Occurrence and Geographic Distribution of Plant-Parasitic Nematodes Associated with Citrus in Morocco and Their Interaction with Soil Patterns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey and Sample Collection
2.2. Nematode Extraction and Identification
2.3. Nematode Community Assessment
2.4. DNA Extraction, PCR, and Sequencing
2.5. Soil Diagnosis
2.6. Taxonomic Diversity
2.7. Data Processing
3. Results
3.1. Distribution and Diversity of Plant-Parasitic Nematode Communities
3.2. Diversity and Community Indices
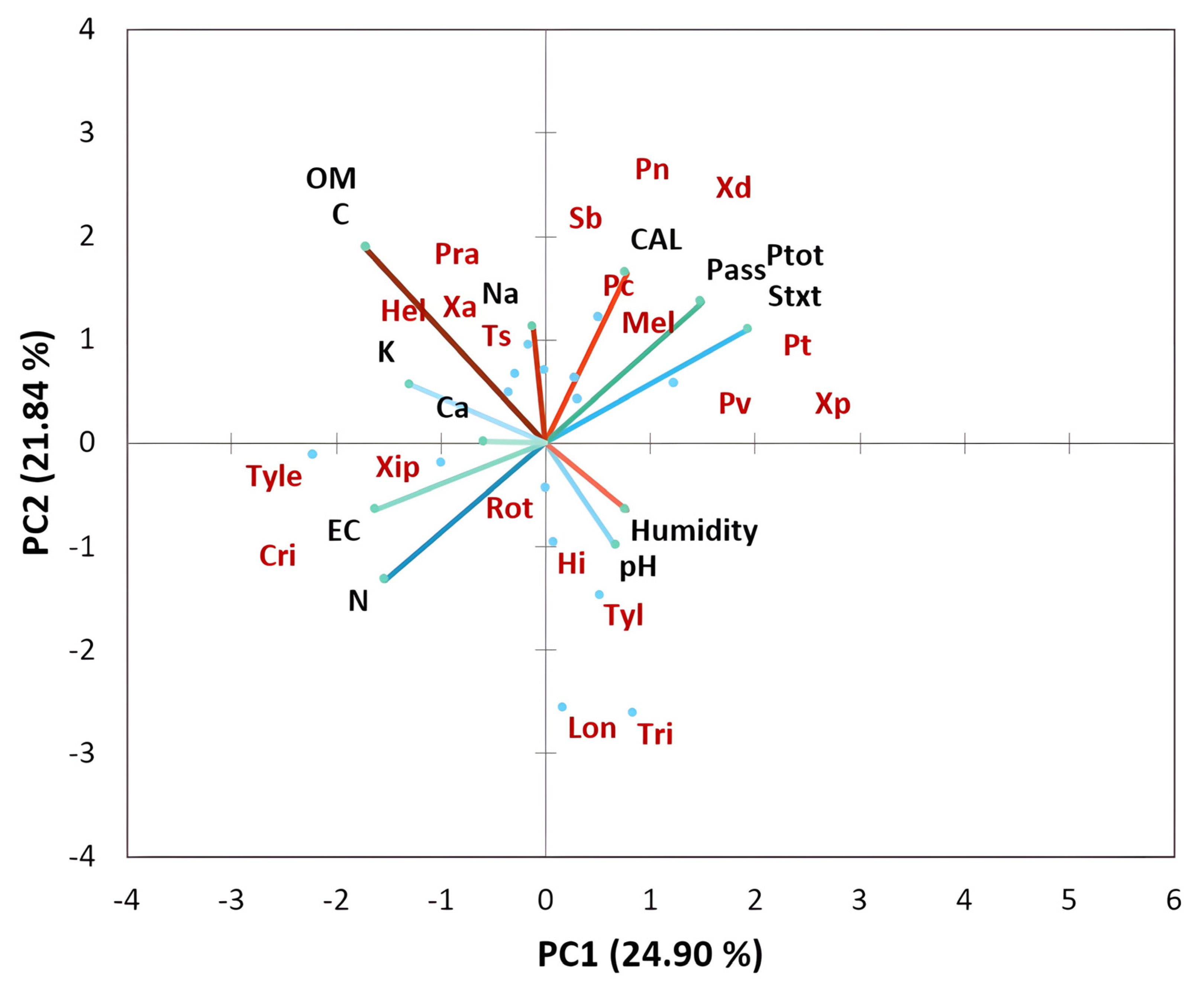
3.3. Physicochemical Properties and Their Interaction with Nematode Communities in Citrus
4. Discussion
4.1. Distribution and Taxonomic Diversity of Plant-Parasitic Nematodes
4.2. Relationship between Plant-Parasitic Nematode Communities and Soil Physicochemical Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ASPAM: Association des Producteurs D’agrumes du Maroc. Division des Statistiques, Maroc. 2019. Available online: www.maroc-citrus.com (accessed on 3 January 2022).
- ADA: Agency for Agricultural. Development Investor’s Guide in the Agricultural Sector in Morocco. 2019. Available online: www.ada.gov.ma (accessed on 7 January 2022).
- Harbouze, R.; Pellissier, J.P.; Rolland, J.P.; Khechimi, W. Rapport de Synthèse sur L’agriculture au Maroc. Ph.D. Dissertation, CIHEAM-IAMM, Montpellier, France, 2019; p. 104. [Google Scholar]
- Abd-Elgawad, M.M.M. Managing nematodes in Egyptian citrus orchards. Bull. Natl. Res. Cent. 2020, 44, 135–136. [Google Scholar] [CrossRef]
- Cohn, E. Nematode diseases of citrus. In Economic Nematology; Webster, J.M., Ed.; Academic Press: London, UK, 1972; pp. 215–244. [Google Scholar]
- Duncan, L.W. Nematode diseases of citrus. In Citrus Health Management; Timmer, L.W., Duncan, L.W., Eds.; APS Press: St. Paul, MN, USA, 1999; pp. 136–148. [Google Scholar]
- Mokrini, F.; Janati, S.; Andaloussi, F.A.; Essarioui, A.; Houari, A.; Sbaghi, M. Importance et répartition des principaux nématodes phytoparasites des agrumes au Maroc. Rev. Maroc. Sci. Agron. Vétérinaires 2018, 6, 558–564. [Google Scholar]
- Hammam, M.; Abdel Gawad, M.; Ruan, W.; El-bahrawy, A. Management of pests and pathogens affecting citrus yield in egypt with special emphasis on nematodes. Egyption J. Agronematology 2021, 20, 64–84. [Google Scholar] [CrossRef]
- Badii, K.B.; Billah, M.K.; Afreh Nuamah, K.; Obeng Ofori, D.; Nyarko, G. Review of the pest status, economic impact and management of fruit-infesting flies (Diptera: Tephritidae) in Africa. Afr. J. Agric. Res. 2015, 10, 1488–1498. [Google Scholar] [CrossRef] [Green Version]
- Bernard, G.C.; Egnin, M.; Bonsi, C. The impact of plant-parasitic nematodes on agriculture and methods of control. Nematol.-Concepts Diagn. Control. 2017, 10, 121–151. [Google Scholar] [CrossRef] [Green Version]
- Duncan, L.W. Managing nematodes in citrus orchards. In Integrated Management of Fruit Crops and Forest Nematodes; Ciancio, A., Mukerji, K.G., Eds.; Springer Science: Dordrecht, The Netherlands, 2009; pp. 135–173. [Google Scholar]
- Sorribas, F.J.; Verdejo-Lucas, S.; Pastor, J.; Ornat, C.; Pons, J.; Valero, J. Population densities of Tylenchulus semipenetrans related to physicochemical properties of soil and yield of clementine mandarin in Spain. Plant Dis. 2008, 92, 445–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milne DL, D.; Villiers, E. Soil application of systemic pesticides for control of thrips and nematodes on citrus. Citrus Subtrop. Fruit J. 1977, 518, 9–18. [Google Scholar]
- Duncan, L.W.; Inserra, I.R. Datasheet for Tylenchulus semipenetrans. In Crop Protection Compendium; CAB International: Wallingford, UK, 2005. [Google Scholar]
- Cohn, E. The occurrence and distribution of species of Xiphinema and Longidorus in Israel. Nematologica 1969, 15, 179–192. [Google Scholar] [CrossRef]
- Hammam, M.M.A.; Wafaa, M.E.N.; Abd-Elgawad, M.M.M. Biological and chemical control of the citrus nematode, Tylenchulus semipenetrans (Cobb, 1913) on mandarin in Egypt. Egypt. J. Biol. Pest Control. 2016, 26, 345–349. [Google Scholar]
- Shokoohi, E.; Duncan, L.W. Nematode parasites of citrus. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 3rd ed.; Sikora, R.A., Coyne, D., Hallmann, J., Timper, P., Eds.; CAB International: Wallingford, UK, 2018; pp. 446–476. [Google Scholar]
- Mokrini, F. Les nématodes de dépérissement lent des agrumes Tylenchulus semipenetrans des agrumes véritabe danger pour les vergers marocains. Agric. Maghreb. 2010, 2, 72–73. [Google Scholar]
- Duncan, L.W.; Cohn, E. Nematode parasites of citrus. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 2nd ed.; Sikora, R.A., Luc, M., Bridge, J., Eds.; CAB International: Wallingford, UK, 2005; pp. 437–466. [Google Scholar]
- Nasiri, M.; Azizi, K.; Hamzehzarghani, H.; Ghaderi, R. Studies on the nematicidal activity of stinging nettle (Urtica Dioica) on plant parasitic nematodes. Arch. Phytopathol. Plant Prot. 2014, 47, 591–599. [Google Scholar] [CrossRef]
- Hooper, D.J. Extraction of free-living nematode stages from soil. In Laboratory Methods for Work with Plant and Soil Nematodes; Southey, J.F., Ed.; Her Majesty’s Stationery Office: London, UK, 1986; pp. 5–22. [Google Scholar]
- Taylor, D.P.; Netscher, C. An improved technique for preparing perineal patterns of Meloidogyne spp. Nematologica 1974, 20, 268–269. [Google Scholar]
- Mai, W.F.; Mullin, P.G. Plant-Parasitic Nematodes: A Pictorial Key to Genera; Cornell University Press: Ithaca, NY, USA, 1996; p. 28. [Google Scholar]
- De Grisse, A.T. Redescription ou modification de quelques techniques utilisées dans l’étude des nématodes phytoparasitaires. Meded. Rijksfakulteit Landbowwetenschappen Gent. 1969, 34, 351–369. [Google Scholar]
- Boag, B. Standardization of ecological terms in nematology. Fundam. Appl. Nematol 1993, 16, 190–191. [Google Scholar]
- Holterman, M.; Van Der Wurff, A.; Van Den Elsen, S.; Van Megen, H.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. Phylum-wide analysis of ssu rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 2006, 23, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- De Ley, P.; Félix, M.A.; Frisse, L.M.; Nadler, S.A.; Sternberg, P.W.; Thomas, W.K. Molecular and morphological characterisation of two reproductively isolated species with mirror-image anatomy (Nematoda: Cephalobidae). Nematology 1999, 1, 591–612. [Google Scholar] [CrossRef]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/ NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Hedges, J.I.; Oades, J.M. Comparative organic geochemistries of soils and marine sediments. Org. Geochem. 1997, 27, 319–361. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and improvement of saline and alkali soils. Soil Sci. 1954, 78, 154. [Google Scholar] [CrossRef]
- Anne, P. Sur le dosage rapide du carbone organique des sols. Ann. Agron. 1945, 15, 161–172. [Google Scholar]
- Olsen, S.R.; Watanabe, F.S.; Bowman, R.A. Evaluation of fertilizer phosphate residues by plant uptake and extractable phosphorous. Soil Sci. Soc. Am. J. 1983, 47, 952–958. [Google Scholar] [CrossRef]
- Barbano, D.M.; Clark, J.L.; Dunham, C.E.; Flemin, R.J. Kjeldahl method for determination of total nitrogen content of milk: Collaborative Study. J. AOAC Int. 1990, 73, 849–859. [Google Scholar] [CrossRef]
- Krebs, J.C. Ecología-estudio de la distribución y la abundancia. Rev. Mex. 1985, 2, 753. [Google Scholar]
- Pielou, E. Mathematical Ecology; John Willey Sons: New York, NY, USA, 1977. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative Studies of Nucleotide Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Kumar, A.; Ravi-Chandar, K.; Lopez-Pamies, O. The configurational-forces view of the nucleation and propagation of fracture and healing in elastomers as a phase transition. Int. J. Fract. 2018, 213, 1–16. [Google Scholar] [CrossRef]
- Eisvand, P.; Farrokhi Nejad, R.; Azimi, S. Plant parasitic nematodes fauna in citrus orchards in Khuzestan province, southwestern Iran. Hell. Plant Prot. J. 2019, 12, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elgawad, M.; Koura, F.; Montasser, S.; Hammam, M.; El-bahrawy, A. Long-term effect of Tylenchulus semipenetrans on citrus tree quality in reclaimed land of Egypt. Egyption J. Agronematology 2016, 15, 53–66. [Google Scholar] [CrossRef] [Green Version]
- Abu Habib, A.; Younes, H.; Ibrahim, I.; Khalil, A. Plant parasitic nematodes associated with citrus trees and reaction of two citrus cultivars to Tylenchulus semipenetrans in northern Egypt. J. Adv. Agric. Res. 2020, 25, 166–175. [Google Scholar] [CrossRef]
- Nisa, R.U.; Tantray, A.Y.; Kouser, N.; Allie, K.A.; Wani, S.M.; Alamri, S.A.; Alyemeni, M.N.; Wijaya, L.; Shah, A.A. Influence of ecological and edaphic factors on biodiversity of soil nematodes. Saudi J. Biol. Sci. 2021, 28, 3049–3059. [Google Scholar] [CrossRef]
- Kumar, K.K.; Das, A.K. Diversity and community analysis of plant parasitic nematodes associated with citrus at citrus research station, Tinsukia, Assam. J. Entomol. Zool. Stud. 2019, 7, 187–189. [Google Scholar]
- AS De Campos, J.M.; Dos Santos, L.D. Nematodes of citrus in open nurseries and orchards in Sao Paulo State, Brazil. Nematology 2002, 4, 263–264. [Google Scholar]
- O’Bannon, J.H.; Tomerlin, A.T. Citrus tree decline caused by Pratylenchus coffeae. J. Nematol. 1973, 5, 311–316. [Google Scholar] [PubMed]
- Bucki, P.; Qing, X.; Castillo, P.; Gamliel, A.; Dobrinin, S.; Alon, T.; Miyara, S.B. The genus Pratylenchus (Nematoda: Pratylenchidae) in Israel: From taxonomy to control practices. Plants 2020, 9, 1475. [Google Scholar] [CrossRef] [PubMed]
- Mokrini, F.; Laasli, S.E.; Iraqui, D.; Wifaya, A.; Mimouni, A.; Erginbas-Orakci, G.; Imren, M.; Dababat, A.A. Distribution and occurrence of plant-parasitic nematodes associated with raspberry (Rubus Idaeus) in Souss-Massa region of Morocco: Relationship with soil physico-chemical factors. Russ. J. Nematol. 2019, 27, 107–121. [Google Scholar] [CrossRef]
- Mokrini, F.; Laasli, S.E.; Karra, Y.; El Aissami, A.; Dababat, A.A. Diversity and incidence of plant-parasitic nematodes associated with saffron (Crocus Sativus L.) in Morocco and their relationship with soil physicochemical properties. Nematology 2020, 22, 87–102. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Adam, M.; Heuer, H.; Ramadan, E.-S.; Huessein, M.; Hallmann, J. Occurrence of Plant-Parasitic Nematodes Survey in Organic Farming in Egypt. Int. J. Nematol. 2013, 23, 82–90. [Google Scholar]
- Mokrini, F.; Abbad Andaloussi, F.; Waeyenberge, L.; Viaene, N.; Moens, M. First report of the dagger nematode Xiphinema diversicaudatum in citrus orchards in Morocco. Plant Dis. 2014, 98, 575. [Google Scholar] [CrossRef] [PubMed]
- AL-hazmi, A.; Abul-hayja, Z.; Trabulsi, I. Plant parasitic nematodes in Al-Kharj region of Saudi Arabia. Nematol. Mediterr. 1983, 11, 209–212. [Google Scholar]
- Hewitt, W.B.; Raski, G.A. Nematode vector of soil-borne fanleaf virus of grapevines. Proc. Helminth. Soc. Wash 1958, 48, 586–595. [Google Scholar]
- Fisher, J.M.; Raski, D.J. Feeding of Xiphinema index and X. diversicaudatum. Proc. Helminth. Soc. Wash 1967, 34, 68–72. [Google Scholar]
- Baines, R.C.; Van Gundy, S.D.; Sher, S.A. Citrus and avocado nematodes. California. Agriculture 1959, 13, 16–18. [Google Scholar]
- Shanmugam, G.; Mohankumar, A.; Sundararaj, P.; Kannan, S. Spatial Distribution of Nematode Genera in Relation to Host Plants of Western Ghats, Tamil Nadu, India. Acta Ecol. Sin. 2021, 41, 189–192. [Google Scholar] [CrossRef]
- Freitas, V.; Cares, J.; Huang, S.P. The Influence of Citrus spp. on the Community of Soil Nematodes in the Dry and Rainy Seasons in Distrito Federal of Brazil. Nematol. Bras. 2008, 32, 20–32. [Google Scholar]
- Benjlil, H.; Elkassemi, K.; Aït Hamza, M.; Mateille, T.; Furze, J.N.; Cherifi, K.; Mayad, E.H.; Ferji, Z. Plant-parasitic nematodes parasitizing saffron in Morocco: Structuring drivers and biological risk identification. Appl. Soil Ecol. 2020, 147, 103362. [Google Scholar] [CrossRef]
- Hu, C.; Qi, Y. Effective microorganisms and compost favor nematodes in wheat crops. Agron. Sustain. Dev. 2013, 33, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Yavuzaslanoglu, E.; Elekcioglu, H.I.; Nicol, J.M.; Yorgancilar, O.; Hodson, D.; Yildirim, A.F.; Yorgancilar, A.; Bolat, N. Distribution, frequency and occurrence of cereal nematodes on the central anatolian plateau in Turkey and their relationship with soil physicochemical properties. Nematology 2012, 14, 839–854. [Google Scholar] [CrossRef]
- Karuri, H.W.; Olago, D.; Neilson, R.; Njeri, E.; Opere, A.; Ndegwa, P. Plant Parasitic Nematode Assemblages Associated with Sweet Potato in Kenya and Their Relationship with Environmental Variables. Trop. Plant Pathol. 2017, 42, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Francl, L.J. Multivariate analysis of selected edaphic factors and their relationship to Heterodera glycines population density. J. Nematol. 1993, 25, 270–276. [Google Scholar]
- Rodríguez-Kábana, R. Organic and inorganic nitrogen amendments to soil as nematode suppressants. J. Nematol. 1986, 18, 129–135. [Google Scholar]
- Wang, C.; Bruening, G.; Williamson, V.M. Determination of preferred ph for root-knot nematode aggregation using pluronic F-127 gel. J. Chem. Ecol. 2009, 35, 1242–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumann, A.W.; Vashisth, T.; Spann, T.M. Mineral Nutrition Contributes to Plant Disease and Pest Resistance; Horticultural Sciences Department, UF/IFAS Extension: Gainesville, FL, USA, 2010. [Google Scholar]
- Norton, D.C. Abiotic Soil Factors and Plant-Parasitic Nematode Communities. J. Nematol. 1989, 21, 299–307. [Google Scholar] [PubMed]
- Cadet, P.; Thioulouse, J.; Albrecht, A. Relationships between Ferrisol Properties and the Structure of Plant Parasitic Nematode Communities on Sugarcane in Martinique (French West Indies). Acta Oecologica 1994, 15, 767–780. [Google Scholar]
- Korthals, G.W.; Alexiev, A.D.; Lexmond, T.M.; Kammenga, J.E.; Bongers, T. Long-term effects of copper and ph on the nematode community in an agroecosystem. Environ. Toxicol. Chem. 1996, 15, 979–985. [Google Scholar] [CrossRef]
- Salahi Ardakani, A.; Tanha Mafi, Z.; Mokaram Hesar, A.; Mohammadi Goltappeh, E. relationship between soil properties and abundance of Tylenchulus semipenetrans in citrus orchards, Kohgilouyeh va Boyerahmad Province. J. Agric. Sci. Technol. 2014, 16, 1699–1710. [Google Scholar]
- Van Gundy, S.D.; Martin, G.P. Soil texture, PH and moisture effect on the development of citrus nematode (Tylenchulus Semipenetrans). Phytopathology 1962, 52, 31. [Google Scholar]
- Laasli, S.-E.; Mokrini, F.; Lahlali, R.; Wuletaw, T.; Paulitz, T.; Dababat, A.A. Biodiversity of Nematode Communities Associated with Wheat (Triticum aestivum L.) in Southern Morocco and Their Contribution as Soil Health Bioindicators. Diversity 2022, 14, 194. [Google Scholar] [CrossRef]
- Kim, E.; Seo, Y.; Kim, Y.S.; Park, Y.; Kim, Y.H. Effects of soil textures on infectivity of root-knot nematodes on carrot. Plant Pathol. J. 2017, 33, 66–74. [Google Scholar] [CrossRef] [Green Version]




| Prospecting Area (Region) | Number of Samples | Rootstock Variety |
|---|---|---|
| Souss-Massa | 32 | Carrizo citrange |
| Sour orange | ||
| macrophylla | ||
| Marrackech-Safi | 60 | Sour orange |
| ‘Australian’ sour orange | ||
| Carrizo citrange | ||
| Volkameriana | ||
| C-35 citranges | ||
| Beni Mellal-Khenifra | 60 | Sour orange |
| Carrizo citrange | ||
| Volkameriana | ||
| C-35 citranges | ||
| ‘Australian’ sour orange | ||
| Gharb | 18 | Troyer citrange |
| Sour orange | ||
| Carrizo citrange | ||
| Berkane | 54 | Sour orange |
| Carrizo citrange | ||
| ‘Australian’ sour orange | ||
| Total | 224 |
| Region | Orchard | Soil Texture | Soil pH |
|---|---|---|---|
| Souss-Massa | Balfa | Sandy loam | 7.65 |
| Taroudant | Sandy loam | 8.32 | |
| Biogra | Sandy clay loam | 7.86 | |
| Marrackech-Safi | Souihla | Sandy loam | 8.57 |
| Es Saada | Sandy loam | 8.27 | |
| Agafai | Sandy loam | 7.42 | |
| Beni Mellal-Khenifra | Souk Sebt | Silty clay | 7.89 |
| Guettaya | Silty clay | 7.03 | |
| Gharb | M’nasra | Sandy | 7.43 |
| Allal Tazi | Sandy | 8.05 | |
| Ouled Azouz | Sandy loam | 7.64 | |
| Sidi Slimane | Sandy clay loam | 7.81 | |
| Sidi Kacem | Sandy loam | 8.23 | |
| Berkane | Zegzel | Sandy clay | 8.13 |
| Aglmine | Sandy loam | 7.59 |
| Nematode Taxa/Genus and Species | Souss-Massa | Marrackech-Safi | Beni Mellal-Khenifra | Gharb | Berkane | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pr | Intensity | Density | Pr | Intensity | Density | Pr | Intensity | Density | Pr | Intensity | Density | Pr | Intensity | Density | |||||||||||
| Root | Soil | Root | Soil | Root | Soil | Root | Soil | Root | Soil | Root | Soil | Root | Soil | Root | Soil | Root | Soil | Root | Soil | ||||||
| T. semipenetrans (Ts) | 28 | 2 | 4 | 5 | 17 | 62 | 11 | 34 | 36 | 121 | 57 | 25 | 49 | 58 | 169 | 67 | 14 | 90 | 34 | 203 | 63 | 14 | 156 | 27 | 213 |
| Pratylenchus spp. (Pra) * | 47 | 2 | 3 | 3 | 8 | 50 | 3 | 4 | 11 | 7 | 43 | 6 | 8 | 16 | 27 | 39 | 3 | 4 | 4 | 6 | 44.4 | 6 | 3 | 17 | 8 |
| P. vulnus (Pv) | - | - | - | - | - | 15 | 2 | 3 | 4 | 5 | 18 | 5 | 4 | 2 | 5 | 17 | 3 | 2 | 3 | 2 | 13 | 3 | 3 | 4 | 4 |
| P. coffeae (Pc) | - | - | - | - | - | 20 | 4 | 3 | 4 | 6 | 20 | 4 | 4 | 5 | 5 | 18 | 1 | 3 | 1 | 4 | 18.8 | 5 | 3 | 5 | 5 |
| P. thornei (Pt) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.9 | - | 3 | - | 3 |
| P. neglectus (Pn) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3.7 | - | 5 | - | 5 |
| Paratylenchus spp. (Par) | 16 | 2 | 3 | 2 | 3 | 15 | 2 | 4 | 4 | 5 | 22 | 4 | 3 | 5 | 5 | 11 | 3 | 4 | 4 | 4 | 14.8 | 3 | 2 | 3 | 3 |
| Helicotylenchus spp. (Hel) | 75 | 1 | 3 | 2 | 5 | 60 | 3 | 5 | 9 | 13 | 38 | 5 | 7 | 11 | 11 | 39 | 2 | 4 | 2 | 7 | 46.3 | 4 | 6 | 11 | 14 |
| Tylenchus spp. (Tyl) | 25 | 3 | 5 | 4 | 7 | 52 | - | 11 | - | 15 | 28 | 4 | 68 | 5 | 15 | 33 | 12 | 9 | 12 | 11 | 24 | 5 | 9 | 8 | 17 |
| Xiphinema spp. (Xip) * | 31 | 0 | 2 | 0 | 2 | 30 | - | 7 | - | 11 | 22 | 5 | 8 | - | 13 | 28 | 3 | 2 | 3 | 3 | 16.7 | - | 5 | - | 11 |
| X. diversicaudatum (Xd) | - | - | - | - | - | 6.6 | - | 3 | - | 4 | 10 | - | 4 | - | 9 | - | - | - | - | - | 7.4 | - | 3 | - | 3 |
| X. americanum (Xa) | - | - | - | - | - | 12 | - | 3 | - | 5 | 6.7 | - | 2 | - | 3 | - | - | - | - | - | 1.9 | - | 3 | - | 1 |
| X. pachtaichum (Xp) | - | - | - | - | - | - | - | - | - | 0 | 3.3 | - | 2 | - | 2 | - | - | - | - | - | 3.7 | - | 5 | - | 2 |
| Hoplolaimus indicus (Hi) | - | - | - | - | - | 5 | - | 2 | - | 3 | 10 | - | 3 | - | 6 | 11 | - | 4 | - | 5 | 5.5 | - | 3 | - | 4 |
| Scutellonema bradys (Sb) | - | - | - | - | - | - | - | - | - | 0 | 1.7 | - | 7 | - | 7 | 5.5 | - | 3 | - | 3 | 1.9 | - | 2 | - | 3 |
| Rotylenchus spp. (Rot) | 25 | 2 | 0 | 0 | 3 | 8.3 | 3 | - | - | 4 | 15 | 3 | 4 | 3 | 7 | 5.5 | - | 3 | - | 3 | 11.1 | - | 4 | - | 7 |
| Tylenchorhynchus spp. (Tyle) | 19 | 0 | 2 | 0 | 3 | 13 | - | 5 | - | 9 | 15 | - | 11 | 0 | 8 | 17 | - | 2 | - | 2 | 16.7 | - | 6 | - | 12 |
| Meloidogyne spp. (Mel) | - | - | - | - | - | 23 | 4 | 3 | 5 | 6 | 25 | 4 | 5 | 7 | 12 | 22 | 3 | 2 | 3 | 3 | 13 | 2 | 3 | 2 | 5 |
| Trichodorus spp. (Tri) | - | - | - | - | - | 8.3 | - | 2 | - | 4 | 6.7 | - | 4 | - | 4 | 11 | - | 2 | - | 2 | 3.7 | - | 3 | - | 2 |
| Criconemoides (Cri) | - | - | - | - | - | 5 | - | 3 | - | 4 | - | - | - | - | - | - | - | - | - | - | 1.9 | - | 2 | - | - |
| Hemicycliophora spp. (Hem) | - | - | - | - | - | 5 | - | 2 | - | 2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Longidorus spp. (Lon) | - | - | - | - | - | 13 | - | 2 | - | 2 | 18 | 7 | 4 | 7 | 7 | 11 | - | 3 | - | 3 | 11.1 | - | - | - | - |
| Nematode Species | Region | Collection Codes for DNA Sequences | Genbank Accession Codes |
|---|---|---|---|
| Pratylenchus vulnus | ITS | MOR-Be1-Pratylenchus MOR-MS1-Pratylenchus MOR-BK1-Pratylenchus MOR-Gh1-Pratylenchus | OM514901 OM514898 OM514899 OM514900 |
| Pratylenchus coffeae | ITS | MOR-MS2-Pratylenchus MOR-BK2-Pratylenchus MOR-Gh9-Pratylenchus MOR-Be2-Pratylenchus | OM514902 OM514903 OM514904 OM514905 |
| Pratylenchus thornei | ITS | MOR-Be4-Pratylenchus | OM514906 |
| Pratylenchus neglectus | ITS | MOR-Be6-Pratylenchus | OM514907 |
| Scutellonema bradys | ITS | MOR-BK15-Scutellonema MOR-Gh10-Scutellonema MOR-Be7-Scutellonema | OM514920 OM514921 OM514922 |
| Hoplolaimus indicus | ITS | MOR-MS6-Hoplolaimus MOR-BK13-Hoplolaimus MOR-Gh14-Hoplolaimus MOR-Be10-Hoplolaimus | OM514916 OM514917 OM514918 OM514919 |
| Xiphinema diversicaudatum | ITS | MOR-MS9-Xiphinema MOR-BK3-Xiphinema MOR-Be5-Xiphinema | OM514908 OM514909 OM514910 |
| Xiphinema americanum | ITS | MOR-MS7-Xiphinema MOR-BK8-Xiphinema MOR-Be16-Xiphinema | OM514911 OM514912 OM514913 |
| Xiphinema pachtaichum | ITS | MOR-BK5-Xiphinema MOR-Be11-Xiphinema | OM514914 OM514915 |
| Regions | Diversity Parameters | ||
|---|---|---|---|
| Number of PPNs * | Shannon Diversity Index (H′) * | Evenness (E) * | |
| Souss-Massa | 2.91 c | 1.36 b | 0.7 a |
| Marrakech-Safi | 12.65 b | 2.09 a | 0.84 a |
| Beni Mellal-Khenifra | 18.05 a | 2.03 a | 0.88 a |
| Gharb | 3.3 c | 2.05 a | 0.89 a |
| Berkane | 3.69 c | 1.4 b | 0.84 a |
| p | <0.05 | <0.05 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoubi, B.; Mokrini, F.; Dababat, A.A.; Amer, M.; Ghoulam, C.; Lahlali, R.; Laasli, S.-E.; Khfif, K.; Imren, M.; Akachoud, O.; et al. Occurrence and Geographic Distribution of Plant-Parasitic Nematodes Associated with Citrus in Morocco and Their Interaction with Soil Patterns. Life 2022, 12, 637. https://doi.org/10.3390/life12050637
Zoubi B, Mokrini F, Dababat AA, Amer M, Ghoulam C, Lahlali R, Laasli S-E, Khfif K, Imren M, Akachoud O, et al. Occurrence and Geographic Distribution of Plant-Parasitic Nematodes Associated with Citrus in Morocco and Their Interaction with Soil Patterns. Life. 2022; 12(5):637. https://doi.org/10.3390/life12050637
Chicago/Turabian StyleZoubi, Btissam, Fouad Mokrini, Abdelfattah A. Dababat, Mohammed Amer, Cherki Ghoulam, Rachid Lahlali, Salah-Eddine Laasli, Khalid Khfif, Mustafa Imren, Oumaima Akachoud, and et al. 2022. "Occurrence and Geographic Distribution of Plant-Parasitic Nematodes Associated with Citrus in Morocco and Their Interaction with Soil Patterns" Life 12, no. 5: 637. https://doi.org/10.3390/life12050637
APA StyleZoubi, B., Mokrini, F., Dababat, A. A., Amer, M., Ghoulam, C., Lahlali, R., Laasli, S.-E., Khfif, K., Imren, M., Akachoud, O., Benkebboura, A., Housseini, A. I., & Qaddoury, A. (2022). Occurrence and Geographic Distribution of Plant-Parasitic Nematodes Associated with Citrus in Morocco and Their Interaction with Soil Patterns. Life, 12(5), 637. https://doi.org/10.3390/life12050637










