The Trinity of Skin: Skin Homeostasis as a Neuro–Endocrine–Immune Organ
Abstract
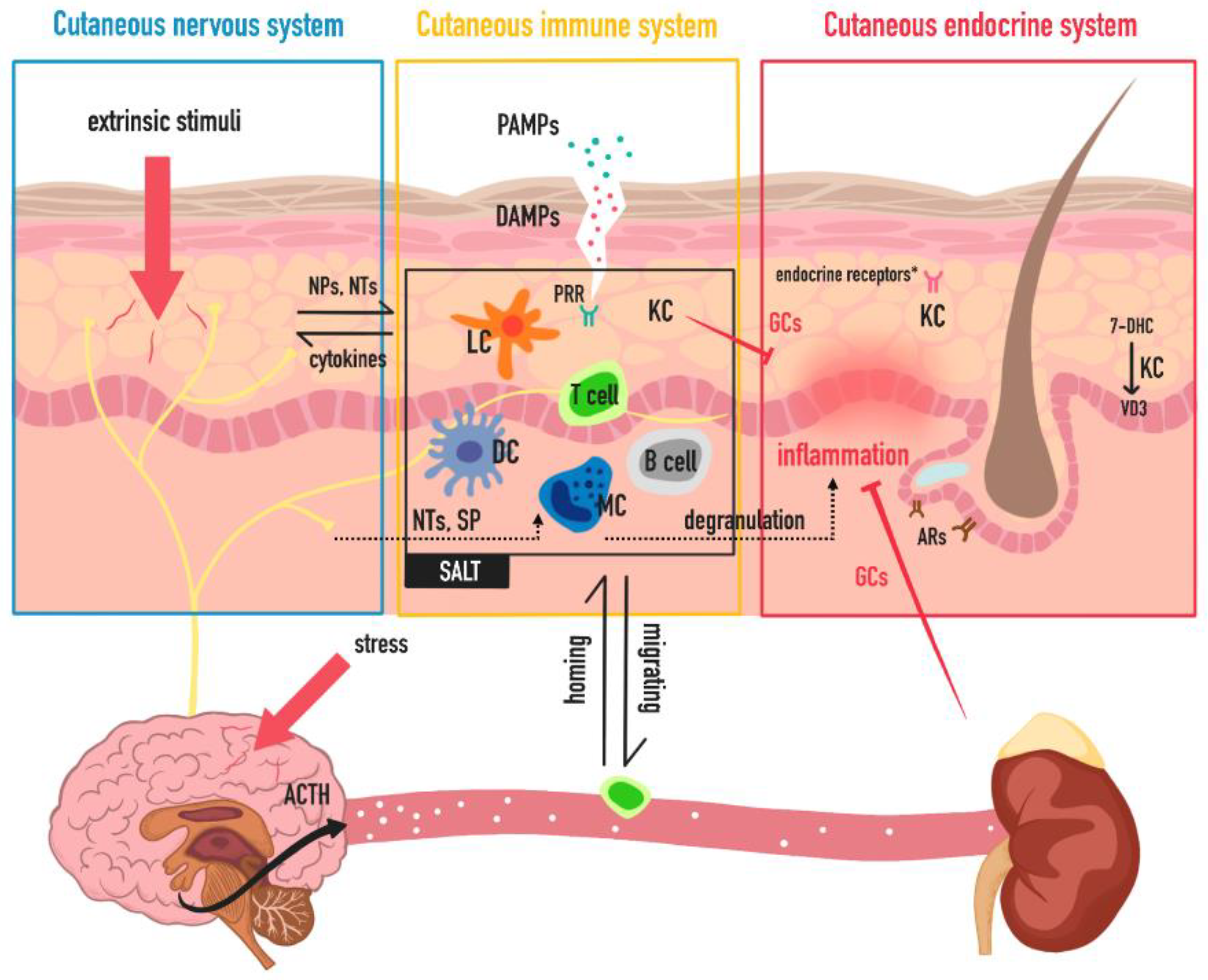
1. Introduction
2. Neuro Function of Skin
2.1. Anatomic Foundation of Cutaneous Nervous System
2.2. Neuroimmune Interactions of Skin
2.3. Skin-CNS Connection
3. Endocrine Function of Skin
3.1. Skin as Endocrine End Organ
3.2. Skin as Endocrine Initiating Organ
4. Immune Function of Skin
4.1. Barrier and Immune Cells Undernease
4.2. Systemic Association with Cutaneous Immue System
5. Rosacea as a Disease Model of the Trinity of Skin
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Sullivan, R.L.; Lipper, G.; Lerner, E.A. The neuro-immuno-cutaneous-endocrine network: Relationship of mind and skin. Arch. Dermatol. 1998, 134, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Association between Stress and the HPA Axis in the Atopic Dermatitis. Int. J. Mol. Sci. 2017, 18, 2131. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Lavery, M.J.; Nattkemper, L.A.; Albornoz, C.; Valdes Rodriguez, R.; Stull, C.; Weaver, L.; Hamsher, J.; Sanders, K.M.; Chan, Y.H.; et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br. J. Dermatol. 2019, 180, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, X.; Zhang, L.; Xue, F.; Zheng, J. Immunomodulatory effects of sleep deprivation at different timing of psoriasiform process on skin inflammation. Biochem. Biophys. Res. Commun. 2019, 513, 452–459. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, J. Influence of stress on the development of psoriasis. Clin. Exp. Dermatol. 2020, 45, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Bozo, R.; Danis, J.; Flink, L.B.; Vidacs, D.L.; Kemeny, L.; Bata-Csorgo, Z. Stress-Related Regulation Is Abnormal in the Psoriatic Uninvolved Skin. Life 2021, 11, 599. [Google Scholar] [CrossRef]
- Glatte, P.; Buchmann, S.J.; Hijazi, M.M.; Illigens, B.M.; Siepmann, T. Architecture of the Cutaneous Autonomic Nervous System. Front. Neurol. 2019, 10, 970. [Google Scholar] [CrossRef]
- Fleming, M.S.; Luo, W. The anatomy, function, and development of mammalian Abeta low-threshold mechanoreceptors. Front. Biol. 2013, 8, 408–420. [Google Scholar] [CrossRef]
- Cobo, R.; Garcia-Piqueras, J.; Cobo, J.; Vega, J.A. The Human Cutaneous Sensory Corpuscles: An Update. J. Clin. Med. 2021, 10, 227. [Google Scholar] [CrossRef]
- Lawson, S.N. Phenotype and Function of Somatic Primary Afferent Nociceptive Neurones with C-, Adelta- or Aalpha/beta-Fibres. Exp. Physiol. 2002, 87, 239–244. [Google Scholar] [CrossRef]
- Djouhri, L. Adelta-fiber low threshold mechanoreceptors innervating mammalian hairy skin: A review of their receptive, electrophysiological and cytochemical properties in relation to Adelta-fiber high threshold mechanoreceptors. Neurosci. Biobehav. Rev. 2016, 61, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, M.; Schmidt, R.; Weidner, C.; Hilliges, M.; Torebjork, H.E.; Handwerker, H.O. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J. Neurophysiol. 2003, 89, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Harvima, I.T. Mast cell-neural interactions contribute to pain and itch. Immunol. Rev. 2018, 282, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Meixiong, J.; Basso, L.; Dong, X.; Gaudenzio, N. Nociceptor-Mast Cell Sensory Clusters as Regulators of Skin Homeostasis. Trends Neurosci. 2020, 43, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Fernandes, H.; Mucida, D. Neuro-Immune Interactions at Barrier Surfaces. Cell 2016, 165, 801–811. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Kolter, J.; Feuerstein, R.; Zeis, P.; Hagemeyer, N.; Paterson, N.; d’Errico, P.; Baasch, S.; Amann, L.; Masuda, T.; Losslein, A.; et al. A Subset of Skin Macrophages Contributes to the Surveillance and Regeneration of Local Nerves. Immunity 2019, 50, 1482–1497.e7. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ricardo-Gonzalez, R.R.; Moro, K. Skin-Resident Innate Lymphoid Cells—Cutaneous Innate Guardians and Regulators. Trends Immunol. 2020, 41, 100–112. [Google Scholar] [CrossRef]
- Marshall, A.S.; Silva, J.R.; Bannerman, C.A.; Gilron, I.; Ghasemlou, N. Skin-Resident gammadelta T Cells Exhibit Site-Specific Morphology and Activation States. J. Immunol. Res. 2019, 2019, 9020234. [Google Scholar] [CrossRef]
- Veiga-Fernandes, H.; Artis, D. Neuronal-immune system cross-talk in homeostasis. Science 2018, 359, 1465–1466. [Google Scholar] [CrossRef]
- Blake, K.J.; Jiang, X.R.; Chiu, I.M. Neuronal Regulation of Immunity in the Skin and Lungs. Trends Neurosci. 2019, 42, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Ordovas-Montanes, J.; Rakoff-Nahoum, S.; Huang, S.; Riol-Blanco, L.; Barreiro, O.; von Andrian, U.H. The Regulation of Immunological Processes by Peripheral Neurons in Homeostasis and Disease. Trends Immunol. 2015, 36, 578–604. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Di Nardo, A. Skin neurogenic inflammation. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 249–259. [Google Scholar]
- Vidal Yucha, S.E.; Tamamoto, K.A.; Kaplan, D.L. The importance of the neuro-immuno-cutaneous system on human skin equivalent design. Cell Prolif. 2019, 52, e12677. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Neuroimmune interactions: From the brain to the immune system and vice versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef] [PubMed]
- Botchkarev, V.A.; Yaar, M.; Peters, E.M.; Raychaudhuri, S.P.; Botchkareva, N.V.; Marconi, A.; Raychaudhuri, S.K.; Paus, R.; Pincelli, C. Neurotrophins in skin biology and pathology. J. Investig. Dermatol. 2006, 126, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.F. Overview of Neuropeptides: Awakening the Senses? Headache 2017, 57 (Suppl. S2), 37–46. [Google Scholar] [CrossRef]
- Li, W.W.; Guo, T.Z.; Liang, D.Y.; Sun, Y.; Kingery, W.S.; Clark, J.D. Substance P signaling controls mast cell activation, degranulation, and nociceptive sensitization in a rat fracture model of complex regional pain syndrome. Anesthesiology 2012, 116, 882–895. [Google Scholar] [CrossRef]
- Theoharides, T.C. The impact of psychological stress on mast cells. Ann. Allergy Asthma Immunol. 2020, 125, 388–392. [Google Scholar] [CrossRef]
- Schlereth, T.; Schukraft, J.; Kramer-Best, H.H.; Geber, C.; Ackermann, T.; Birklein, F. Interaction of calcitonin gene related peptide (CGRP) and substance P (SP) in human skin. Neuropeptides 2016, 59, 57–62. [Google Scholar] [CrossRef]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, L.; Bekaert, T.; Chau, T.L.; Tavernier, J.; Chariot, A.; Beyaert, R. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: Variations on a common theme. Cell Mol. Life Sci. 2008, 65, 2964–2978. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-kappaB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Maier, E.; Werner, D.; Duschl, A.; Bohle, B.; Horejs-Hoeck, J. Human Th2 but not Th9 cells release IL-31 in a STAT6/NF-kappaB-dependent way. J. Immunol. 2014, 193, 645–654. [Google Scholar] [CrossRef]
- Roosterman, D.; Goerge, T.; Schneider, S.W.; Bunnett, N.W.; Steinhoff, M. Neuronal control of skin function: The skin as a neuroimmunoendocrine organ. Physiol. Rev. 2006, 86, 1309–1379. [Google Scholar] [CrossRef]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int. J. Mol. Sci. 2017, 18, 1028. [Google Scholar] [CrossRef]
- Marz, P.; Heese, K.; Dimitriades-Schmutz, B.; Rose-John, S.; Otten, U. Role of interleukin-6 and soluble IL-6 receptor in region-specific induction of astrocytic differentiation and neurotrophin expression. Glia 1999, 26, 191–200. [Google Scholar] [CrossRef]
- Furue, M.; Yamamura, K.; Kido-Nakahara, M.; Nakahara, T.; Fukui, Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy 2018, 73, 29–36. [Google Scholar] [CrossRef]
- Stander, S.; Steinhoff, M. Pathophysiology of pruritus in atopic dermatitis: An overview. Exp. Dermatol. 2002, 11, 12–24. [Google Scholar] [CrossRef]
- Mochizuki, H.; Tashiro, M.; Kano, M.; Sakurada, Y.; Itoh, M.; Yanai, K. Imaging of central itch modulation in the human brain using positron emission tomography. Pain 2003, 105, 339–346. [Google Scholar] [CrossRef]
- Najafi, P.; Dufor, O.; Ben Salem, D.; Misery, L.; Carre, J.L. Itch processing in the brain. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Golpanian, R.S.; Kim, H.S.; Yosipovitch, G. Effects of Stress on Itch. Clin. Ther. 2020, 42, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, J.B.; Lee, J.H.; Kim, I.H. How stress triggers itch: A preliminary study of the mechanism of stress-induced pruritus using fMRI. Int. J. Dermatol. 2016, 55, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, L.M.; Perez, P. Roles of the Glucocorticoid and Mineralocorticoid Receptors in Skin Pathophysiology. Int. J. Mol. Sci. 2018, 19, 1906. [Google Scholar] [CrossRef]
- Mancino, G.; Miro, C.; Di Cicco, E.; Dentice, M. Thyroid hormone action in epidermal development and homeostasis and its implications in the pathophysiology of the skin. J. Endocrinol. Investig. 2021, 44, 1571–1579. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Picardo, M.; Ju, Q.; Kurokawa, I.; Torocsik, D.; Biro, T.; Schneider, M.R. Beyond acne: Current aspects of sebaceous gland biology and function. Rev. Endocr. Metab. Disord. 2016, 17, 319–334. [Google Scholar] [CrossRef]
- Wertheimer, E.; Trebicz, M.; Eldar, T.; Gartsbein, M.; Nofeh-Moses, S.; Tennenbaum, T. Differential roles of insulin receptor and insulin-like growth factor-1 receptor in differentiation of murine skin keratinocytes. J. Investig. Dermatol. 2000, 115, 24–29. [Google Scholar] [CrossRef]
- Daye, M.; Temiz, S.A.; Isik, B.; Durduran, Y. Relationship between acanthosis nigricans, acrochordon and metabolic syndrome in patients with lichen planus. Int. J. Clin. Pract. 2021, 75, e14687. [Google Scholar] [CrossRef]
- Saylam Kurtipek, G.; Cihan, F.G.; Erayman Demirbas, S.; Ataseven, A. The Frequency of Autoimmune Thyroid Disease in Alopecia Areata and Vitiligo Patients. Biomed. Res. Int. 2015, 2015, 435947. [Google Scholar] [CrossRef]
- Kubba, R. Insulin Resistance Associated Acne. In Acne: Current Concepts and Management; Suh, D.H., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 95–110. [Google Scholar]
- Terao, M.; Katayama, I. Local cortisol/corticosterone activation in skin physiology and pathology. J. Dermatol. Sci. 2016, 84, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.S.; Schink, L.; Mann, J.; Merk, V.M.; Zwicky, P.; Mundt, S.; Simon, D.; Kulms, D.; Abraham, S.; Legler, D.F.; et al. Keratinocytes control skin immune homeostasis through de novo-synthesized glucocorticoids. Sci. Adv. 2021, 7, eabe0337. [Google Scholar] [CrossRef] [PubMed]
- Mancino, G.; Sibilio, A.; Luongo, C.; Di Cicco, E.; Miro, C.; Cicatiello, A.G.; Nappi, A.; Sagliocchi, S.; Ambrosio, R.; De Stefano, M.A.; et al. The Thyroid Hormone Inactivator Enzyme, Type 3 Deiodinase, Is Essential for Coordination of Keratinocyte Growth and Differentiation. Thyroid 2020, 30, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, J.M.; Leiros, G.J.; Balana, M.E. Androgens and androgen receptor action in skin and hair follicles. Mol. Cell Endocrinol. 2018, 465, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.L.; Deak, T. A users guide to HPA axis research. Physiol. Behav. 2017, 178, 43–65. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef]
- Slominski, A.T.; Manna, P.R.; Tuckey, R.C. Cutaneous glucocorticosteroidogenesis: Securing local homeostasis and the skin integrity. Exp. Dermatol. 2014, 23, 369–374. [Google Scholar] [CrossRef]
- Hannen, R.F.; Michael, A.E.; Jaulim, A.; Bhogal, R.; Burrin, J.M.; Philpott, M.P. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem. Biophys. Res. Commun. 2011, 404, 62–67. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef]
- Samuel, S.; Sitrin, M.D. Vitamin D’s role in cell proliferation and differentiation. Nutr. Rev. 2008, 66, S116–S124. [Google Scholar] [CrossRef]
- Demay, M.B. The hair cycle and Vitamin D receptor. Arch. Biochem. Biophys. 2012, 523, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Schauber, J.; Dorschner, R.A.; Coda, A.B.; Büchau, A.S.; Liu, P.T.; Kiken, D.; Helfrich, Y.R.; Kang, S.; Elalieh, H.Z.; Steinmeyer, A. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D–dependent mechanism. J. Clin. Investig. 2007, 117, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Feldman, D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Kader, S.; Temiz, S.A.; Akdag, T.; Unlu, A. Evaluation of vitamin D and calcium mineral metabolism in patients with chronic urticaria. Int. J. Med. Biochem. 2021, 4, 157–160. [Google Scholar] [CrossRef]
- Ekiz, Ö.; Balta, I.; Şen, B.B.; Dikilitaş, M.C.; Özuğuz, P.; Rifaioğlu, E.N. Vitamin D status in patients with rosacea. Cutan. Ocul. Toxicol. 2014, 33, 60–62. [Google Scholar] [CrossRef]
- Bosko, C.A. Skin Barrier Insights: From Bricks and Mortar to Molecules and Microbes. J. Drugs Dermatol. 2019, 18, s63–s67. [Google Scholar]
- Juráňová, J.; Franková, J.; Ulrichová, J. The role of keratinocytes in inflammation. J. Appl. Biomed. 2017, 15, 169–179. [Google Scholar] [CrossRef]
- Abdallah, F.; Mijouin, L.; Pichon, C. Skin Immune Landscape: Inside and Outside the Organism. Mediat. Inflamm. 2017, 2017, 5095293. [Google Scholar] [CrossRef]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef]
- Girardi, M.; Lewis, J.M.; Filler, R.B.; Hayday, A.C.; Tigelaar, R.E. Environmentally responsive and reversible regulation of epidermal barrier function by gammadelta T cells. J. Investig. Dermatol. 2006, 126, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Bashiardes, S.; Levy, M.; Elinav, E. The Role of the Immune System in Metabolic Health and Disease. Cell Metab. 2017, 25, 506–521. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, D.R.; Hart, R.; Bah, N.; Ushakov, D.S.; Munoz-Ruiz, M.; Feederle, R.; Hayday, A.C. Normality sensing licenses local T cells for innate-like tissue surveillance. Nat. Immunol. 2022, 23, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.S. Organization of the Skin Immune System and Compartmentalized Immune Responses in Infectious Diseases. Clin. Microbiol. Rev. 2019, 32, e00034-18. [Google Scholar] [CrossRef]
- Ono, S.; Kabashima, K. Proposal of inducible skin-associated lymphoid tissue (iSALT). Exp. Dermatol. 2015, 24, 630–631. [Google Scholar] [CrossRef][Green Version]
- Gröne, A. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 2002, 88, 1–12. [Google Scholar] [CrossRef]
- Stoitzner, P.; Stössel, H.; Romani, N.; Pfaller, K. A close-up view of migrating Langerhans cells in the skin. J. Investig. Dermatol. 2002, 118, 117–125. [Google Scholar] [CrossRef]
- Suto, H.; Nakae, S.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast cell-associated TNF promotes dendritic cell migration. J. Immunol. 2006, 176, 4102–4112. [Google Scholar] [CrossRef]
- Byrne, S.N.; Limón-Flores, A.Y.; Ullrich, S.E. Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J. Immunol. 2008, 180, 4648–4655. [Google Scholar] [CrossRef]
- Masopust, D.; Schenkel, J.M. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 2013, 13, 309–320. [Google Scholar] [CrossRef]
- Xu, Q.; Zheng, J. γδT cells as a potential candidate contributing to the development of psoriatic cardiovascular disease. Clin. Exp. Dermatol. 2021, 46, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Elnabawi, Y.A.; Garshick, M.S.; Tawil, M.; Barrett, T.J.; Fisher, E.A.; Lo Sicco, K.; Neimann, A.L.; Scher, J.U.; Krueger, J.; Berger, J.S. CCL20 in psoriasis: A potential biomarker of disease severity, inflammation, and impaired vascular health. J. Am. Acad. Dermatol. 2021, 84, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Kadono, T. Inflammatory skin march” in atopic dermatitis and psoriasis. Inflamm. Res. 2017, 66, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Spergel, J.M. The atopic march: Critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 2018, 120, 131–137. [Google Scholar] [CrossRef]
- Worbs, T.; Hammerschmidt, S.I.; Forster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017, 17, 30–48. [Google Scholar] [CrossRef]
- Gregor, C.E.; Foeng, J.; Comerford, I.; McColl, S.R. Chemokine-Driven CD4(+) T Cell Homing: New Concepts and Recent Advances. Adv. Immunol. 2017, 135, 119–181. [Google Scholar] [CrossRef]
- Islam, S.A.; Luster, A.D. T cell homing to epithelial barriers in allergic disease. Nat. Med. 2012, 18, 705–715. [Google Scholar] [CrossRef]
- Campbell, J.J.; Ebsworth, K.; Ertl, L.S.; McMahon, J.P.; Newland, D.; Wang, Y.; Liu, S.; Miao, Z.; Dang, T.; Zhang, P.; et al. IL-17-Secreting gammadelta T Cells Are Completely Dependent upon CCR6 for Homing to Inflamed Skin. J. Immunol. 2017, 199, 3129–3136. [Google Scholar] [CrossRef]
- Czarnowicki, T.; He, H.Y.; Wen, H.C.; Hashim, P.W.; Nia, J.K.; Malik, K.; Estrada, Y.; Kimmel, G.W.; Taliercio, M.; Krueger, J.G.; et al. Alopecia areata is characterized by expansion of circulating Th2/Tc2/Th22, within the skin-homing and systemic T-cell populations. Allergy 2018, 73, 713–723. [Google Scholar] [CrossRef]
- Li, S.; Zhu, G.; Yang, Y.; Jian, Z.; Guo, S.; Dai, W.; Shi, Q.; Ge, R.; Ma, J.; Liu, L.; et al. Oxidative stress drives CD8(+) T-cell skin trafficking in patients with vitiligo through CXCL16 upregulation by activating the unfolded protein response in keratinocytes. J. Allergy Clin. Immunol. 2017, 140, 177–189.e9. [Google Scholar] [CrossRef]
- Gazi, U.; Gureser, A.S.; Oztekin, A.; Karasartova, D.; Kosar-Acar, N.; Derici, M.K.; Artuz, F.; Mumcuoglu, K.Y.; Taylan-Ozkan, A. Skin-homing T-cell responses associated with Demodex infestation and rosacea. Parasite Immunol. 2019, 41, e12658. [Google Scholar] [CrossRef] [PubMed]
- Yumeen, S.; Girardi, M. Insights Into the Molecular and Cellular Underpinnings of Cutaneous T Cell Lymphoma. Yale J. Biol. Med. 2020, 93, 111–121. [Google Scholar] [PubMed]
- Saraceno, R.; Kleyn, C.; Terenghi, G.; Griffiths, C. The role of neuropeptides in psoriasis. Br. J. Dermatol. 2006, 155, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Ascenso, A.; Ribeiro, H.M.; Marto, J. The Brain–skin connection and the pathogenesis of psoriasis: A review with a focus on the serotonergic system. Cells 2020, 9, 796. [Google Scholar] [CrossRef] [PubMed]
- Rousset, L.; Halioua, B. Stress and psoriasis. Int. J. Dermatol. 2018, 57, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Schwab, V.D.; Sulk, M.; Seeliger, S.; Nowak, P.; Aubert, J.; Mess, C.; Rivier, M.; Carlavan, I.; Rossio, P.; Metze, D.; et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J. Investig. Dermatol. Symp. Proc. 2011, 15, 53–62. [Google Scholar] [CrossRef]
- Muto, Y.; Wang, Z.; Vanderberghe, M.; Two, A.; Gallo, R.L.; Di Nardo, A. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J. Investig. Dermatol. 2014, 134, 2728–2736. [Google Scholar] [CrossRef]
- Rainer, B.M.; Kang, S.; Chien, A.L. Rosacea: Epidemiology, pathogenesis, and treatment. Dermatoendocrinol 2017, 9, e1361574. [Google Scholar] [CrossRef]
- Kulka, M.; Sheen, C.H.; Tancowny, B.P.; Grammer, L.C.; Schleimer, R.P. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008, 123, 398–410. [Google Scholar] [CrossRef]
- Meylan, E.; Tschopp, J.; Karin, M. Intracellular pattern recognition receptors in the host response. Nature 2006, 442, 39–44. [Google Scholar] [CrossRef]
- Yoon, S.H.; Hwang, I.; Lee, E.; Cho, H.J.; Ryu, J.H.; Kim, T.G.; Yu, J.W. Antimicrobial Peptide LL-37 Drives Rosacea-Like Skin Inflammation in an NLRP3-Dependent Manner. J. Investig. Dermatol. 2021, 141, 2885–2894.e5. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Di Nardo, A.; Bardan, A.; Murakami, M.; Ohtake, T.; Coda, A.; Dorschner, R.A.; Bonnart, C.; Descargues, P.; Hovnanian, A.; et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2007, 13, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Nagata, H.; Umemura, S.; Kawana, S.; Osamura, R.Y. In situ expression of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skin. FASEB J. 2001, 15, 2297–2299. [Google Scholar] [CrossRef] [PubMed]
- Fimmel, S.; Glass, E.; Zouboulis, C. Neuropeptides and UV radiation are possible mediators of inflammation in rosacea. J. Investig. Dermatol. 2005, 124, A16. [Google Scholar]
- Fimmel, S.; Abdel-Naser, M.B.; Kutzner, H.; Kligman, A.M.; Zouboulis, C.C. New aspects of the pathogenesis of rosacea. Drug Discov. Today Dis. Mech. 2008, 5, e103–e111. [Google Scholar] [CrossRef]
- Wang, W.; Nan, X.; Ji, P.; Dow, K. Corticotropin releasing hormone modulates endotoxin-induced inflammatory cytokine expression in human trophoblast cells. Placenta 2007, 28, 1032–1038. [Google Scholar] [CrossRef]
- Jamieson, B.B.; Kim, J.S.; Iremonger, K.J. Cannabinoid and vanilloid pathways mediate opposing forms of synaptic plasticity in corticotropin-releasing hormone neurons. J. Neuroendocrinol. 2021, 34, e13084. [Google Scholar] [CrossRef]
- Suresh, R.; Mosser, D.M. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv. Physiol. Educ. 2013, 37, 284–291. [Google Scholar] [CrossRef]
- Paus, R.; Theoharides, T.C.; Arck, P.C. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006, 27, 32–39. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, R.; Luo, L.; Zheng, J. The Trinity of Skin: Skin Homeostasis as a Neuro–Endocrine–Immune Organ. Life 2022, 12, 725. https://doi.org/10.3390/life12050725
Jin R, Luo L, Zheng J. The Trinity of Skin: Skin Homeostasis as a Neuro–Endocrine–Immune Organ. Life. 2022; 12(5):725. https://doi.org/10.3390/life12050725
Chicago/Turabian StyleJin, Rong, Lan Luo, and Jie Zheng. 2022. "The Trinity of Skin: Skin Homeostasis as a Neuro–Endocrine–Immune Organ" Life 12, no. 5: 725. https://doi.org/10.3390/life12050725
APA StyleJin, R., Luo, L., & Zheng, J. (2022). The Trinity of Skin: Skin Homeostasis as a Neuro–Endocrine–Immune Organ. Life, 12(5), 725. https://doi.org/10.3390/life12050725




