Prevention and Treatment of Life-Threatening COVID-19 May Be Possible with Oxygen Treatment
Abstract
:1. Introduction
2. Potential Pathophysiological Mechanisms of COVID-19
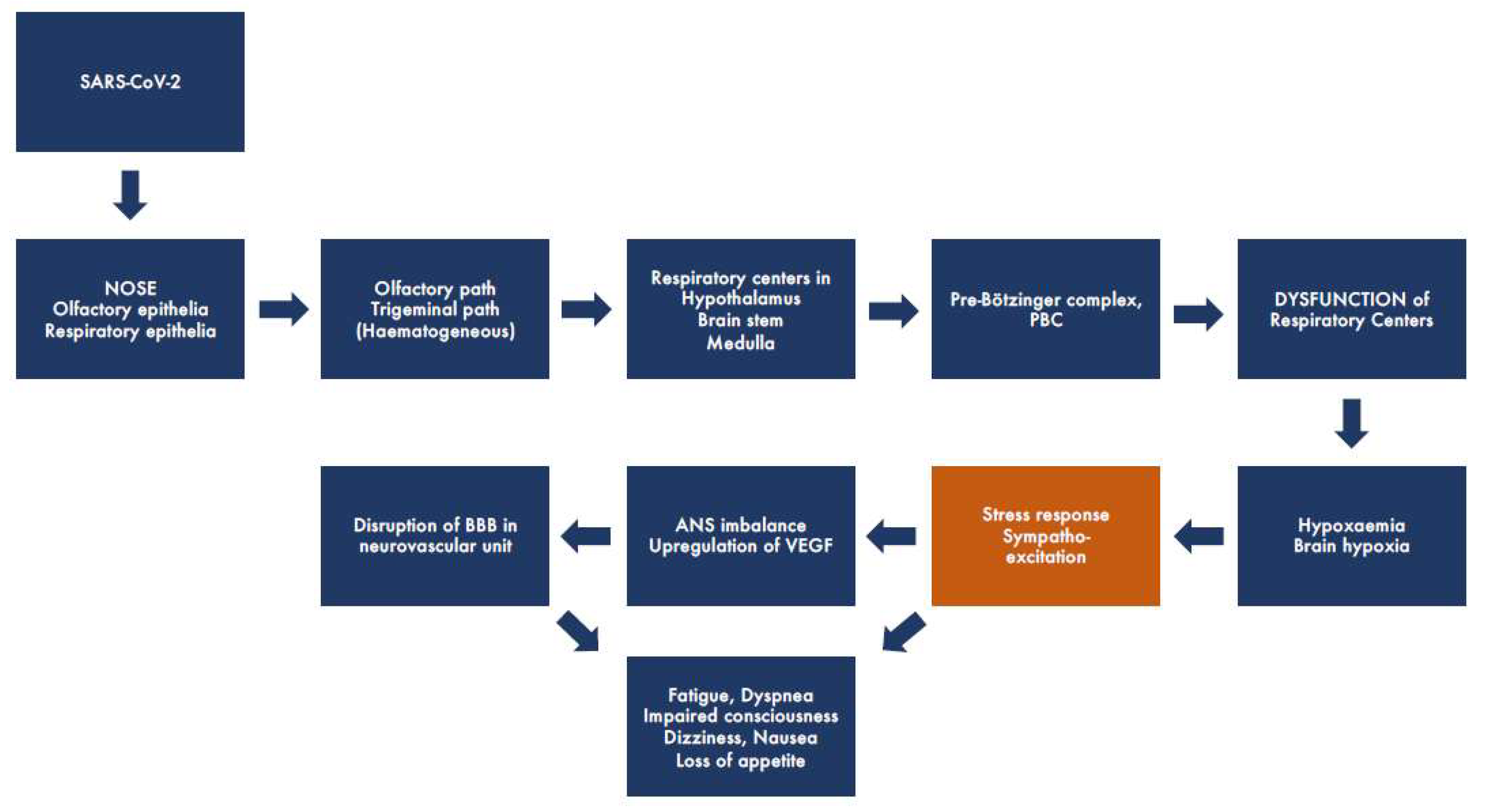
2.1. Early Neurological (Extrapulmonary) Symptoms
2.2. Significance of Virus Genotypes for Virulence Symptoms and Neurotropism
2.3. The Role of the Blood-Brain Barrier
2.4. Role of Autonomic Dysfunction in Symptoms of COVID-19 and Long COVID-19
3. Therapies of COVID-19 and Long COVID-19
4. Oxygen Treatment
4.1. Principles of the Delivery of Oxygen into the Body and Its Administration
4.2. Oxygen Toxicity
4.3. Oxygen Treatment under Increased Ambient Pressure
5. Reversing the ANS/CAN Imbalance in COVID-19 and Long COVID-19
Inflammation and VNS
6. Brain Hypoxia May Be the Major Cause for COVID-19 and Long COVID-19 Symptoms
7. Significance of Chest CT in COVID-19
8. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perlman, S.; Netland, J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 439–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.-Y.; Chen, S.-D.; Jin, H.-J.; Tan, K.-S.; Wang, D.-Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Desai, I.; Manchanda, R.; Kumar, N.; Tiwari, A.; Kumar, M. Neurological manifestations of coronavirus disease 2019: Exploring past to understand present. Neurol. Sci. 2021, 42, 773–785. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Roman, G.C.; Spencer, P.S.; Reis, J.; Buguet, A.; Faris, M.E.A.; Katrak, S.M.; Lainez, M.; Medina, M.T.; Meshram, C.; Mizusawa, H.; et al. WFN Environmental Neurology Specialty Group The neurology of COVID-19 revisited: A proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J. Neurol. Sci. 2020, 414, 116884. [Google Scholar] [CrossRef]
- Ylikoski, J.; Markkanen, M.; Mäkitie, A. Pathophysiology of the COVID-19—Entry to the CNS through the nose. Acta Oto-Laryngol. 2020, 140, 886–889. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Qu, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552–555. [Google Scholar] [CrossRef]
- Chang, R.B.; Strochlic, D.E.; Williams, E.K.; Umans, B.; Liberles, S.D. Vagal sensory neuron subtypes that differentially control breathing. Cell 2015, 161, 622–633. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Cardona, G.C.; Pájaro, L.D.; Marzola, I.D.; Villegas, Y.R.; Salazar, L.R. Neurotropism of SARS-CoV-2: Mechanisms and manifestations. J. Neurol. Sci. 2020, 412, 116824. [Google Scholar] [CrossRef] [PubMed]
- Bougakov, D.; Podell, K.; Goldberg, E. Multiple Neuroinvasive Pathways in COVID-19. Mol. Neurobiol. 2020, 58, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Srivastava, A.K.; Ray, U.; Tripathi, P.P. Is the collapse of the respiratory center in the brain responsible for respiratory breakdown in COVID-19 patients? ACS Chem. Neurosci. 2020, 11, 1379–1381. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.M.; Levi, M.; McKee, M.; Schilling, R.; Lim, W.S.; Grocott, M.P. Covid-19: A complex multisystem clinical syndrome. Br. J. Anaesth. 2020, 125, 238. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multi-center European study. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- van Riel, D.; Verdijk, R.; Kuiken, T. The olfactory nerve: A shortcut for influenza and other viral diseases into the central nervous system. J. Pathol. 2015, 235, 277–287. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and is detected in Specific Cell Subsets Across Tissues. Cell 2020, 181, 1016–1035. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2020, 24, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Desforges, M.; Le Coupanec, A.; Brison, É.; Meessen-Pinard, M.; Talbot, P.J. Neuroinvasive and neurotropic human respiratory coronaviruses: Potential neurovirulent agents in humans. In Infectious Diseases and Nanomedicine I; Adhikari, R., Thapa, S., Eds.; Springer: New Delhi, India, 2014; Volume 807, pp. 75–96. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Y.; Li, X.L.; Yan, Z.R.; Sun, X.P.; Han, J.; Zhang, B.W. Potential neurological symptoms of COVID-19. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420917830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.C.; Ellenberger, H.H.; Ballanyi, K.; Richter, D.W.; Feldman, J.L. PreBötzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science 1991, 254, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Burgold, T.; Voituron, N.; Caganova, M.; Tripathi, P.P.; Menuet, C.; Tusi, B.K.; Spreafico, F.; Bévengut, M.; Gestreau, C.; Buontempo, S.; et al. The H3K27 demethylase JMJD3 is required for maintenance of the embryonic respiratory neuronal network, neonatal breathing, and survival. Cell Rep. 2012, 2, 1244–1258. [Google Scholar] [CrossRef] [Green Version]
- Chigr, F.; Merzouki, M.; Najimi, M. Comment on “The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients”. J. Med. Virol. 2020, 92, 703–704. [Google Scholar] [CrossRef]
- Chigr, F.; Rachidi, F.; Tardivel, C.; Najimi, M.; Moyse, E. Modulation of orexigenic and anorexigenic peptides gene expression in the rat DVC and hypothalamus by acute immobilization stress. Front. Cell. Neurosci. 2014, 8, 198. [Google Scholar] [CrossRef] [Green Version]
- Babic, T.; Browning, K.N. the role of vagal neurocircuits in the regulation of nausea and vomiting. Eur. J. Pharmacol. 2014, 722, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Benarroch, E.E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Benvenuto, D.; Giovanetti, M.; Ciccozzi, A.; Spoto, S.; Angeletti, S.; Ciccozzi, M. The 2019-new coronavirus epidemic: Evidence for virus evolution. J. Med. Virol. 2020, 92, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.-E.; Kavanagh Williamson, M.; Antón-Plágaro, C.; Shoemark, D.K.; Simón-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; Van Der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Molina-Mora, J.A.; González, A.; Jiménez-Morgan, S.; Cordero-Laurent, E.; Brenes, H.; Soto-Garita, C.; Sequeira-Soto, J.; Duarte-Martínez, F. Clinical profiles at the time of diagnosis of COVID-19 in Costa Rica during the pre-vaccination period using a machine learning approach. medRxiv 2021. [Google Scholar] [CrossRef]
- Molina-Mora, J.A.; González, A.; Jiménez-Morgan, S.; Cordero-Laurent, E.; Brenes, H.; Soto-Garita, C.; Sequeira-Soto, J.; Duarte-Martínez, F. Metagenomic Pipeline for Identifying Co-Infections among Distinct SARS-CoV-2 Variants of Concern: Study Cases from Alpha to Omicron; Research Square: Durham, NC, USA, 2022. [Google Scholar] [CrossRef]
- Molina-Mora, J.A. Insights into the mutation T1117I in the spike and the lineage B.1.1.389 of SARS-CoV-2 circulating in Costa Rica. Gene Rep. 2022, 27, 101554. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.; Huang, S.F.; Patkar, S.; Gassmann, M.; Ogunshola, O.O. Differential responses of blood-brain barrier associated cells to hypoxia and ischemia: A comparative study. Fluids Barriers CNS 2015, 12, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood–Brain Barrier Dynamics to Maintain Brain Homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef]
- Kaur, C.; Ling, E.A. Blood brain barrier in hypoxic-ischemic conditions. Curr. Neurovascular Res. 2008, 5, 71–81. [Google Scholar] [CrossRef]
- Lüscher, T.F. Understanding COVID-19: In the end it is the endothelium—What else? Eur. Heart J. 2020, 41, 3023–3027. [Google Scholar] [CrossRef]
- Jarrott, B.; Head, R.; Pringle, K.G.; Lumbers, E.R.; Martin, J.H. “LONG COVID”—A hypothesis for understanding the biological basis and pharmacological treatment strategy. Pharmacol. Res. Perspect. 2022, 10, e00911. [Google Scholar] [CrossRef]
- Yu, F.; Yan, L.; Wang, N.; Yang, S.; Wang, L.; Tang, Y.; Gao, G.; Wang, S.; Ma, C.; Xie, R.; et al. Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin. Infect. Dis. 2020, 71, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Jiang, L.; Huang, G.; Pu, H.; Gong, B.; Lin, H.; Ma, S.; Chen, X.; Long, B.; Si, G.; et al. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int. J. Infect. Dis. 2020, 93, 264–267. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Li, S.; Peng, Z.; Xiao, Z.; Wang, X.; Yan, R.; Luo, J. Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. Travel Med. Infect. Dis. 2020, 37, 101673. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.I.; Arancibia-Carcamo, C.V.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beneke, T.; Bibi, S.; Brooks, T.; Carroll, M.; Crook, D.; et al. SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus. Wellcome Open Res. 2020, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.-E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef] [PubMed]
- Krasemann, S.; Haferkamp, U.; Pfefferle, S.; Woo, M.S.; Heinrich, F.; Schweizer, M.; Appelt-Menzel, A.; Cubukova, A.; Barenberg, J.; Leu, J.; et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Rep. 2022, 17, 307–320. [Google Scholar] [CrossRef]
- Liu, X.; Wen, Y.Z.; Huang, Z.L.; Shen, X.; Wang, J.H.; Luo, Y.H.; Chen, W.X.; Lun, Z.R.; Li, H.B.; Qu, L.H.; et al. SARS-CoV-2 Causes a Significant Stress Response Mediated by Small RNAs in the Blood of COVID-19 Patients. Mol. Ther. Nucleic Acids 2022, 27, 751–762. [Google Scholar] [CrossRef]
- Selye, H. Stress and the General Adaptation Syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Akselrod, S.; Gordon, D.; Ubel, F.A.; Shannon, D.C.; Berger, A.C.; Cohen, R.J. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science 1981, 213, 220–222. [Google Scholar] [CrossRef]
- Maurier, F.; Godbert, B.; Perrin, J. Respiratory Distress in SARS-CoV-2 without Lung Damage: Phrenic Paralysis Should Be Considered in COVID-19 Infection. Eur. J. Case Rep. Intern. Med. 2020, 21, 7:001728. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Elder, G.; Hallett, M.; Ravits, J.; Baker, M.; Papadopoulos, N.; Albrecht, P.; Sever, J. A long-term follow-up study of patients with post-poliomyelitis neuromuscular symptoms. N. Engl. J. Med. 1986, 314, 959–963. [Google Scholar] [CrossRef]
- Herridge, M.S.; Cheung, A.M.; Tansey, C.M.; Matte-Martyn, A.; Diaz-Granados, N.; Al-Saidi, F.; Cooper, A.B.; Guest, C.B.; Mazer, C.D.; Mehta, S.; et al. One-Year Outcomes In Survivors Of The Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2003, 348, 683–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldofsky, H.; Patcai, J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236,379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent Neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Benarroch, E.E. Postural tachycardia syndrome: A heterogeneous and multifactorial disorder. Mayo Clin. Proc. 2012, 87, 1214–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamm, K.; Lamm, C.; Arnold, W. Effect of isobaric oxygen versus hyperbaric oxygen on the normal and noise-damaged hypoxic and ischemic guinea pig inner ear. Hyperb. Oxyg. Ther. Otorhinolaryngol. 1998, 54, 59–85. [Google Scholar]
- Thom, S.R. Hyperbaric oxygen therapy. J. Intensive Care Med. 1989, 4, 58–74. [Google Scholar] [CrossRef]
- James, P.B. The Ultimate Oxygen Machine. Oxygen and the Brain: The Journey of Our Lifetime the Brain; Best Publishing Company: North Palm Beach, FL, USA, 2014; pp. 23–29. [Google Scholar]
- Montgomery, D.; Goldberg, J.; Amar, M.; Lacroix, V.; Lecomte, J.; Lambert, J.; Vanasse, M.; Marois, P. Effects of hyperbaric oxygen therapy on children with spastic diplegic cerebral palsy: A pilot project. Undersea Hyperb. Med. 1999, 26, 235–242. [Google Scholar]
- Mellemgaard, K. The alveolar-arterial oxygen difference: Its size and components in normal man. Acta Physiol. Scand. 1966, 67, 10–20. [Google Scholar] [CrossRef]
- Nunn, J.F.; Bergman, N.A.; Coleman, A.J. Factors influencing the arterial oxygen tension during anaesthesia with artificial ventilation. Br. J. Anaesth. 1965, 37, 898–914. [Google Scholar] [CrossRef] [Green Version]
- Comroe, J.H., Jr.; Dripps, R.D., Jr. The oxygen tension of arterial blood and alveolar air in normal subjects. Am. J. Physiol. 1944, 142, 700–706. [Google Scholar] [CrossRef]
- Crapo, R.O.; Jensen, R.L.; Hegewald, M.; Tashkin, D.P. Arterial bood gas reference values for sea level and an altitude of 1400 m. Am. J. Respir. Crit. Care Med. 1999, 160, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial pressure of oxygen in the human body: A general review. Am. J. Blood Res. 2019, 9, 1–14. [Google Scholar] [PubMed]
- O’Driscoll, B.R.; Howard, L.S.; Davison, A.G. British Thoracic Society Guideline for emergency oxygen use in adult patients. Thorax 2008, 63, 734–735. [Google Scholar]
- Matthay, M.A. Saving Lives with High-Flow Nasal Oxygen. N. Engl. J. Med. 2015, 372, 2225–2226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazuaye, E.A.; Stone, T.N.; Corris, P.A.; Gibson, G.J. Variability of inspired oxygen concentration with nasal cannulas. Thorax 1992, 47, 609–611. [Google Scholar] [CrossRef] [Green Version]
- Haldane, J.S. The therapeutic administration of oxygen. Br. Med. J. 1917, 1, 181–183. [Google Scholar] [CrossRef] [Green Version]
- Nunn, J.F.; Williams, I.P.; Jones, J.G.; Hewlett, A.M.; Hulands, G.H.; Minty, B.D. Detection and reversal of pulmonary absorption collapse. Br. J. Anaesth. 1978, 50, 91–99. [Google Scholar] [CrossRef]
- Tobin, M.J.; Laghi, F.; Jubran, A. Ventilatory failure, ventilator support, and ventilator weaning. Compr. Physiol. 2012, 2, 2871–2921. [Google Scholar]
- Tobin, M.J. Basing respiratory management of COVID-19 on physiological principles. Am. J. Respir. Crit. Care Med. 2020, 201, 1319–1320. [Google Scholar] [CrossRef] [Green Version]
- Harch, P.G. Hyperbaric oxygen treatment of novel coronavirus (COVID-19) respiratory failure. Med. Gas Res. 2020, 10, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Nunn, J.F.; Coleman, A.J.; Sachithanandan, T.; Bergman, N.A.; Laws, J.W. Hypoxaemia and atelectasis produced by forced inspiration. Brit. J. Anaesth. 1965, 37, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Oxygen is Toxic! Bioscience 1977, 27, 462–466. [Google Scholar] [CrossRef]
- Fridovich, I. Hypoxia and oxygen toxicity. Adv. Neurol. 1979, 26, 255–259. [Google Scholar]
- Glaisher, J. Notes of effects experienced during recent balloon ascents. Lancet 1862, 80, 559–560. [Google Scholar] [CrossRef] [Green Version]
- Benni, P.B.; MacLeod, D.; Ikeda, K.; Lin, H.M. A validation method for near-infrared spectroscopy based tissue oximeters for cerebral and somatic tissue oxygen saturation measurements. J. Clin. Monit. Comput. 2018, 32, 269–284. [Google Scholar] [CrossRef] [Green Version]
- James, P.B. Intermittent high dosage oxygen treats COVID-19 infection: The Chinese studies. Med. Gas Res. 2020, 10, 63. [Google Scholar] [CrossRef]
- Thibodeaux, K.; Speyrer, M.; Raza, A.; Yaakov, R.; E Serena, T. Hyperbaric oxygen therapy in preventing mechanical ventilation in COVID-19 patients: A retrospective case series. J. Wound Care 2020, 29, S4–S8. [Google Scholar] [CrossRef]
- Kjellberg, A.; De Maio, A.; Lindholm, P. Can hyperbaric oxygen safely serve as an anti-inflammatory treatment for COVID-19? Med. Hypotheses 2020, 144, 110224. [Google Scholar] [CrossRef]
- Guo, D.; Pan, S.; Wang, M.; Guo, Y. Hyperbaric oxygen therapy may be effective to improve hypoxemia in patients with severe COVID-2019 pneumonia: Two case reports. Undersea Hyperb. Med. 2020, 47, 181–187. [Google Scholar] [CrossRef]
- Gorenstein, S.A.; Castellano, M.L.; Slone, E.S.; Gillette, B.; Liu, H.; Alsamarraie, C.; Jacobson, A.M.; Wall, S.P.; Adhikari, S.; Swartz, J.L.; et al. Hyperbaric oxygen therapy for COVID-19 patients with respiratory distress: Treated cases versus propensity-matched controls. Undersea Hyperb. Med. 2020, 47, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Boet, S.; Etherington, C.; Djaiani, G.; Tricco, A.C.; Sikora, L.; Katznelson, R. Efficacy and safety of hyperbaric oxygen treatment in SARS-COV-2 (COVID-19) pneumonia: A systematic review. Diving Hyperb. Med. J. 2021, 51, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Cannellotto, M.; Duarte, M.; Keller, G.; Larrea, R.; Cunto, E.; Chediack, V.; Mansur, M.; Brito, D.M.; García, E.; Di Salvo, H.E.; et al. Hyperbaric oxygen as an adjuvant treatment for patients with COVID-19 severe hypoxaemia: A randomised controlled trial. Emerg. Med. J. 2022, 39, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Robbins, T.; Gonevski, M.; Clark, C.; Baitule, S.; Sharma, K.; Magar, A.; Patel, K.; Sankar, S.; Kyrou, I.; Ali, A.; et al. Hyperbaric oxygen therapy for the treatment of long COVID: Early evaluation of a highly promising intervention. Clin. Med. 2021, 21, e629–e632. [Google Scholar] [CrossRef] [PubMed]
- Bhaiyat, A.M.; Sasson, E.; Wang, Z.; Khairy, S.; Ginzarly, M.; Qureshi, U.; Fikree, M.; Efrati, S. Hyperbaric oxygen treatment for long coronavirus disease-19: A case report. J. Med. Case Rep. 2022, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Fischer, I.; Barak, B. Molecular and Therapeutic Aspects of Hyperbaric Oxygen Therapy in Neurological Conditions. Biomolecules 2020, 10, 1247. [Google Scholar] [CrossRef]
- Efrati, S.; Fishlev, G.; Bechor, Y.; Volkov, O.; Bergan, J.; Kliakhandler, K.; Kamiager, I.; Gal, N.; Friedman, M.; Ben-Jacob, E.; et al. Hyperbaric oxygen induces late neuroplasticity in post stroke patients—randomized, prospective trial. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Boussi-Gross, R.; Golan, H.; Fishlev, G.; Bechor, Y.; Volkov, O.; Bergan, J.; Friedman, M.; Hoofien, D.; Shlamkovitch, N.; Ben-Jacob, E.; et al. Hyperbaric oxygen therapy can improve post concussion syndrome years after mild traumatic brain injury—Randomized prospective trial. PLoS ONE 2013, 8, e79995. [Google Scholar] [CrossRef] [Green Version]
- Efrati, S.; Ben-Jacob, E. Reflections on the neurotherapeutic effects of hyperbaric oxygen. Expert Rev. Neurother. 2014, 14, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Boussi-Gross, R.; Golan, H.; Volkov, O.; Bechor, Y.; Hoofien, D.; Beeri, M.S.; Ben-Jacob, E.; Efrati, S. Improvement of memory impairments in poststroke patients by hyperbaric oxygen therapy. Neuropsychology 2015, 29, 610–621. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. Neural circuitry and immunity. Immunol. Res. 2015, 63, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, T.R. Therapeutic mechanisms of vagus nerve stimulation. Neurology 2002, 59, S3–S14. [Google Scholar] [CrossRef]
- Ressler, K.J.; Mayberg, H.S. Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nat. Neurosci. 2007, 10, 1116–1124. [Google Scholar] [CrossRef]
- Cao, J.; Lu, K.-H.; Powley, T.L.; Liu, Z. Vagal nerve stimulation triggers widespread responses and alters large-scale functional connectivity in the rat brain. PLoS ONE 2017, 12, e0189518. [Google Scholar] [CrossRef] [Green Version]
- Tracey, K.J. Neurons Are the Inflammatory Problem. Cell 2018, 173, 1066–1068. [Google Scholar] [CrossRef] [Green Version]
- Kraus, T.; Hossl, K.; Kiess, O.; Schanze, A.; Kornhuber, J.; Forster, C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J. Neural. Transm. 2007, 114, 1485–1493. [Google Scholar] [CrossRef]
- Frangos, E.; Ellrich, J.; Komisaruk, B.R. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015, 8, 624–636. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.F.; Chen, Y.J.; Lin, Y.J.; Chen, S.A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 2015, 12, 230–243. [Google Scholar] [CrossRef]
- Stavrakis, S.; Stoner, J.A.; Humphrey, M.B.; Morris, L.; Filiberti, A.; Reynolds, J.C.; Elkholey, K.; Javed, I.; Twidale, N.; Riha, P.; et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A Randomized Clinical Trial. Clin. Electrophysiol. 2020, 6, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Sclocco, R.; Garcia, R.G.; Kettner, N.W.; Isenburg, K.; Fisher, H.P.; Hubbard, C.S.; Ay, I.; Polimeni, J.R.; Goldstein, J.; Makris, N.; et al. The influence of respiration on brainstem and cardiovagal response to auricular vagus nerve stimulation: A multimodal ultrahigh-field (7T) fMRI study. Brain Stimul. 2019, 12, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, R.J.S.; Band, G.P.H. Breath of Life: The Respiratory Vagal Stimulation Model of Contemplative Activity. Front. Hum. Neurosci. 2018, 12, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbanck, P.; Clarinval, A.M.; Burton, F.; Corazza, F.; Nagant, C.; Cheron, G. Transcutaneous Auricular Vagus Nerve Stimulation (tVNS) can Reverse the Manifestations of the Long-COVID Syndrome: A Pilot Study. Front. Neurology Neurosci. Res. 2021, 2, 100011. [Google Scholar] [CrossRef]
- Ylikoski, J.; Markkanen, M.; Pirvola, U.; Lehtimäki, J.A.; Ylikoski, M.; Jing, Z.; Sinkkonen, S.T.; Mäkitie, A. Stress and Tinnitus; Transcutaneous Auricular Vagal Nerve Stimulation Attenuates Tinnitus-Triggered Stress Reaction. Front. Psychol. 2020, 11, 570196. [Google Scholar] [CrossRef]
- Basnyat, B. Acute high-altitude illnesses. N. Engl. J. Med. 2013, 369, 1664–1667. [Google Scholar]
- Schoene, R. Illnesses at high altitude. Chest 2008, 134, 402–416. [Google Scholar] [CrossRef]
- Bhagi, S.; Srivastava, S.; Singh, S.B. High-altitude pulmonary edema: Review. J. Occup. Health 2014, 56, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Haldane, J.S. The action of carbonic oxide on man. J. Physiol. 1895, 18, 430–462. [Google Scholar] [CrossRef] [Green Version]
- WHO Guidelines for Indoor Air Quality: Selected PollutantsCarbon Monoxide; World Health Organization: Geneva, Switzerland, 2010.
- Shaw, D.M.; Cabre, G.; Gant, N. Hypoxic Hypoxia and Brain Function in Military Aviation: Basic Physiology and Applied Perspectives. Front. Physiol. 2021, 12, 665821. [Google Scholar] [CrossRef]
- West, J.B. Early history of high-altitude physiology. Ann. N. Y. Acad. Sci. 2016, 1365, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Virués-Ortega, J.; Buela-Casal, G.; Garrido, E.; Alcázar, B. Neuropsychological functioning associated with high-altitude exposure. Neuropsychol. Rev. 2004, 14, 197–224. [Google Scholar] [CrossRef] [PubMed]
- Monge, C.C.; Whittembury, J. Chronic mountain sickness. Johns Hopkins Med. J. 1976, 139, 87–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arons, M.M.; Hatfield, K.M.; Reddy, S.C.; Kimball, A.; James, A.; Jacobs, J.R.; Taylor, J.; Spicer, K.; Bardossy, A.C.; Oakley, L.P.; et al. Presymptomatic SARS-CoV-2 infections and transmission in a nursing facility. N. Engl. J. Med. 2020, 382, 2081–2090. [Google Scholar] [CrossRef]
- Whittaker, A.; Anson, M.; Harky, A. Neurological manifestations of COVID-19: A systematic review and current update. Acta Neurol. Scand. 2020, 142, 14–22. [Google Scholar] [CrossRef]
- Chuang, D.T.; Aydemir, S.; Magda, P.; Thomas, C.; Zarnegar, R. Neurological manifestations as primary presentation of COVID-19 in hospitalized patients. Acta Neurol. Scand. 2021, 143, 569–574. [Google Scholar] [CrossRef]
- WHO. Naming the Coronavirus Disease (COVID-19) and the Virus that Causes it. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(COVID-2019)-and-the-virus-that-causes-it/ (accessed on 20 November 2020).
- Romero-Sanchez, C.M.; Diaz-Maroto, I.; Fernandez-Diaz, E.; Sánchez-Larsen, Á.; Layos-Romero, A.; García-García, J. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology 2020, 95, e1060–e1070. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Olesen, S.-P. Rapid increase in blood-brain barrier permeability during severe hypoxia and metabolic inhibition. Brain Res. 1986, 368, 24–29. [Google Scholar] [CrossRef]
- Nzou, G.; Wicks, R.T.; VanOstrand, N.R.; Mekky, G.A.; Seale, S.A.; El-Taibany, A.; Wicks, E.E.; Nechtman, C.M.; Marrotte, E.J.; Makani, V.S.; et al. Multicellular 3D Neurovascular Unit Model for Assessing Hypoxia and Neuroinflammation Induced Blood-Brain Barrier Dysfunction. Sci. Rep. 2020, 10, 9766. [Google Scholar] [CrossRef]
- Shupak, A.; Weiler-Ravell, D.; Adir, Y.; Daskalovic, Y.I.; Ramon, Y.; Kerem, D. Pulmonary oedema induced by strenuous swimming: A field study. Respir. Physiol. 2000, 121, 25–31. [Google Scholar] [CrossRef]
- Adir, Y.; Shupak, A.; Gil, A.; Peled, N.; Keynan, Y.; Domachevsky, L.; Weiler-Ravell, D. Swimming-induced pulmonary edema: Clinical presentation and serial lung function. Chest 2004, 126, 394–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slade, J.B., Jr.; Hattori, T.; Ray, C.S.; Bove, A.A.; Cianci, P. Pulmonary edema associated with scuba diving: Case reports and review. Chest 2001, 120, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Yamanashi, H.; Koyamatsu, J.; Nobuyoshi, M.; Murase, K.; Maeda, T. Exercise-Induced Pulmonary Edema in a Triathlon. Case Rep. Med. 2015, 2015, 968152. [Google Scholar] [CrossRef] [Green Version]
- Manne, J.R.; Kasirye, Y.; Epperla, N.; Garcia-Montilla, R.J. Non-cardiogenic pulmonary edema complicating electroconvulsive therapy: Short review of the pathophysiology and diagnostic approach Clin. Med. Res. 2012, 10, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Arakaki, W.; Kinjo, T.; Nakamura, H.; Fujita, J. Seizure followed by lung edema: An intriguing link between the brain and the lung. Clin. Case Rep. 2020, 8, 2291–2292. [Google Scholar] [CrossRef]
- Abdennour, L.; Zeghal, C.; Dème, M.; Puybasset, L. Interaction brain-lungs. Ann. Fr. D’anesthesie Reanim. 2012, 31, e101–e107. [Google Scholar] [CrossRef]
- Kong, W.; Wang, Y.; Hu, J.; Chughtai, A.; Pu, H. Comparison of clinical and epidemiological characteristics of asymptomatic and symptomatic SARS-CoV-2 infection: A multi-center study in Sichuan Province, China. Travel Med. Infect. Dis. 2020, 37, 101754. [Google Scholar] [CrossRef]
- De Smet, K.; De Smet, D.; Ryckaert, T.; Laridon, E.; Heremans, B.; Vandenbulcke, R.; Demedts, I.; Bouckaert, B.; Gryspeerdt, S.; Martens, G.A. Diagnostic performance of chest CT for SARS-CoV-2 infection in individuals with or without COVID-19 symptoms. Radiology 2021, 298, E30–E37. [Google Scholar] [CrossRef]
- Uysal, E.; Kilinçer, A.; Cebeci, H.; Özer, H.; Demir, N.A.; Öztürk, M.; Koplay, M. Chest CT findings in RT-PCR positive asymptomatic COVID-19 patients. Clin. Imaging 2021, 77, 37–42. [Google Scholar] [CrossRef]
- Kramer, M.R.; Springer, C.; Berkman, N.; Glazer, M.; Bublil, M.; Bar-Yishay, E.; Godfrey, S. Rehabilitation of hypoxemic patients with COPD at low altitude at the Dead Sea, the lowest place on earth. Chest 1998, 113, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Goldbart, A.D.; Cohen, A.D.; Weitzman, D.; Tal, A. Effects of rehabilitation winter camps at the Dead Sea on European cystic fibrosis patients. Isr. Med. Assoc. J. 2007, 9, 806–809. [Google Scholar] [PubMed]

| Atmospheric/Ambient | Oxygen Tension of Inspired Air, PO2 | Oxygen Tension in Alvoli, PAO2 | Oxygen Tension in Arteries, PaO2 | Oxygen Tension in Tissues, PtO2 | Oxygen Tension in Mitochondria, PmO2 | |
|---|---|---|---|---|---|---|
| Pressure (AP) mmHg | mmHg | mmHg | mmHg | mmHg | mmHg | |
| 2.5 ATA, AP 1875 | ||||||
| 15 m diving, 100% O2 breathing | 1875 | 1284 * | 1274 | 250–500 [58,59] | 80–125 * | |
| 236 | 162 * | 152 | 30–60 * | |||
| 5 m diving, 100% O2 breathing | 1125 | 771 * | 761 (59) | 150–304 * | 50–76 * | |
| 1.3 ATA, AP 988 air breathing | 207 | 158 * | 148 [60,61] | 30–60 * | 10–15 * | |
| 3 m diving, 100% O2 breathing | 975 | 668 * | 658 | 130–263 * | 45–65 * | |
| Sea level (1.0 ATA), AP 760 mmHg | ||||||
| Air breathing [62,63,64] 20–50 y | 160 | 102–110 | 97–99 | 20–40 | 7.5–11 | |
| >64 y | -”- | -”- | 82–93 | 16–37 * | ||
| 100% O2 breathing [63,64] | 760 | 674 | 516 | 207 * | 77 * | |
| Dead Sea * −457 m AP 802 mmHg | 167 | 114 | 104 | 42 | 15 | |
| Air | ||||||
| Mild CO-Poisoning [112,113] | Aviation/Ballooning/Mountain Climbing | COVID-19/Long Covid |
|---|---|---|
| (in order of prevalence) | (Hypobaric hypoxia) [114,115,116,117] | [55,56,118,119,120,121,122,123] |
| Fatigue/lethargy | Visual disturbances | Anosmia |
| Headache | Headache | Fatigue |
| Numbness and tingling | Fatigue, lethargy | Headache |
| “Brain fog” | Dizziness, nausea | Dyspnea |
| Dizziness, nausea | Impaired fine touch & motor skills | “Brain fog” |
| Sleep disturbances | Personality & mood changes | Impaired consciousness |
| Palpitations | Sensory loss | Dizziness, nausea, tinnitus |
| Visual impairments | Confusion | Palpitations |
| Loss of consciousness | Loss of consciousness | Sleep disturbances |
| Neuropsychological symptoms |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ylikoski, J.; Lehtimäki, J.; Pääkkönen, R.; Mäkitie, A. Prevention and Treatment of Life-Threatening COVID-19 May Be Possible with Oxygen Treatment. Life 2022, 12, 754. https://doi.org/10.3390/life12050754
Ylikoski J, Lehtimäki J, Pääkkönen R, Mäkitie A. Prevention and Treatment of Life-Threatening COVID-19 May Be Possible with Oxygen Treatment. Life. 2022; 12(5):754. https://doi.org/10.3390/life12050754
Chicago/Turabian StyleYlikoski, Jukka, Jarmo Lehtimäki, Rauno Pääkkönen, and Antti Mäkitie. 2022. "Prevention and Treatment of Life-Threatening COVID-19 May Be Possible with Oxygen Treatment" Life 12, no. 5: 754. https://doi.org/10.3390/life12050754
APA StyleYlikoski, J., Lehtimäki, J., Pääkkönen, R., & Mäkitie, A. (2022). Prevention and Treatment of Life-Threatening COVID-19 May Be Possible with Oxygen Treatment. Life, 12(5), 754. https://doi.org/10.3390/life12050754





