A Seed-Borne Bacterium of Rice, Pantoea dispersa BB1, Protects Rice from the Seedling Rot Caused by the Bacterial Pathogen Burkholderia glumae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Bacterial Materials
2.2. Inoculation Tests
2.3. Isolation and Characterization of the Seed-Borne Bacteria of Rice
2.4. Toxoflavin Tolerance Tests
2.5. Antibacterial and Biocontrol Analyses of the Culture Filtrate
3. Results
3.1. Isolation of Seed-Borne Bacteria
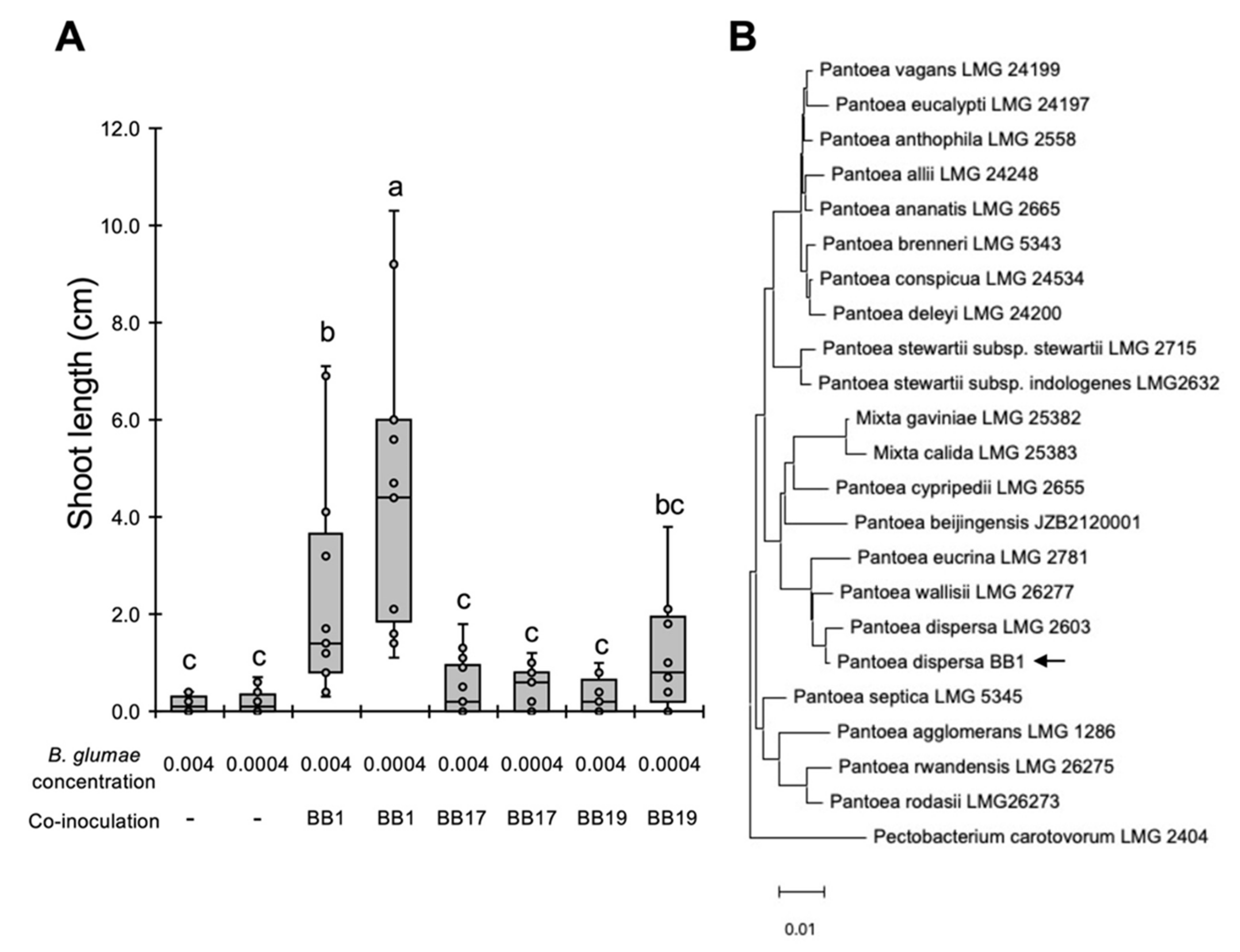
3.2. Screening of Biocontrol Bacteria
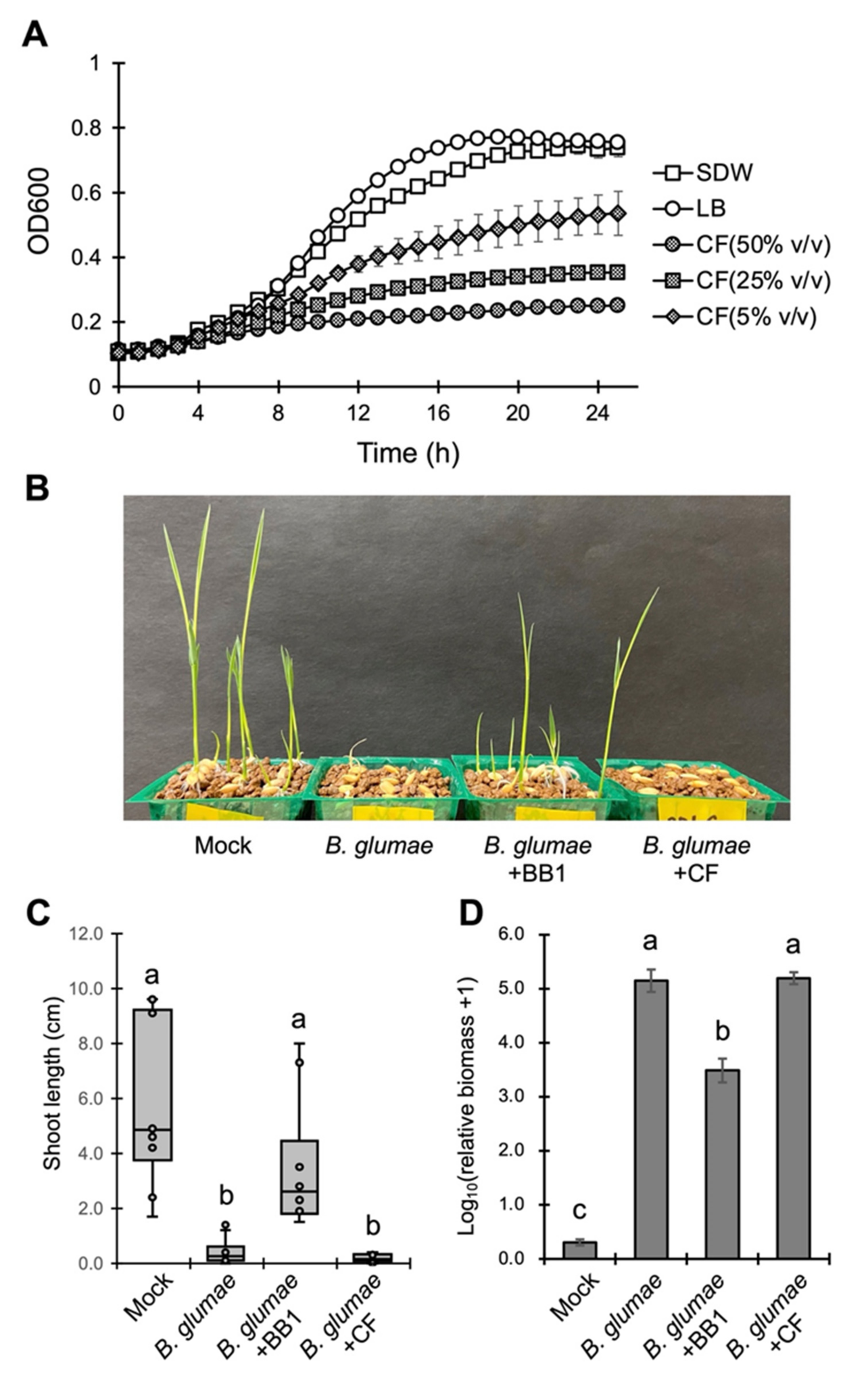
3.3. Biocontrol Mechanisms of P. dispersa BB1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ham, J.H.; Melanson, R.A.; Rush, M.C. Burkholderia glumae: Next major pathogen of Rice? Mol. Plant Pathol. 2011, 12, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhu, B.; Xie, G.; Li, B.; Huang, S. Research status and prospect of Burkholderia glumae, the pathogen causing bacterial panicle blight. Rice Sci. 2016, 23, 111–118. [Google Scholar]
- Hikichi, Y.; Okuno, T.; Furusawa, I. Immunofluorescent antibody technique for detecting Pseudomonas glumae on rice plants. Jpn. J. Phytopathol. 1993, 59, 477–480. [Google Scholar] [CrossRef]
- Fory, P.A.; Triplett, L.; Ballen, C.; Abello, J.F.; Duitama, J.; Aricapa, M.G.; Prado, G.A.; Correa, F.; Hamilton, J.; Leach, J.E.; et al. Comparative analysis of two emerging rice seed bacterial pathogens. Phytopathology 2014, 104, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, S.; Naito, H. Spatial distribution and dissemination of bacterial grain rot of rice caused by Pseudomonas glumae. Jpn. J. Phytopathol. 1991, 57, 180–187. [Google Scholar] [CrossRef]
- Hikichi, Y.; Tsujiguchi, K.; Maeda, Y.; Okuno, T. Development of increased oxolinic acid-resistance in Burkholderia glumae. J. Gen. Plant Pathol. 2001, 67, 58–62. [Google Scholar] [CrossRef]
- Mizobuchi, R.; Fukuoka, S.; Tsuiki, C.; Tsushima, S.; Sato, H. Evaluation of major rice cultivars for resistance to bacterial seedling rot caused by Burkholderia glumae and identification of Japanese standard cultivars for resistance assessments. Breed. Sci. 2020, 70, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.; Kim, J.; Kim, S.; Kang, Y.; Nagamatsu, T.; Hwang, I. Toxoflavin produced by Burkholderia glumae causing rice grain rot is responsible for inducing bacterial wilt in many field crops. Plant Dis. 2003, 87, 890–895. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, J.-G.; Kang, Y.; Jang, J.Y.; Jog, G.J.; Lim, J.Y.; Kim, S.; Suga, H.; Nagamatsu, T.; Hwang, I. Quorum sensing and the LysR-type transcriptional activator ToxR regulate toxoflavin biosynthesis and transport in Burkholderia glumae. Mol. Microbiol. 2004, 54, 921–934. [Google Scholar] [CrossRef]
- Akimoto-Tomiyama, C. Multiple endogenous seed-borne bacteria recovered rice growth disruption caused by Burkholderia glumae. Sci. Rep. 2021, 11, 4177. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Wang, R.; Wang, Q.; Lu, L. Toxoflavin produced by Burkholderia gladioli from Lycoris aurea is a new broad-spectrum fungicide. Appl. Environ. Microbiol. 2019, 85, e00106-19. [Google Scholar] [CrossRef] [Green Version]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [Green Version]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Miyagawa, H.; Takaya, S. Biological control of bacterial grain rot of rice by avirulent strain of Burkholderia gladioli. Bull. Chugoku Natl. Agric. Exp. Stn. 2000, 1–12. [Google Scholar]
- Walterson, A.M.; Stavrinides, J. Pantoea: Insights into a highly versatile and diverse genus within the Enterobacteriaceae. FEMS Microbiol. Rev. 2015, 39, 968–984. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Lee, C.; Lee, H.-H.; Mannaa, M.; Kim, N.; Park, J.; Kim, J.; Seo, Y.-S. Genomics-based sensitive and specific novel primers for simultaneous detection of Burkholderia glumae and Burkholderia gladioli in rice seeds. Plant Pathol. J. 2018, 34, 490–498. [Google Scholar] [CrossRef]
- Kouzai, Y.; Kimura, M.; Watanabe, M.; Kusunoki, K.; Osaka, D.; Suzuki, T.; Matsui, H.; Yamamoto, M.; Ichinose, Y.; Toyoda, K.; et al. Salicylic acid-dependent immunity contributes to resistance against Rhizoctonia solani, a necrotrophic fungal agent of sheath blight, in rice and Brachypodium distachyon. New Phytol. 2018, 217, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Kouzai, Y.; Shimizu, M.; Inoue, K.; Uehara-Yamaguchi, Y.; Takahagi, K.; Nakayama, R.; Matsuura, T.; Mori, I.C.; Hirayama, T.; Abdelsalam, S.S.H.; et al. BdWRKY38 is required for the incompatible interaction of Brachypodium distachyon with the necrotrophic fungus Rhizoctonia solani. Plant J. 2020, 104, 995–1008. [Google Scholar] [CrossRef]
- Saito, K.; Watanabe, M.; Matsui, H.; Yamamoto, M.; Ichinose, Y.; Toyoda, K.; Kawaguchi, A.; Noutoshi, Y. Characterization of the suppressive effects of the biological control strain VAR03-1 of Rhizobium vitis on the virulence of tumorigenic R. vitis. J. Gen. Plant Pathol. 2018, 84, 58–64. [Google Scholar] [CrossRef]
- Coutinho, T.A.; Venter, S.N. Pantoea ananatis: An unconventional plant pathogen. Mol. Plant Pathol. 2009, 10, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, O.; Kim, T.-S. An outbreak of onion center rot caused by Pantoea ananatis in Korea. Plant Dis. 2012, 96, 1576. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.K.; Loh, P.C.; Wong, H.L. First report of leaf blight of rice caused by Pantoea ananatis and Pantoea dispersa in Malaysia. Plant Dis. 2019, 103, 1764. [Google Scholar] [CrossRef]
- Chang, C.-P.; Sung, I.-H.; Huang, C.-J. Pantoea dispersa causing bulb decay of onion in Taiwan. Australas. Plant Pathol. 2018, 47, 609–613. [Google Scholar] [CrossRef]
- Gasser, F.; Cardinale, M.; Schildberger, B.; Berg, G. Biocontrol of Botrytis cinerea by successful introduction of Pantoea ananatis in the grapevine phyllosphere. IJWR 2012, 4, 53–63. [Google Scholar]
- Verma, S.K.; Kingsley, K.; Irizarry, I.; Bergen, M.; Kharwar, R.N.; White, J.F., Jr. Seed-vectored endophytic bacteria modulate development of rice seedlings. J. Appl. Microbiol. 2017, 122, 1680–1691. [Google Scholar] [CrossRef]
- Jiang, L.; Jeong, J.C.; Lee, J.-S.; Park, J.M.; Yang, J.-W.; Lee, M.H.; Choi, S.H.; Kim, C.Y.; Kim, D.-H.; Kim, S.W.; et al. Potential of Pantoea dispersa as an effective biocontrol agent for black rot in sweet potato. Sci. Rep. 2019, 9, 16354. [Google Scholar]
- Smits, T.H.M.; Duffy, B.; Blom, J.; Ishimaru, C.A.; Stockwell, V.O. Pantocin A, a peptide-derived antibiotic involved in biological control by plant-associated Pantoea species. Arch. Microbiol. 2019, 201, 713–722. [Google Scholar] [CrossRef]
- Walterson, A.M.; Smith, D.D.N.; Stavrinides, J. Identification of a Pantoea biosynthetic cluster that directs the synthesis of an antimicrobial natural product. PLoS ONE 2014, 9, e96208. [Google Scholar] [CrossRef] [Green Version]
- Raaijmakers, J.M.; Mazzola, M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 2012, 50, 403–424. [Google Scholar] [CrossRef]
- Arseneault, T.; Filion, M. Biocontrol through antibiosis: Exploring the role played by subinhibitory concentrations of antibiotics in soil and their impact on plant pathogens. Can. J. Plant Pathol. 2017, 39, 267–274. [Google Scholar] [CrossRef]
- Paasch, B.C.; He, S.Y. Toward understanding microbiota homeostasis in the plant kingdom. PLoS Pathog. 2021, 17, e1009472. [Google Scholar] [CrossRef] [PubMed]
- Walitang, D.I.; Kim, C.-G.; Kim, K.; Kang, Y.; Kim, Y.K.; Sa, T. The influence of host genotype and salt stress on the seed endophytic community of salt-sensitive and salt-tolerant rice cultivars. BMC Plant Biol. 2018, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Abdullaeva, Y.; Ratering, S.; Ambika Manirajan, B.; Rosado-Porto, D.; Schnell, S.; Cardinale, M. Domestication impacts the wheat-associated microbiota and the rhizosphere colonization by seed- and soil-originated microbiomes, across different fields. Front. Plant Sci. 2021, 12, 806915. [Google Scholar] [CrossRef]
- Dudenhöffer, J.-H.; Scheu, S.; Jousset, A. Systemic enrichment of antifungal traits in the rhizosphere microbiome after pathogen attack. J. Ecol. 2016, 104, 1566–1575. [Google Scholar]
- Gao, M.; Xiong, C.; Gao, C.; Tsui, C.K.M.; Wang, M.-M.; Zhou, X.; Zhang, A.-M.; Cai, L. Disease-induced changes in plant microbiome assembly and functional adaptation. Microbiome 2021, 9, 187. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouzai, Y.; Akimoto-Tomiyama, C. A Seed-Borne Bacterium of Rice, Pantoea dispersa BB1, Protects Rice from the Seedling Rot Caused by the Bacterial Pathogen Burkholderia glumae. Life 2022, 12, 791. https://doi.org/10.3390/life12060791
Kouzai Y, Akimoto-Tomiyama C. A Seed-Borne Bacterium of Rice, Pantoea dispersa BB1, Protects Rice from the Seedling Rot Caused by the Bacterial Pathogen Burkholderia glumae. Life. 2022; 12(6):791. https://doi.org/10.3390/life12060791
Chicago/Turabian StyleKouzai, Yusuke, and Chiharu Akimoto-Tomiyama. 2022. "A Seed-Borne Bacterium of Rice, Pantoea dispersa BB1, Protects Rice from the Seedling Rot Caused by the Bacterial Pathogen Burkholderia glumae" Life 12, no. 6: 791. https://doi.org/10.3390/life12060791





