Timing Is Everything. Epigenetics 2011, 6, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Kalhan, S.C.; Marczewski, S.E. Methionine, Homocysteine, One Carbon Metabolism and Fetal Growth. Rev. Endocr. Metab. Disord. 2012, 13, 109–119. [Google Scholar] [CrossRef]
- Radziejewska, A.; Chmurzynska, A. Folate and Choline Absorption and Uptake: Their Role in Fetal Development. Biochimie 2019, 158, 10–19. [Google Scholar] [CrossRef]
- Rush, E.C.; Katre, P.; Yajnik, C.S. Vitamin B12: One Carbon Metabolism, Fetal Growth and Programming for Chronic Disease. Eur. J. Clin. Nutr. 2014, 68, 2–7. [Google Scholar] [CrossRef]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and Epigenetics: An Interplay of Dietary Methyl Donors, One-Carbon Metabolism and DNA Methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Niculescu, M.D.; Zeisel, S.H. Diet, Methyl Donors and DNA Methylation: Interactions between Dietary Folate, Methionine and Choline. J. Nutr. 2002, 132, 2333S–2335S. [Google Scholar] [CrossRef]
- Fekete, K.; Berti, C.; Cetin, I.; Hermoso, M.; Koletzko, B.v.; Decsi, T. Perinatal Folate Supply: Relevance in Health Outcome Parameters. Matern. Child Nutr. 2010, 6, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Picciano, M.F. Folate and Human Reproduction. Am. J. Clin. Nutr. 2006, 83, 993–1016. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Kuizon, S.; Junaid, M.A. Folic Acid Supplementation in Pregnancy and Implications in Health and Disease. J. Biomed. Sci. 2014, 21, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero, A.M.; Crider, K.S.; Rogers, L.M.; Cannon, M.J.; Berry, R.J. Optimal Serum and Red Blood Cell Folate Concentrations in Women of Reproductive Age for Prevention of Neural Tube Defects: World Health Organization Guidelines. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 421–423. [Google Scholar] [PubMed]
- Ueland, P.M. Choline and Betaine in Health and Disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Mar, M.H.; Zhou, Z.; da Costa, K.A. Pregnancy and Lactation Are Associated with Diminished Concentrations of Choline and Its Metabolites in Rat Liver. J. Nutr. 1995, 125, 3049–3054. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.A. Betaine in Human Nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; West, A.A.; Caudill, M.A. Maternal Choline Supplementation: A Nutritional Approach for Improving Offspring Health? Trends Endocrinol. Metab. 2014, 25, 263–273. [Google Scholar] [CrossRef]
- Wolff, G.L.; Kodell, R.L.; Moore, S.R.; Cooney, C.A. Maternal Epigenetics and Methyl Supplements Affect Agouti Gene Expression in Avy/a Mice. FASEB J. 1998, 12, 949–957. [Google Scholar] [CrossRef] [Green Version]
- Miltenberger, R.J.; Mynatt, R.L.; Wilkinson, J.E.; Woychik, R.P. The Role of the Agouti Gene in the Yellow Obese Syndrome. J. Nutr. 1997, 127, 1902S–1907S. [Google Scholar] [CrossRef]
- Yi, P.; Melnyk, S.; Pogribna, M.; Pogribny, I.P.; Hine, R.J.; James, S.J. Increase in Plasma Homocysteine Associated with Parallel Increases in Plasma S-Adenosylhomocysteine and Lymphocyte DNA Hypomethylation. J. Biol. Chem. 2000, 275, 29318–29323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterland, R.A. Do Maternal Methyl Supplements in Mice Affect DNA Methylation of Offspring? J. Nutr. 2003, 133, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ba, Y.; Yu, H.; Liu, F.; Geng, X.; Zhu, C.; Zhu, Q.; Zheng, T.; Ma, S.; Wang, G.; Li, Z.; et al. Relationship of Folate, Vitamin B12 and Methylation of Insulin-like Growth Factor-II in Maternal and Cord Blood. Eur. J. Clin. Nutr. 2011, 65, 480–485. [Google Scholar] [CrossRef] [Green Version]
- Hoyo, C.; Daltveit, A.K.; Iversen, E.; Benjamin-Neelon, S.E.; Fuemmeler, B.; Schildkraut, J.; Murtha, A.P.; Overcash, F.; Vidal, A.C.; Wang, F.; et al. Erythrocyte Folate Concentrations, CpG Methylation at Genomically Imprinted Domains, and Birth Weight in a Multiethnic Newborn Cohort. Epigenetics 2014, 9, 1120–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzi, S.; Sas, T.C.; Koudou, Y.; le Bouc, Y.; Souberbielle, J.-C.; Dargent-Molina, P.; Netchine, I.; Charles, M.-A. Degree of Methylation of ZAC1 (PLAGL1) Is Associated with Prenatal and Post-Natal Growth in Healthy Infants of the EDEN Mother Child Cohort. Epigenetics 2014, 9, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Quan, S.; Yang, G.; Ye, Q.; Chen, M.; Yu, H.; Wang, G.; Wang, Y.; Zeng, X.; Qiao, S. One Carbon Metabolism and Mammalian Pregnancy Outcomes. Mol. Nutr. Food Res. 2021, 65, 2000734. [Google Scholar] [CrossRef] [PubMed]
- Lassi, Z.S.; Salam, R.A.; Haider, B.A.; Bhutta, Z.A. Folic Acid Supplementation during Pregnancy for Maternal Health and Pregnancy Outcomes. Cochrane Database Syst. Rev. 2013, 3, CD006896. [Google Scholar] [CrossRef]
- Ebisch, I.M.W.; Thomas, C.M.G.; Peters, W.H.M.; Braat, D.D.M.; Steegers-Theunissen, R.P.M. The Importance of Folate, Zinc and Antioxidants in the Pathogenesis and Prevention of Subfertility. Hum. Reprod. Update 2007, 13, 163–174. [Google Scholar] [CrossRef]
- Smallwood, S.A.; Kelsey, G. De Novo DNA Methylation: A Germ Cell Perspective. Trends Genet. 2012, 28, 33–42. [Google Scholar] [CrossRef]
- Messerschmidt, D.M.; Knowles, B.B.; Solter, D. DNA Methylation Dynamics during Epigenetic Reprogramming in the Germline and Preimplantation Embryos. Genes Dev. 2014, 28, 812–828. [Google Scholar] [CrossRef] [Green Version]
- Lane, N.; Dean, W.; Erhardt, S.; Hajkova, P.; Surani, A.; Walter, J.; Reik, W. Resistance of IAPs to Methylation Reprogramming May Provide a Mechanism for Epigenetic Inheritance in the Mouse. Genesis 2003, 35, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Brandeis, M.; Kafri, T.; Ariel, M.; Chaillet, J.R.; McCarrey, J.; Razin, A.; Cedar, H. The Ontogeny of Allele-Specific Methylation Associated with Imprinted Genes in the Mouse. EMBO J. 1993, 12, 3669–3677. [Google Scholar] [CrossRef] [PubMed]
- Ly, L.; Chan, D.; Landry, M.; Angle, C.; Martel, J.; Trasler, J. Impact of Mothers’ Early Life Exposure to Low or High Folate on Progeny Outcome and DNA Methylation Patterns. Environ. Epigenetics 2020, 6, dvaa018. [Google Scholar] [CrossRef] [PubMed]
- Hoorsan, H.; Mirmiran, P.; Chaichian, S.; Moradi, Y.; Hoorsan, R.; Jesmi, F. Congenital Malformations in Infants of Mothers Undergoing Assisted Reproductive Technologies: A Systematic Review and Meta-Analysis Study. J. Prev. Med. Public Health 2017, 50, 347–360. [Google Scholar] [CrossRef]
- Cortessis, V.K.; Azadian, M.; Buxbaum, J.; Sanogo, F.; Song, A.Y.; Sriprasert, I.; Wei, P.C.; Yu, J.; Chung, K.; Siegmund, K.D. Comprehensive Meta-Analysis Reveals Association between Multiple Imprinting Disorders and Conception by Assisted Reproductive Technology. J. Assist. Reprod. Genet. 2018, 35, 943–952. [Google Scholar] [CrossRef]
- Heber, M.F.; Ptak, G.E. The Effects of Assisted Reproduction Technologies on Metabolic Health and Disease†. Biol. Reprod. 2021, 104, 734–744. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Liu, X.-M.; Jin, L.; Wang, T.-T.; Ullah, K.; Sheng, J.-Z.; Huang, H.-F. Cardiovascular and Metabolic Profiles of Offspring Conceived by Assisted Reproductive Technologies: A Systematic Review and Meta-Analysis. Fertil. Steril. 2017, 107, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, S.; Martel, J.; Karahan, G.; Angle, C.; Behan, N.A.; Chan, D.; MacFarlane, A.J.; Trasler, J.M. Moderate Maternal Folic Acid Supplementation Ameliorates Adverse Embryonic and Epigenetic Outcomes Associated with Assisted Reproduction in a Mouse Model. Hum. Reprod. 2019, 34, 851–862. [Google Scholar] [CrossRef]
- Breton-Larrivée, M.; Elder, E.; McGraw, S. DNA Methylation, Environmental Exposures and Early Embryo Development. Anim. Reprod. 2019, 16, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, K.D.; Allegrucci, C.; Singh, R.; Gardner, D.S.; Sebastian, S.; Bispham, J.; Thurston, A.; Huntley, J.F.; Rees, W.D.; Maloney, C.A.; et al. DNA Methylation, Insulin Resistance, and Blood Pressure in Offspring Determined by Maternal Periconceptional B Vitamin and Methionine Status. Proc. Natl. Acad. Sci. USA 2007, 104, 19351–19356. [Google Scholar] [CrossRef] [Green Version]
- Menezo, Y.; Elder, K.; Benkhalifa, M.; Dale, B. DNA Methylation and Gene Expression in IVF. Reprod. Biomed. Online 2010, 20, 709–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauwels, S.; Duca, R.; Devlieger, R.; Freson, K.; Straetmans, D.; van Herck, E.; Huybrechts, I.; Koppen, G.; Godderis, L. Maternal Methyl-Group Donor Intake and Global DNA (Hydroxy)Methylation before and during Pregnancy. Nutrients 2016, 8, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez-Salas, P.; Cox, S.E.; Prentice, A.M.; Hennig, B.J.; Moore, S.E. Maternal Nutritional Status, C1 Metabolism and Offspring DNA Methylation: A Review of Current Evidence in Human Subjects. Proc. Nutr. Soc. 2012, 71, 154–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, R.O.; Chellappan, S.; Kukshal, P. Exploring the Role of Maternal Nutritional Epigenetics in Congenital Heart Disease. Curr. Dev. Nutr. 2020, 4, nzaa166. [Google Scholar] [CrossRef] [PubMed]
- Gonseth, S.; Shaw, G.M.; Roy, R.; Segal, M.R.; Asrani, K.; Rine, J.; Wiemels, J.; Marini, N.J. Epigenomic Profiling of Newborns with Isolated Orofacial Clefts Reveals Widespread DNA Methylation Changes and Implicates Metastable Epiallele Regions in Disease Risk. Epigenetics 2019, 14, 198–213. [Google Scholar] [CrossRef] [Green Version]
- De-Regil, L.M.; Peña-Rosas, J.P.; Fernández-Gaxiola, A.C.; Rayco-Solon, P. Effects and Safety of Periconceptional Oral Folate Supplementation for Preventing Birth Defects. Cochrane Database Syst. Rev. 2015, 2015, CD007950. [Google Scholar] [CrossRef]
- Zeisel, S.H. Importance of Methyl Donors during Reproduction. Am. J. Clin. Nutr. 2009, 89, 673S–677S. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Jiang, X.; Caudill, M.A. Choline: Exploring the Growing Science on Its Benefits for Moms and Babies. Nutrients 2019, 11, 1823. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S. Choline, Other Methyl-Donors and Epigenetics. Nutrients 2017, 9, 445. [Google Scholar] [CrossRef]
- Pauwels, S.; Ghosh, M.; Duca, R.C.; Bekaert, B.; Freson, K.; Huybrechts, I.; Langie, S.A.S.; Koppen, G.; Devlieger, R.; Godderis, L. Maternal Intake of Methyl-Group Donors Affects DNA Methylation of Metabolic Genes in Infants. Clin. Epigenetics 2017, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Rees, W.D.; Wilson, F.A.; Maloney, C.A. Sulfur Amino Acid Metabolism in Pregnancy: The Impact of Methionine in the Maternal Diet. J. Nutr. 2006, 136, 1701S–1705S. [Google Scholar] [CrossRef] [PubMed]
- Joubert, B.R.; den Dekker, H.T.; Felix, J.F.; Bohlin, J.; Ligthart, S.; Beckett, E.; Tiemeier, H.; van Meurs, J.B.; Uitterlinden, A.G.; Hofman, A.; et al. Maternal Plasma Folate Impacts Differential DNA Methylation in an Epigenome-Wide Meta-Analysis of Newborns. Nat. Commun. 2016, 7, 10577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küpers, L.K.; Monnereau, C.; Sharp, G.C.; Yousefi, P.; Salas, L.A.; Ghantous, A.; Page, C.M.; Reese, S.E.; Wilcox, A.J.; Czamara, D.; et al. Meta-Analysis of Epigenome-Wide Association Studies in Neonates Reveals Widespread Differential DNA Methylation Associated with Birthweight. Nat. Commun. 2019, 10, 1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Wu, Q.; Huang, Y.; Wang, L.; Su, Z.; Ye, H. The Role of DNA Methylation in Syndromic and Non-Syndromic Congenital Heart Disease. Clin. Epigenetics 2021, 13, 93. [Google Scholar] [CrossRef]
- Elhamamsy, A.R. Role of DNA Methylation in Imprinting Disorders: An Updated Review. J. Assist. Reprod. Genet. 2017, 34, 549–562. [Google Scholar] [CrossRef] [Green Version]
- Cooney, C.A.; Dave, A.A.; Wolff, G.L. Maternal Methyl Supplements in Mice Affect Epigenetic Variation and DNA Methylation of Offspring. J. Nutr. 2002, 132, 2393S–2400S. [Google Scholar] [CrossRef] [Green Version]
- Bartolomei, M.S.; Webber, A.L.; Brunkow, M.E.; Tilghman, S.M. Epigenetic Mechanisms Underlying the Imprinting of the Mouse H19 Gene. Genes Dev. 1993, 7, 1663–1673. [Google Scholar] [CrossRef] [Green Version]
- Brenner’s Encyclopedia of Genetics. Brenner’s Encyclopedia of Genetics, 2nd ed. Elsevier/Academic Press: Amsterdam, The Netherlands, 2013.
- Hoyo, C.; Murtha, A.P.; Schildkraut, J.M.; Jirtle, R.L.; Demark-Wahnefried, W.; Forman, M.R.; Iversen, E.S.; Kurtzberg, J.; Overcash, F.; Huang, Z.; et al. Methylation Variation at IGF2 Differentially Methylated Regions and Maternal Folic Acid Use before and during Pregnancy. Epigenetics 2011, 6, 928–936. [Google Scholar] [CrossRef] [Green Version]
- Steegers-Theunissen, R.P.; Obermann-Borst, S.A.; Kremer, D.; Lindemans, J.; Siebel, C.; Steegers, E.A.; Slagboom, P.E.; Heijmans, B.T. Periconceptional Maternal Folic Acid Use of 400 Μg per Day Is Related to Increased Methylation of the IGF2 Gene in the Very Young Child. PLoS ONE 2009, 4, e7845. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Chang, S.; Liu, C.; Zhang, M.; Zhang, L.; Liang, L.; Li, R.; Wang, X.; Qin, C.; Zhang, T.; et al. Low Maternal Dietary Folate Alters Retrotranspose by Methylation Regulation in Intrauterine Growth Retardation (IUGR) Fetuses in a Mouse Model. Med Sci. Monit. 2019, 25, 3354–3365. [Google Scholar] [CrossRef]
- Fryer, A.A.; Nafee, T.M.; Ismail, K.M.K.; Carroll, W.D.; Emes, R.D.; Farrell, W.E. LINE-1 DNA Methylation Is Inversely Correlated with Cord Plasma Homocysteine in Man: A Preliminary Study. Epigenetics 2009, 4, 394–398. [Google Scholar] [CrossRef] [Green Version]
- Fryer, A.A.; Emes, R.D.; Ismail, K.M.K.; Haworth, K.E.; Mein, C.; Carroll, W.D.; Farrell, W.E. Quantitative, High-Resolution Epigenetic Profiling of CpG Loci Identifies Associations with Cord Blood Plasma Homocysteine and Birth Weight in Humans. Epigenetics 2011, 6, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arima, T. A Conserved Imprinting Control Region at the HYMAI/ZAC Domain Is Implicated in Transient Neonatal Diabetes Mellitus. Hum. Mol. Genet. 2001, 10, 1475–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Jiang, Y.; Yan, T.; Chen, Y.; Yang, M.; Lv, M.; Xi, F.; Lu, J.; Zhao, B.; Luo, Q. Placental Maternally Expressed Gene 3 Differentially Methylated Region Methylation Profile Is Associated with Maternal Glucose Concentration and Newborn Birthweight. J. Diabetes Investig. 2021, 12, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.J.; Marazita, M.L.; Beaty, T.H.; Murray, J.C. Cleft Lip and Palate: Understanding Genetic and Environmental Influences. Nat. Rev. Genet. 2011, 12, 167–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahanbin, A.; Shadkam, E.; Miri, H.H.; Shirazi, A.S.; Abtahi, M. Maternal Folic Acid Supplementation and the Risk of Oral Clefts in Offspring. J. Craniofacial Surg. 2018, 29, e534–e541. [Google Scholar] [CrossRef]
- Sharp, G.C.; Ho, K.; Davies, A.; Stergiakouli, E.; Humphries, K.; McArdle, W.; Sandy, J.; Davey Smith, G.; Lewis, S.J.; Relton, C.L. Distinct DNA Methylation Profiles in Subtypes of Orofacial Cleft. Clin. Epigenetics 2017, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Alvizi, L.; Ke, X.; Brito, L.A.; Seselgyte, R.; Moore, G.E.; Stanier, P.; Passos-Bueno, M.R. Differential Methylation Is Associated with Non-Syndromic Cleft Lip and Palate and Contributes to Penetrance Effects. Sci. Rep. 2017, 7, 2441. [Google Scholar] [CrossRef]
- Serra-Juhé, C.; Cuscó, I.; Homs, A.; Flores, R.; Torán, N.; Pérez-Jurado, L.A. DNA Methylation Abnormalities in Congenital Heart Disease. Epigenetics 2015, 10, 167–177. [Google Scholar] [CrossRef] [Green Version]
- González-Peña, S.M.; Calvo-Anguiano, G.; Martínez-de-Villarreal, L.E.; Ancer-Rodríguez, P.R.; Lugo-Trampe, J.J.; Saldivar-Rodríguez, D.; Hernández-Almaguer, M.D.; Calzada-Dávila, M.; Guerrero-Orjuela, L.S.; Campos-Acevedo, L.D. Maternal Folic Acid Intake and Methylation Status of Genes Associated with Ventricular Septal Defects in Children: Case–Control Study. Nutrients 2021, 13, 2071. [Google Scholar] [CrossRef]
- Hernández-Almaguer, M.D.; Calvo-Anguiano, G.; Cerda-Flores, R.M.; Salinas-Torres, V.M.; Orozco-Galicia, F.; Glenn, E.; García-Guerra, J.; Sánchez-Cortés, G.; Lugo-Trampe, J.; Martínez-Garza, L.E. Genetic Variants at the Rs4720169 Locus of TBX20 and the Rs12921862 Locus of AXIN1 May Increase the Risk of Congenital Heart Defects in the Mexican Population: A Pilot Study. Genet. Test. Mol. Biomark. 2019, 23, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-L.; Wang, L.; Chang, S.-Y.; Shangguan, S.-F.; Wang, Z.; Wu, L.-H.; Zou, J.-Z.; Xiao, P.; Li, R.; Bao, Y.-H.; et al. Sonic Hedgehog Signaling Affected by Promoter Hypermethylation Induces Aberrant Gli2 Expression in Spina Bifida. Mol. Neurobiol. 2016, 53, 5413–5424. [Google Scholar] [CrossRef] [PubMed]
- Rochtus, A.; Jansen, K.; Geet, C.; Freson, K. Nutri-Epigenomic Studies Related to Neural Tube Defects: Does Folate Affect Neural Tube Closure Via Changes in DNA Methylation? Mini-Rev. Med. Chem. 2015, 15, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shangguan, S.; Xin, Y.; Chang, S.; Wang, Z.; Lu, X.; Wu, L.; Niu, B.; Zhang, T. Folate Deficiency Disturbs Hsa-Let-7 g Level through Methylation Regulation in Neural Tube Defects. J. Cell. Mol. Med. 2017, 21, 3244–3253. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Guan, J.; Le, J.; Wu, L.; Zou, J.; Zhao, H.; Pei, L.; Zheng, X.; Zhang, T. Relation between Hypomethylation of Long Interspersed Nucleotide Elements and Risk of Neural Tube Defects. Am. J. Clin. Nutr. 2010, 91, 1359–1367. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Wang, L.; Guan, Y.; Shangguan, S.; Du, Q.; Wang, Y.; Zhang, T.; Zhang, Y. Long Interspersed Nucleotide Element-1 Hypomethylation in Folate-Deficient Mouse Embryonic Stem Cells. J. Cell. Biochem. 2013, 114, 1549–1558. [Google Scholar] [CrossRef]
- Mastrototaro, G.; Zaghi, M.; Sessa, A. Epigenetic Mistakes in Neurodevelopmental Disorders. J. Mol. Neurosci. 2017, 61, 590–602. [Google Scholar] [CrossRef]
- Sable, P.; Randhir, K.; Kale, A.; Chavan-Gautam, P.; Joshi, S. Maternal Micronutrients and Brain Global Methylation Patterns in the Offspring. Nutr. Neurosci. 2015, 18, 30–36. [Google Scholar] [CrossRef]
- Caffrey, A.; Irwin, R.E.; McNulty, H.; Strain, J.J.; Lees-Murdock, D.J.; McNulty, B.A.; Ward, M.; Walsh, C.P.; Pentieva, K. Gene-Specific DNA Methylation in Newborns in Response to Folic Acid Supplementation during the Second and Third Trimesters of Pregnancy: Epigenetic Analysis from a Randomized Controlled Trial. Am. J. Clin. Nutr. 2018, 107, 566–575. [Google Scholar] [CrossRef]
- Navarro, E.; Funtikova, A.N.; Fíto, M.; Schröder, H. Prenatal Nutrition and the Risk of Adult Obesity: Long-Term Effects of Nutrition on Epigenetic Mechanisms Regulating Gene Expression. J. Nutr. Biochem. 2017, 39, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J. The Fetal and Infant Origins of Adult Disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterland, R.A.; Jirtle, R.L. Transposable Elements: Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Mol. Cell. Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fall, C. Maternal Nutrition: Effects on Health in the next Generation. Indian J. Med. Res. 2009, 130, 593–599. [Google Scholar] [PubMed]
- Kantake, M.; Ikeda, N.; Nakaoka, H.; Ohkawa, N.; Tanaka, T.; Miyabayashi, K.; Shoji, H.; Shimizu, T. IGF1 Gene Is Epigenetically Activated in Preterm Infants with Intrauterine Growth Restriction. Clin. Epigenetics 2020, 12, 108. [Google Scholar] [CrossRef]
- Giudicelli, F.; Brabant, A.-L.; Grit, I.; Parnet, P.; Amarger, V. Excess of Methyl Donor in the Perinatal Period Reduces Postnatal Leptin Secretion in Rat and Interacts with the Effect of Protein Content in Diet. PLoS ONE 2013, 8, e68268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Yan, J.; West, A.A.; Perry, C.A.; Malysheva, O.v.; Devapatla, S.; Pressman, E.; Vermeylen, F.; Caudill, M.A. Maternal Choline Intake Alters the Epigenetic State of Fetal Cortisol-regulating Genes in Humans. FASEB J. 2012, 26, 3563–3574. [Google Scholar] [CrossRef] [Green Version]
- Xiong, F.; Zhang, L. Role of the Hypothalamic–Pituitary–Adrenal Axis in Developmental Programming of Health and Disease. Front. Neuroendocrinol. 2013, 34, 27–46. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokor, S.; Vass, R.A.; Funke, S.; Ertl, T.; Molnár, D. Epigenetic Effect of Maternal Methyl-Group Donor Intake on Offspring’s Health and Disease. Life 2022, 12, 609. https://doi.org/10.3390/life12050609
Bokor S, Vass RA, Funke S, Ertl T, Molnár D. Epigenetic Effect of Maternal Methyl-Group Donor Intake on Offspring’s Health and Disease. Life. 2022; 12(5):609. https://doi.org/10.3390/life12050609
Chicago/Turabian StyleBokor, Szilvia, Réka A. Vass, Simone Funke, Tibor Ertl, and Dénes Molnár. 2022. "Epigenetic Effect of Maternal Methyl-Group Donor Intake on Offspring’s Health and Disease" Life 12, no. 5: 609. https://doi.org/10.3390/life12050609






