Effects of Hypoxia Stress on Growth, Root Respiration, and Metabolism of Phyllostachys praecox
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Hydroponics Experiment
2.3. Physiological and Elemental Determination
2.3.1. Determination of Leaf Chlorophyll Contents (SPAD)
2.3.2. Determination of Malondialdehyde (MDA)
2.3.3. Determination of Plant Root Vitality and Respiratory Products
2.3.4. Element Analysis in Pants
2.3.5. Metabolic Profiling
2.4. Statistical Analysis
3. Result
3.1. Effects of Hypoxia Stress on Growth
3.1.1. Changes of Crop Growth and Chlorophyll Content
3.1.2. Effects of Hypoxia Stress on Nutrient Absorption of Phyllostachys Praecox
3.1.3. Effects of Hypoxia Stress on MDA in Roots
3.2. Effects of Hypoxia Stress on Root Activity and Respiratory Products
3.3. Metabolome Analysis
3.3.1. PCA Analysis
3.3.2. Metabolites
3.3.3. Metabolic Pathway
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Niu, W.; Cao, X.; Wang, J.; Zhang, M.; Duan, X.; Zhang, Z. Effect of soil aeration on root morphology and photosynthetic characteristics of potted tomato plants (Solanum lycopersicum) at different nacl salinity levels. BMC Plant Biol. 2019, 19, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Sui, X.; Guo, J.; Wang, Z.; Cheng, J.; Ma, S.; Li, X.; Zhang, Z. Antisense suppression of cucumber (Cucumis sativus L.) sucrose synthase 3 (cssus3) reduces hypoxic stress tolerance. Plant Cell Environ. 2014, 37, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cheng, W.; Hou, H.; Peng, R.; Hai, N.; Bian, Z.; Jiao, C.; Wang, C. Antioxidative responses and morpho-anatomical alterations for coping with flood-induced hypoxic stress in grass pea (Lathyrus sativus L.) in comparison with pea (pisum sativum). J. Plant Growth Regul. 2016, 35, 690–700. [Google Scholar] [CrossRef]
- Gao, H.; Jia, Y.; Guo, S.; Lv, G.; Wang, T.; Juan, L. Exogenous calcium affects nitrogen metabolism in root-zone hypoxia-stressed muskmelon roots and enhances short-term hypoxia tolerance. J. Plant Physiol. 2011, 168, 1217–1225. [Google Scholar] [CrossRef]
- Salvatierra, A.; Toro, G.; Mateluna, P.; Opazo, I.; Ortiz, M.; Pimentel, P. Keep calm and survive: Adaptation strategies to energy crisis in fruit trees under root hypoxia. Plants 2020, 9, 1108. [Google Scholar] [CrossRef]
- Xu, M.; Zhuang, S.; Gui, R. Soil hypoxia induced by an organic-material mulching technique stimulates the bamboo rhizome up-floating of phyllostachys praecox. Sci. Rep. 2017, 7, 14353. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.; Zhuang, S.; Gui, R.; Tang, L. Effect of soil aeration treatment on the physiological and biochemical characteristics of Phyllostachys praecox under the organic material mulching. Plant Soil 2021, 459, 357–369. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chang, S.X.; Jiang, P.; Zhou, G.; Fu, S.; Yan, E.; Wu, J.; Lin, L. Long-term intensive management effects on soil organic carbon pools and chemical composition in moso bamboo (Phyllostachys pubescens) forests in subtropical china. For. Ecol. Manag. 2013, 303, 121–130. [Google Scholar] [CrossRef]
- Loreti, E.; Perata, P. The many facets of hypoxia in plants. Plants 2020, 9, 745. [Google Scholar] [CrossRef]
- Safavi-Rizi, V.; Herde, M.; Stöhr, C. Rna-seq reveals novel genes and pathways associated with hypoxia duration and tolerance in tomato root. Sci. Rep. 2020, 10, 1692. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Kumari, A.; Mur, L.A.; Gupta, K.J. A discrete role for alternative oxidase under hypoxia to increase nitric oxide and drive energy production. Free. Radic. Biol. Med. 2018, 122, 40–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Chen, D.; Zhang, X.; Liu, D.; Cheng, Y.; Shen, F. Role of plant respiratory burst oxidase homologs in stress responses. Free. Radic. Res. 2018, 52, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Dat, J.; Folzer, H.; Parent, C.; Badot, P.-M.; Capelli, N. Hypoxia stress. Current understanding and perspectives. Floric. Ornam. Plant Biotechnol. Adv. Top. Issues 2006, 3, 664–674. [Google Scholar]
- Sasidharan, R.; Schippers, J.H.; Schmidt, R.R. Redox and low-oxygen stress: Signal integration and interplay. Plant Physiol. 2021, 186, 66–78. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.-H.; Shabala, S. Hypoxia sensing in plants: On a quest for ion channels as putative oxygen sensors. Plant Cell Physiol. 2017, 58, 1126–1142. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef]
- Hossain, M.A.; Uddin, S.N. Mechanisms of waterlogging tolerance in wheat: Morphological and metabolic adaptations under hypoxia or anoxia. Aust. J. Crop Sci. 2011, 5, 1094–1101. [Google Scholar]
- Zhong, B.; Chen, J.; Shafi, M.; Guo, J.; Wang, Y.; Wu, J.; Ye, Z.; He, L.; Liu, D. Effect of lead (pb) on antioxidation system and accumulation ability of moso bamboo (Phyllostachys pubescens). Ecotoxicol. Environ. Saf. 2017, 138, 71–77. [Google Scholar] [CrossRef]
- Pagola, M.; Ortiz, R.; Irigoyen, I.; Bustince, H.; Barrenechea, E.; Aparicio-Tejo, P.; Lamsfus, C.; Lasa, B. New method to assess barley nitrogen nutrition status based on image colour analysis: Comparison with spad-502. Comput. Electron. Agric. 2009, 65, 213–218. [Google Scholar] [CrossRef]
- Chen, J.; Shafi, M.; Li, S.; Wang, Y.; Wu, J.; Ye, Z.; Peng, D.; Yan, W.; Liu, D. Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of moso bamboo (Phyllostachys pubescens). Sci. Rep. 2015, 5, 13554. [Google Scholar] [CrossRef] [Green Version]
- Peng, D.; Shafi, M.; Wang, Y.; Li, S.; Yan, W.; Chen, J.; Ye, Z.; Liu, D. Effect of zn stresses on physiology, growth, zn accumulation, and chlorophyll of Phyllostachys pubescens. Environ. Sci. Pollut. Res. 2015, 22, 14983–14992. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Wang, S.; Xin, H. Toxicity of cuo nanoparticles to structure and metabolic activity of allium cepa root tips. Bull. Environ. Contam. Toxicol. 2016, 97, 702–708. [Google Scholar] [CrossRef]
- Good, A.G.; Muench, D.G. Long-term anaerobic metabolism in root tissue (metabolic products of pyruvate metabolism). Plant Physiol. 1993, 101, 1163–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmeyer, H.-U. Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Bailey-Serres, J.; Fukao, T.; Gibbs, D.J.; Holdsworth, M.J.; Lee, S.C.; Licausi, F.; Perata, P.; Voesenek, L.A.; van Dongen, J.T. Making sense of low oxygen sensing. Trends Plant Sci. 2012, 17, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.A.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Wang, M.; He, D.; Shen, F.; Huang, J.; Zhang, R.; Liu, W.; Zhu, M.; Zhou, L.; Wang, L.; Zhou, Q. Effects of soil compaction on plant growth, nutrient absorption, and root respiration in soybean seedlings. Environ. Sci. Pollut. Res. 2019, 26, 22835–22845. [Google Scholar] [CrossRef]
- Najeeb, U.; Bange, M.P.; Tan, D.K.; Atwell, B.J. Consequences of waterlogging in cotton and opportunities for mitigation of yield losses. AoB Plants 2015, 7, plv080. [Google Scholar] [CrossRef]
- Alkhatib, R.; Abdo, N.; Mheidat, M. Photosynthetic and ultrastructural properties of eggplant (Solanum melongena) under salinity stress. Horticulturae 2021, 7, 181. [Google Scholar] [CrossRef]
- Mielecki, J.; Gawroński, P.; Karpiński, S. Retrograde signaling: Understanding the communication between organelles. Int. J. Mol. Sci. 2020, 21, 6173. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucciariello, C.; Perata, P. The oxidative paradox in low oxygen stress in plants. Antioxidants 2021, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, J.; Faist, H.; Saupe, S.; Lambertz, S.; Krischke, M.; Stingl, N.; Fekete, A.; Mueller, M.J.; Feussner, I.; Hedrich, R. Two fatty acid desaturases, stearoyl-acyl carrier protein Δ9-desaturase6 and fatty acid desaturase3, are involved in drought and hypoxia stress signaling in arabidopsis crown galls. Plant Physiol. 2014, 164, 570–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.J.; Yu, L.J.; Chen, Q.F.; Wang, F.Z.; Huang, L.; Xia, F.N.; Zhu, T.R.; Wu, J.X.; Yin, J.; Liao, B. Arabidopsis acyl-c o a-binding protein acbp 3 participates in plant response to hypoxia by modulating very-long-chain fatty acid metabolism. Plant J. 2015, 81, 53–67. [Google Scholar] [CrossRef]
- Nakajima, Y.; Umena, Y.; Nagao, R.; Endo, K.; Kobayashi, K.; Akita, F.; Suga, M.; Wada, H.; Noguchi, T.; Shen, J.-R. Thylakoid membrane lipid sulfoquinovosyl-diacylglycerol (sqdg) is required for full functioning of photosystem ii in thermosynechococcus elongatus. J. Biol. Chem. 2018, 293, 14786–14797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.-J.; Zhou, Y.; Chen, Q.-F.; Xiao, S. New insights into the role of lipids in plant hypoxia responses. Prog. Lipid Res. 2021, 81, 101072. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, D.-M.; Yu, W.-W.; Shi, L.-L.; Zhang, Y.; Lai, Y.-X.; Huang, L.-P.; Qi, H.; Chen, Q.-F.; Yao, N. Phosphatidic acid modulates mpk3-and mpk6-mediated hypoxia signaling in arabidopsis. Plant Cell 2022, 34, 889–909. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.; Russell, D.W.; Seyama, Y.; Shaw, W. A comprehensive classification system for lipids1. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef] [Green Version]
- Ackah, M.; Shi, Y.; Wu, M.; Wang, L.; Guo, P.; Guo, L.; Jin, X.; Li, S.; Zhang, Q.; Qiu, C. Metabolomics response to drought stress in morus alba l. Variety yu-711. Plants 2021, 10, 1636. [Google Scholar] [CrossRef]
- Narsai, R.; Rocha, M.; Geigenberger, P.; Whelan, J.; van Dongen, J.T. Comparative analysis between plant species of transcriptional and metabolic responses to hypoxia. New Phytol. 2011, 190, 472–487. [Google Scholar] [CrossRef]
- Kafer, C.; Zhou, L.; Santoso, D.; Guirgis, A.; Weers, B.; Park, S.; Thornburg, R. Regulation of pyrimidine metabolism in plants. Front. Biosci. 2004, 9, 1611–1625. [Google Scholar] [PubMed]
- Patel, M.K.; Pandey, S.; Burritt, D.J.; Tran, L.-S.P. Plant responses to low-oxygen stress: Interplay between ros and no signaling pathways. Environ. Exp. Bot. 2019, 161, 134–142. [Google Scholar] [CrossRef]
- Yuenyong, W.; Chinpongpanich, A.; Comai, L.; Chadchawan, S.; Buaboocha, T. Downstream components of the calmodulin signaling pathway in the rice salt stress response revealed by transcriptome profiling and target identification. BMC Plant Biol. 2018, 18, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Mao, C.; Shi, Z.; Kou, X. The amino acid metabolic and carbohydrate metabolic pathway play important roles during salt-stress response in tomato. Front. Plant Sci. 2017, 8, 1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shingaki-Wells, R.N.; Huang, S.; Taylor, N.L.; Carroll, A.J.; Zhou, W.; Millar, A.H. Differential molecular responses of rice and wheat coleoptiles to anoxia reveal novel metabolic adaptations in amino acid metabolism for tissue tolerance. Plant Physiol. 2011, 156, 1706–1724. [Google Scholar] [CrossRef] [Green Version]
- Rocha, M.; Sodek, L.; Licausi, F.; Hameed, M.W.; Dornelas, M.C.; Van Dongen, J.T. Analysis of alanine aminotransferase in various organs of soybean (Glycine max) and in dependence of different nitrogen fertilisers during hypoxic stress. Amino Acids 2010, 39, 1043–1053. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, D.; Zhou, R.; Wang, X.; Dossa, K.; Wang, L.; Zhang, Y.; Yu, J.; Gong, H.; Zhang, X. Transcriptome and metabolome analyses of two contrasting sesame genotypes reveal the crucial biological pathways involved in rapid adaptive response to salt stress. BMC Plant Biol. 2019, 19, 66. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, J.; Yang, F.; Zhou, W.; Mao, S.; Lin, J.; Yan, X. Untargeted lc-ms-based metabolomics revealed specific metabolic changes in cotyledons and roots of ricinus communis during early seedling establishment under salt stress. Plant Physiol. Biochem. 2021, 163, 108–118. [Google Scholar] [CrossRef]
- Xiao, S.; Chye, M.-L. New roles for acyl-coa-binding proteins (acbps) in plant development, stress responses and lipid metabolism. Prog. Lipid Res. 2011, 50, 141–151. [Google Scholar] [CrossRef]
- Lung, S.C.; Chye, M.L. Arabidopsis acyl-coa-binding proteins regulate the synthesis of lipid signals. New Phytol. 2019, 223, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, Y.; Toya, Y.; Kurokawa, H.; Fukano, Y.; Sato, A.; Umemura, H.; Yamada, K.; Iwasaki, H.; Tobori, N.; Shimizu, H. Characterization of oil-producing yeast lipomyces starkeyi on glycerol carbon source based on metabolomics and 13c-labeling. Appl. Microbiol. Biotechnol. 2018, 102, 8909–8920. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.-Y.; Li, J.; Wang, P.-Y.; Qin, F. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Mutale-joan, C.; Rachidi, F.; Mohamed, H.A.; Mernissi, N.E.; Aasfar, A.; Barakate, M.; Mohammed, D.; Sbabou, L.; Arroussi, H.E. Microalgae-cyanobacteria–based biostimulant effect on salinity tolerance mechanisms, nutrient uptake, and tomato plant growth under salt stress. J. Appl. Phycol. 2021, 33, 3779–3795. [Google Scholar] [CrossRef]
- Li, P.; Yang, X.; Wang, H.; Pan, T.; Yang, J.; Wang, Y.; Xu, Y.; Yang, Z.; Xu, C. Metabolic responses to combined water deficit and salt stress in maize primary roots. J. Integr. Agric. 2021, 20, 109–119. [Google Scholar] [CrossRef]
- Xu, K.; Xu, X.; Fukao, T.; Canlas, P.; Maghirang-Rodriguez, R.; Heuer, S.; Ismail, A.M.; Bailey-Serres, J.; Ronald, P.C.; Mackill, D.J. Sub1a is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef] [Green Version]
- Baena-González, E.; Rolland, F.; Thevelein, J.M.; Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 2007, 448, 938–942. [Google Scholar] [CrossRef]
- Fukao, T.; Bailey-Serres, J. Submergence tolerance conferred by sub1a is mediated by slr1 and slrl1 restriction of gibberellin responses in rice. Proc. Natl. Acad. Sci. USA 2008, 105, 16814–16819. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, R.; Hartman, S.; Liu, Z.; Martopawiro, S.; Sajeev, N.; van Veen, H.; Yeung, E.; Voesenek, L.A. Signal dynamics and interactions during flooding stress. Plant Physiol. 2018, 176, 1106–1117. [Google Scholar] [CrossRef] [Green Version]
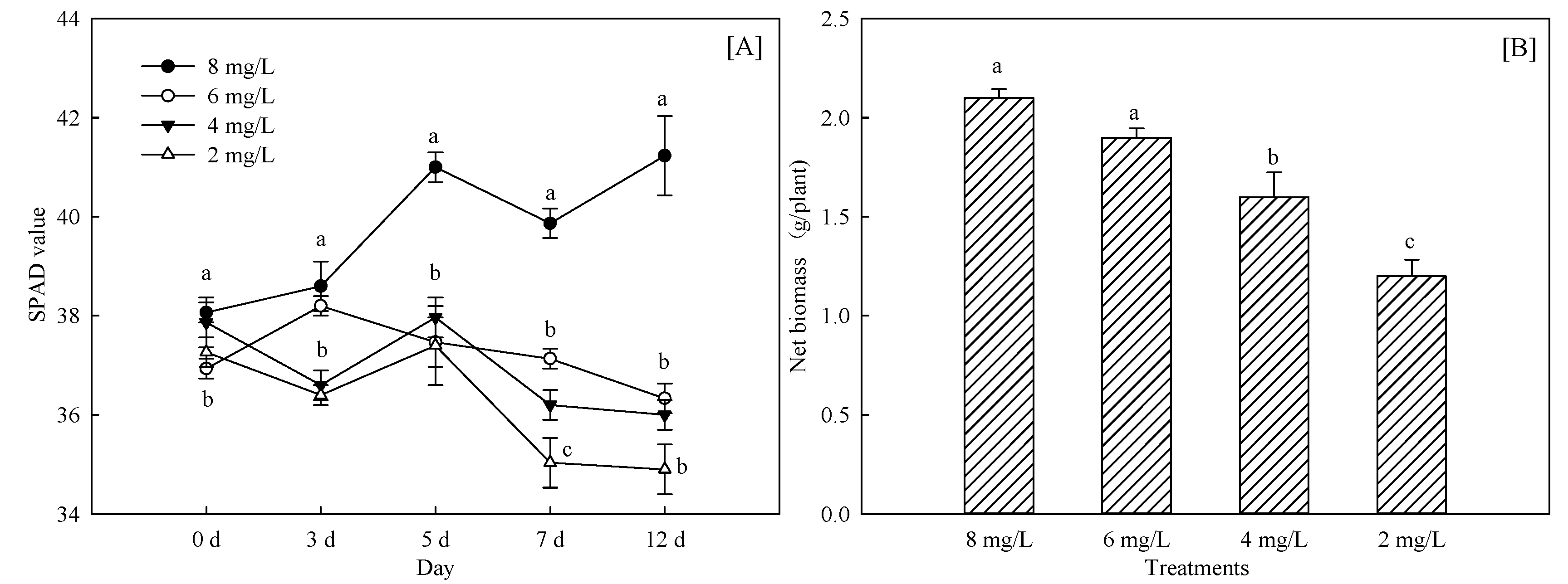
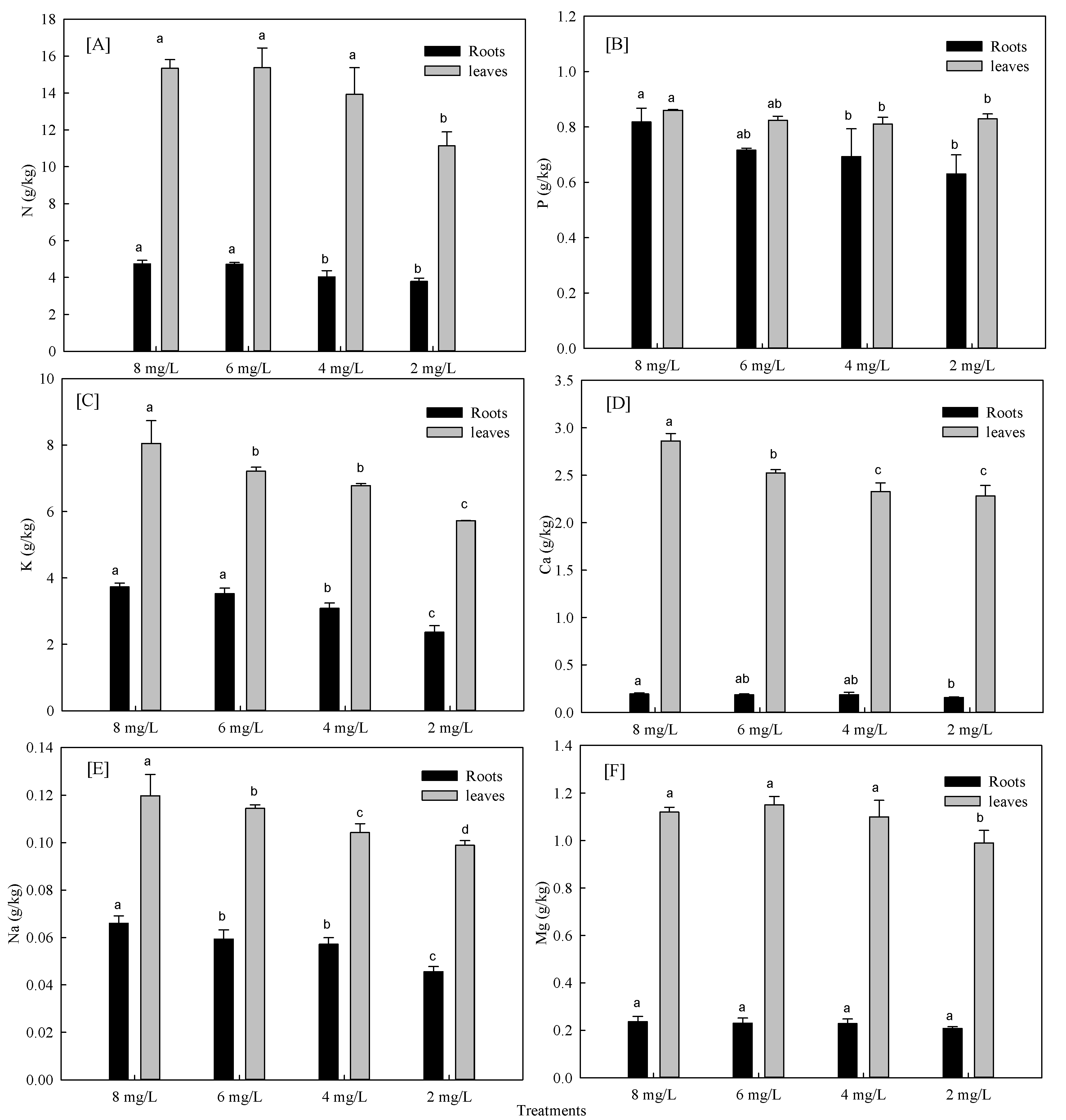

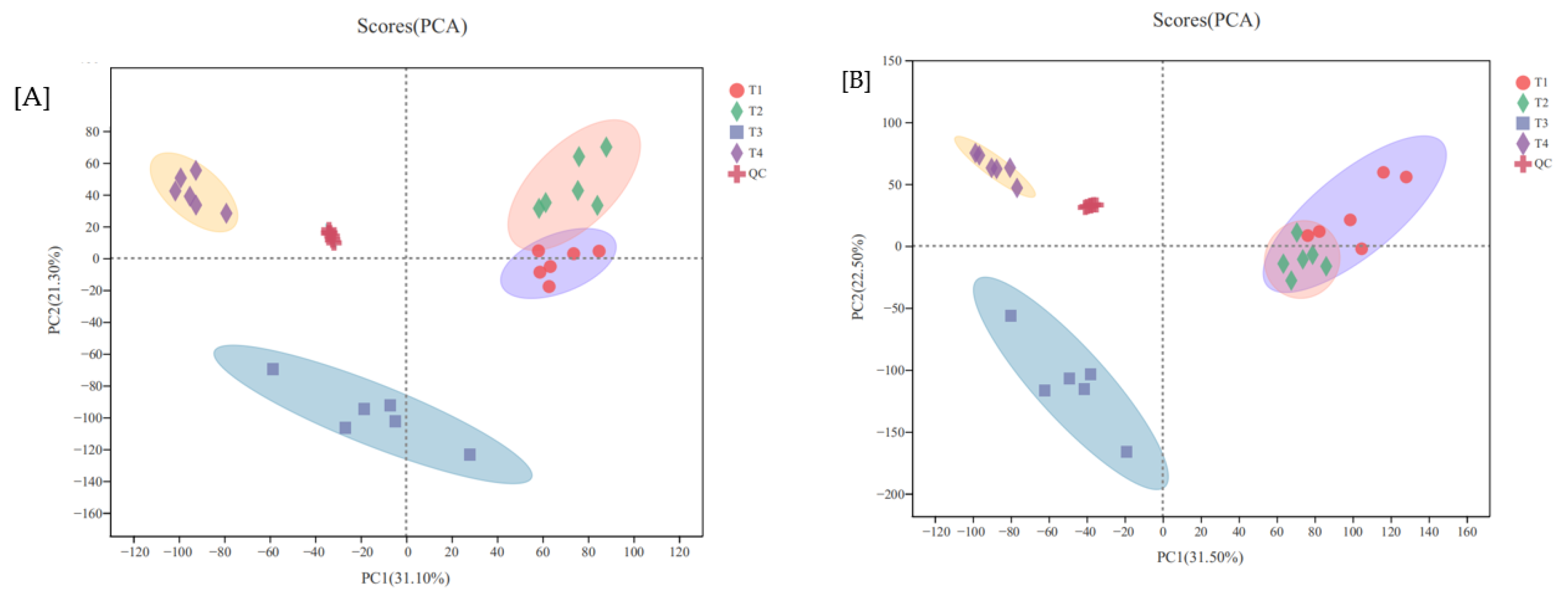


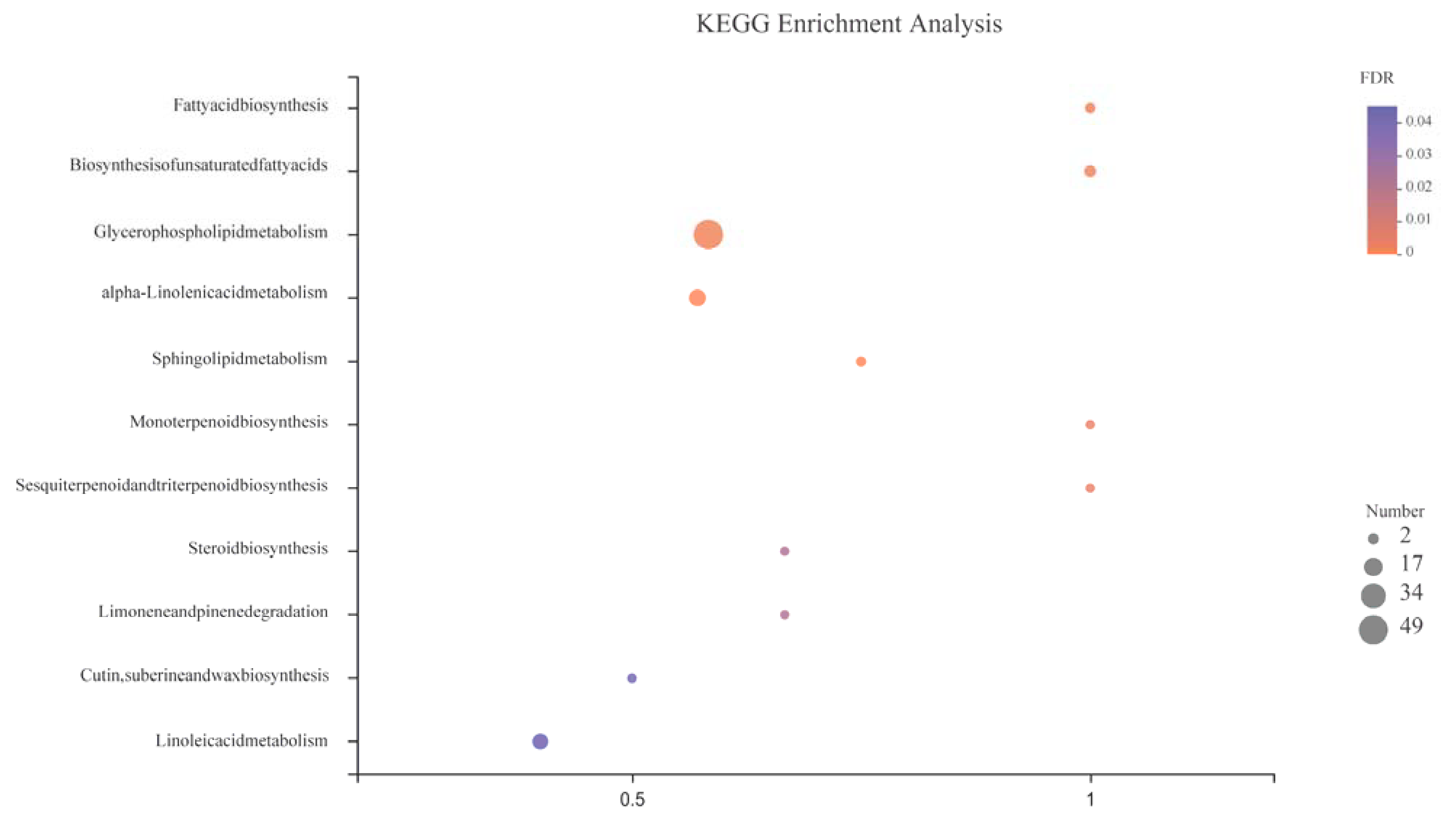
| Treatments | 0 d | 3 d | 5 d | 7 d | 12 d |
|---|---|---|---|---|---|
| 8 mg/L | 5.50 ± 0.32 a | 5.02 ± 0.29 c | 5.02 ± 0.41 d | 4.7 ± 0.33 c | 4.6 ± 0.13 d |
| 6 mg/L | 4.87 ± 0.37 a | 5.42 ± 0.82 c | 6.04 ± 0.49 c | 6.87 ± 0.65 b | 7.13 ± 0.39 c |
| 4 mg/L | 4.97 ± 0.24 a | 6.42 ± 0.12 b | 7.32 ± 0.44 b | 11.03 ± 0.84 a | 14.26 ± 1.04 b |
| 2 mg/L | 5.04 ± 0.69 a | 7.98 ± 0.76 a | 9.20 ± 0.91 a | 12.85 ± 0.96 a | 16.82 ± 1.26 a |
| Treatments | Ethanol | Acetaldehyde | Lactic Acid |
|---|---|---|---|
| 8 mg/L | 23.4 ± 2.3 d | 52.1 ± 5.3 c | 335.5 ± 19.4 d |
| 6 mg/L | 60.5 ± 5.7 c | 96.9 ± 7.5 b | 643.7 ± 63.5 c |
| 4 mg/L | 120.0 ± 10.5 b | 93.1 ± 4.7 b | 1126.8 ± 106.2 b |
| 2 mg/L | 243.0 ± 12.6 a | 109.7 ± 10.3 a | 1309.0 ± 125.5 a |
| Mode | Total Number | ||
|---|---|---|---|
| A (T1 vs. T2) | B (T1 vs. T3) | C (T1 vs. T4) | |
| pos | 21 (210) | 42 (282) | 23 (162) |
| neg | 20 (368) | 18 (391) | 15 (298) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Rukh, G.; Ruan, Z.; Xie, X.; Ye, Z.; Liu, D. Effects of Hypoxia Stress on Growth, Root Respiration, and Metabolism of Phyllostachys praecox. Life 2022, 12, 808. https://doi.org/10.3390/life12060808
Ma J, Rukh G, Ruan Z, Xie X, Ye Z, Liu D. Effects of Hypoxia Stress on Growth, Root Respiration, and Metabolism of Phyllostachys praecox. Life. 2022; 12(6):808. https://doi.org/10.3390/life12060808
Chicago/Turabian StyleMa, Jiawei, Gul Rukh, Zhongqiang Ruan, Xiaocui Xie, Zhengqian Ye, and Dan Liu. 2022. "Effects of Hypoxia Stress on Growth, Root Respiration, and Metabolism of Phyllostachys praecox" Life 12, no. 6: 808. https://doi.org/10.3390/life12060808
APA StyleMa, J., Rukh, G., Ruan, Z., Xie, X., Ye, Z., & Liu, D. (2022). Effects of Hypoxia Stress on Growth, Root Respiration, and Metabolism of Phyllostachys praecox. Life, 12(6), 808. https://doi.org/10.3390/life12060808





