Hemostatic Biomarkers and Volumetry Help to Identify High-Risk Abdominal Aortic Aneurysms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Analysis and Hemostatic Parameters
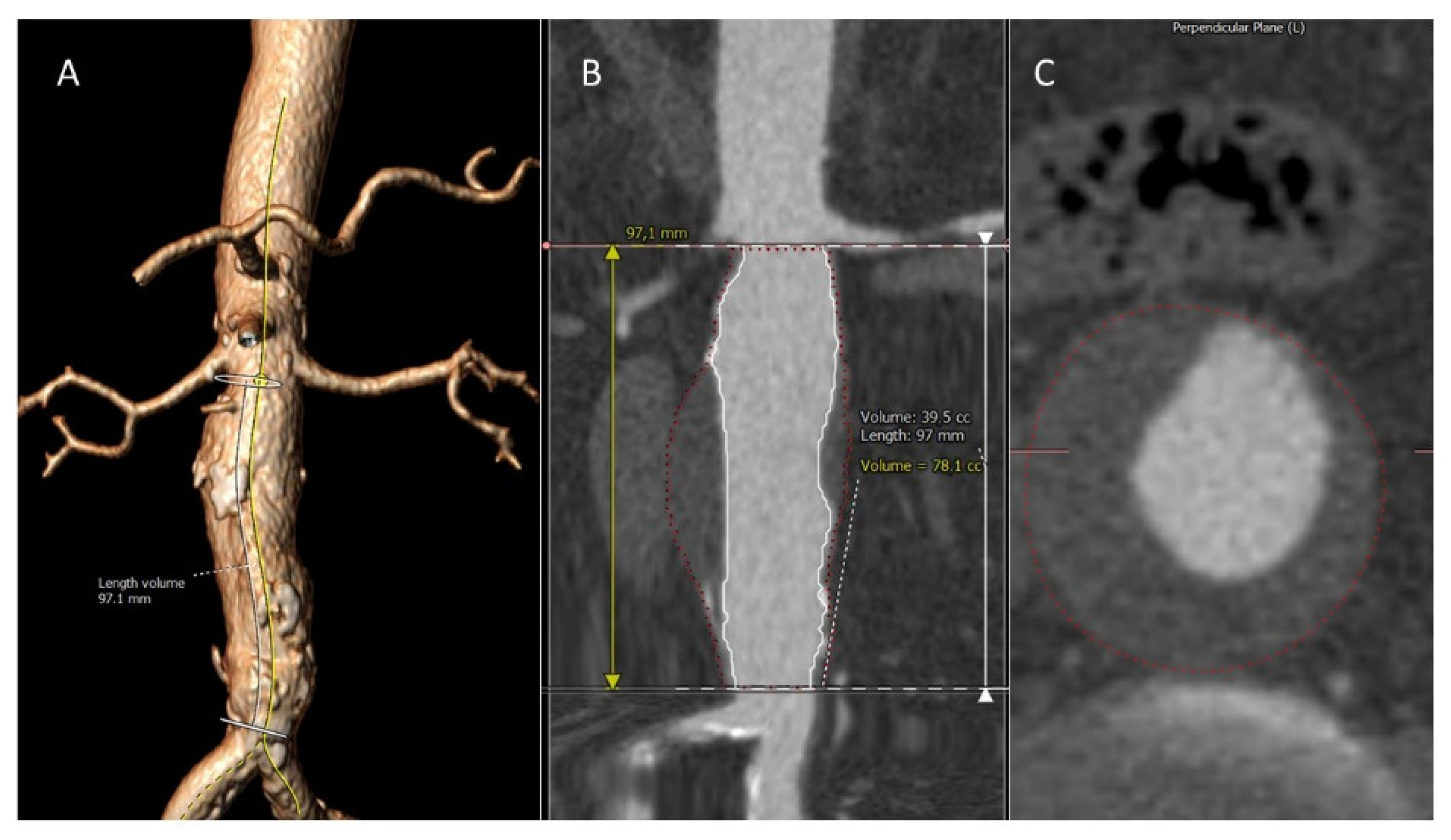
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Hemostatic Parameters in Patients with AAA
3.2. AAA Growth Assessment at 1-Year
3.3. Associations of Hemostatic Factors with Image Parameters during AAA Progression
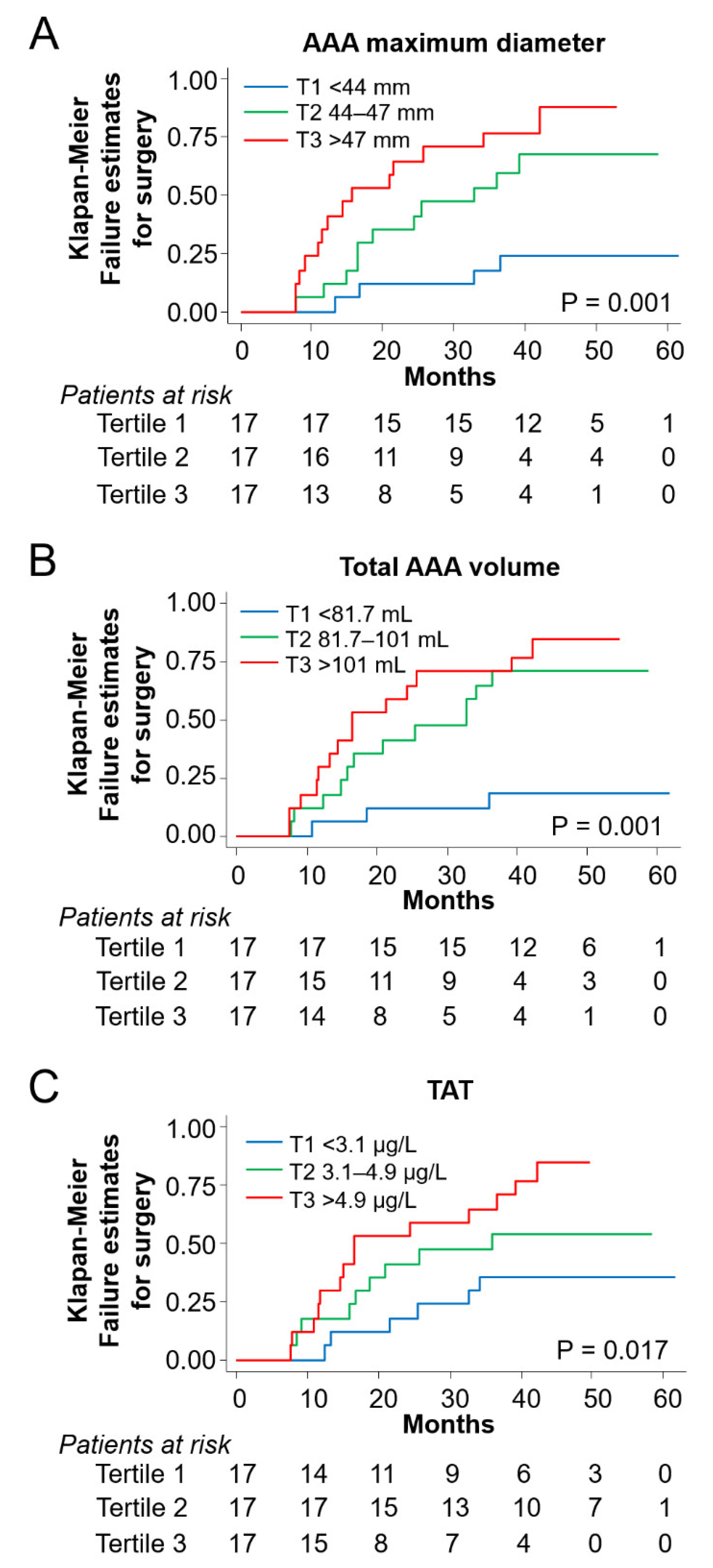
3.4. Risk Prediction for Aneurysm Surgery in the Follow Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acosta, S.; Ögren, M.; Bengtsson, H.; Bergqvist, D.; Lindblad, B.; Zdanowski, Z. Increasing incidence of ruptured abdominal aortic aneurysm: A population-based study. J. Vasc. Surg. 2006, 44, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, L.; Atallah, E.; Stansby, G. Pharmacological treatment of vascular risk factors for reducing mortality and cardiovascular events in patients with abdominal aortic aneurysm. Cochrane Database Syst. Rev. 2017, 2017, CD010447. [Google Scholar] [CrossRef] [PubMed]
- Long, A.; Rouet, L.; Lindholt, J.S.; Allaire, E. Measuring the maximum diameter of native abdominal aortic aneurysms: Review and critical analysis. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 515–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef] [Green Version]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice–European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [Green Version]
- Van Keulen, J.W.; Van Prehn, J.; Prokop, M.; Moll, F.L.; Van Herwaarden, J.A. Potential value of aneurysm sac volume measurements in addition to diameter measurements after endovascular aneurysm repair. J. Endovasc. Ther. 2009, 16, 506–513. [Google Scholar] [CrossRef]
- Bley, T.A.; Chase, P.J.; Reeder, S.B.; François, C.J.; Shinki, K.; Tefera, G.; Ranallo, F.N.; Grist, T.M.; Pozniak, M. Endovascular abdominal aortic aneurysm repair: Nonenhanced volumetric CT for follow-up. Radiology 2009, 253, 253–262. [Google Scholar] [CrossRef]
- Kontopodis, N.; Metaxa, E.; Papaharilaou, Y.; Georgakarakos, E.; Tsetis, D.; Ioannou, C.V. Value of volume measurements in evaluating abdominal aortic aneurysms growth rate and need for surgical treatment. Eur. J. Radiol. 2014, 83, 1051–1056. [Google Scholar] [CrossRef]
- Kontopodis, N.; Lioudaki, S.; Pantidis, D.; Papadopoulos, G.; Georgakarakos, E.; Ioannou, C. V Advances in determining abdominal aortic aneurysm size and growth. World J. Radiol. 2016, 8, 148. [Google Scholar] [CrossRef]
- Fillinger, M. Three-dimensional analysis of enlarging aneurysms after endovascular abdominal aortic aneurysm repair in the Gore Excluder Pivotal clinical trial. J. Vasc. Surg. 2006, 43, 888–895. [Google Scholar] [CrossRef] [Green Version]
- Prinssen, M.; Verhoeven, E.L.G.; Verhagen, H.J.M.; Blankensteijn, J.D. Decision-making in follow-up after endovascular aneurysm repair based on diameter and volume measurements: A blinded comparison. Eur. J. Vasc. Endovasc. Surg. 2003, 26, 184–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidloff, D.A.; Stather, P.W.; Choke, E.; Bown, M.J.; Sayers, R.D. A systematic review and meta-analysis of the association between markers of hemostasis and abdominal aortic aneurysm presence and size. J. Vasc. Surg. 2014, 59, 528–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, A.R.; Fowkes, F.G.R.; Greenhalgh, R.M.; Powell, J.T.; Ruckley, C.V.; Thompson, S.G. Risk factors for postoperative death following elective surgical repair of abdominal aortic aneurysm: Results from the UK Small Aneurysm Trial. Br. J. Surg. 2000, 87, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.R.; Fowkes, F.G.R.; Thompson, S.G.; Powell, J.T. Aortic aneurysm diameter and risk of cardiovascular mortality. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1203–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. Editor’s Choice-2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef] [Green Version]
- Pollock, J.G.; Travis, S.J.; Whitaker, S.C.; Davidson, I.R.; Gregson, R.H.; Hopkinson, B.R.; Wenham, P.W.; MacSweeney, S.T. Endovascular AAA repair: Classification of aneurysm sac volumetric change using spiral computed tomographic angiography. J. Endovasc. Ther. 2002, 9, 185–193. [Google Scholar] [CrossRef]
- Speelman, L.; Schurink, G.W.H.; Bosboom, E.M.H.; Buth, J.; Breeuwer, M.; van de Vosse, F.N.; Jacobs, M.H. The mechanical role of thrombus on the growth rate of an abdominal aortic aneurysm. J. Vasc. Surg. 2010, 51, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Vorp, D.A.; Lee, P.C.; Wang, D.H.J.; Makaroun, M.S.; Nemoto, E.M.; Ogawa, S.; Webster, M.W. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J. Vasc. Surg. 2001, 34, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Parr, A.; McCann, M.; Bradshaw, B.; Shahzad, A.; Buttner, P.; Golledge, J. Thrombus volume is associated with cardiovascular events and aneurysm growth in patients who have abdominal aortic aneurysms. J. Vasc. Surg. 2011, 53, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Parr, A.; Jayaratne, C.; Buttner, P.; Golledge, J. Comparison of volume and diameter measurement in assessing small abdominal aortic aneurysm expansion examined using computed tomographic angiography. Eur. J. Radiol. 2011, 79, 42–47. [Google Scholar] [CrossRef]
- Yamazumi, K.; Ojiro, M.; Okumura, H.; Aikou, T. An activated state of blood coagulation and fibrinolysis in patients with abdominal aortic aneurysm. Am. J. Surg. 1998, 175, 297–301. [Google Scholar] [CrossRef]
- Sundermann, A.C.; Saum, K.; Conrad, K.A.; Russell, H.M.; Edwards, T.L.; Mani, K.; Björck, M.; Wanhainen, A.; Phillip Owens, A. Prognostic value of D-dimer and markers of coagulation for stratification of abdominal aortic aneurysm growth. Blood Adv. 2018, 2, 3088–3096. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.J.; Al-Barjas, H.S.; Chappell, L.; Rashid, T.; Ariëns, R.A.S.; Scott, D.J.A. Haemostatic and fibrinolytic factors in men with a small abdominal aortic aneurysm. Br. J. Surg. 2009, 96, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Manabe, H.; Kawai, N.; Goto, S.; Umemoto, T. Plasma Fibrinogen and D-dimer Concentrations are Associated with the Presence of Abdominal Aortic Aneurysm: A Systematic Review and Meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 273–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simes, J.; Robledo, K.P.; White, H.D.; Espinoza, D.; Stewart, R.A.; Sullivan, D.R.; Zeller, T.; Hague, W.; Nestel, P.J.; Glasziou, P.P.; et al. D-dimer predicts long-term cause-specific mortality, cardiovascular events, and cancer in patients with stable coronary heart disease LIPID study. Circulation 2018, 138, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Muller, R.; Clancy, P.; McCann, M.; Norman, P.E. Evaluation of the diagnostic and prognostic value of plasma D-dimer for abdominal aortic aneurysm. Eur. Hear. J. 2011, 32, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.N.; Ke, X.; Yi, Z.L.; Lin, Y.Q.; Deng, B.Q.; Shu, X.R.; Yang, D.H.; Liao, Z.Y.; Nie, R.Q. Plasma D-dimer as a predictor of intraluminal thrombus burden and progression of abdominal aortic aneurysm. Life Sci. 2020, 240, 117069. [Google Scholar] [CrossRef]
- van Prehn, J.; van der Wal, M.B.A.; Vincken, K.; Bartels, L.W.; Moll, F.L.; van Herwaarden, J.A. Intra- and interobserver variability of aortic aneurysm volume measurement with fast CTA postprocessing software. J. Endovasc. Ther. 2008, 15, 504–510. [Google Scholar] [CrossRef]
- Kauffmann, C.; Tang, A.; Therasse, É.; Giroux, M.F.; Elkouri, S.; Melanson, P.; Melanson, B.; Oliva, V.L.; Soulez, G. Measurements and detection of abdominal aortic aneurysm growth: Accuracy and reproducibility of a segmentation software. Eur. J. Radiol. 2012, 81, 1688–1694. [Google Scholar] [CrossRef]
- Meyrignac, O.; Bal, L.; Zadro, C.; Vavasseur, A.; Sewonu, A.; Gaudry, M.; Saint-Lebes, B.; De Masi, M.; Revel-Mouroz, P.; Sommet, A.; et al. Combining volumetric and wall shear stress analysis from CT to assess risk of abdominal aortic aneurysm progression. Radiology 2020, 295, 722–729. [Google Scholar] [CrossRef]
- Kent, K.C. Clinical practice. Abdominal aortic aneurysms. N. Engl. J. Med. 2014, 371, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, M.F.; Marra, S.P.; Raghavan, M.L.; Kennedy, F.E. Prediction of rupture risk in abdominal aortic aneurysm during observation: Wall stress versus diameter. J. Vasc. Surg. 2003, 37, 724–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosla, S.; Morris, D.R.; Moxon, J.V.; Walker, P.J.; Gasser, T.C.; Golledge, J. Meta-analysis of peak wall stress in ruptured, symptomatic and intact abdominal aortic aneurysms. Br. J. Surg. 2014, 101, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Leemans, E.L.; Willems, T.P.; Van Der Laan, M.J.; Slump, C.H.; Zeebregts, C.J. Biomechanical Indices for Rupture Risk Estimation in Abdominal Aortic Aneurysms. J. Endovasc. Ther. 2017, 24, 254–261. [Google Scholar] [CrossRef]

| Group 1 (n = 52) | Group 2 (n = 51) | Group 3 (n = 50) | p for Trend | |
|---|---|---|---|---|
| Demographical and clinical parameters | ||||
| Sex (male, n, %) | 40(77) | 46(90) | 50(100) | 0.001 |
| Age (years) | 75(6) | 72(8) | 73(8) | 0.161 |
| BMI | 27(4) | 28(5) | 28(4) | 0.872 |
| Smokers (n, %) | 31(60) | 45(88) | 45(90) | <0.001 |
| DM (n, %) | 11(21) | 12(24) | 8(16) | 0.630 |
| Hypertension (n, %) | 32(61) | 34(67) | 33(66) | 0.839 |
| Dyslipidemia (n, %) | 38(73) | 41(80) | 38(76) | 0.679 |
| Biochemical parameters | ||||
| Total cholesterol (mg/mL) | 180(37) | 175(39) | 175(44) | 0.453 |
| LDL (mg/dL) | 109(33) | 105(33) | 107(38) | 0.865 |
| HDL (mg/dL) | 48 ± 13 | 42 ± 10 | 40 ± 11 | 0.002 |
| Treatment (n, %) | ||||
| Antiplatelets | 31(60) | 28(55) | 41(82) | 0.009 |
| Anticoagulants | 10(19) | 11(22) | 9(18) | 0.900 |
| ACE inhibitors | 16(31) | 14(28) | 10(20) | 0.450 |
| ARA-2 | 12(23) | 13(25) | 11(22) | 0.914 |
| Calcium antagonists | 8(15) | 18(35) | 11(22) | 0.056 |
| Vasodilators | 3(6) | 5(10) | 6(12) | 0.541 |
| β-Blockers | 10(19) | 19(37) | 20(40) | 0.049 |
| Statins | 34(65) | 41(80) | 39(78) | 0.171 |
| Metformin | 10(19) | 10(20) | 4(8) | 0.190 |
| Previous history of CVD (n, %) | ||||
| Coronary disease | 11(21) | 11(22) | 15(30) | 0.503 |
| Stroke | 5(10) | 2(4) | 3(6) | 0.496 |
| COPD | 12(23) | 11(22) | 10(20) | 0.931 |
| CKD | 2(4) | 8(16) | 4(8) | 0.108 |
| PAD | 28(54) | 9(18) | 11(22) | <0.001 |
| Functional parameters | ||||
| ABI | 0.83(0.2) | 0.92(0.2) | 0.93(0.2) | 0.016 |
| Hemostatic parameters | ||||
| TAT (µg/L) * | 2.2 (2.1) | 4.1(3.7) | 4.7(6.9) | <0.001 |
| D-dimer (ng/mL) * | 869 (1047) | 1683(1958) | 1832(1606) | <0.001 |
| Image Parameter | Recruitment | 1 Year | p | δ 1 Year- Recruitment |
|---|---|---|---|---|
| Maximum aortic diameter (mm) | 45.04 (2.82) | 47.63 (3.43) | <0.001 | 2.59 (2.53) |
| AAA volume (mL) | 95.36 (24.25) | 104.75 (28.75) | <0.001 | 9.40 (7.75) |
| Aortic thrombus volume (mL) | 44.05 (22.87) | 50.04 (27.59) | <0.001 | 5.98 (10.45) |
| Aortic lumen volume (mL) | 51.30 (15.34) | 54.72 (17.23) | 0.001 | 3.41 (6.92) |
| Image Parameter | TAT (µg/L) * | D-dimer (ng/mL) * | ||||
|---|---|---|---|---|---|---|
| Mean Difference | 95% CI | p | Mean Difference | 95% CI | p | |
| AAA diameter (mm) | ||||||
| Unadjusted | 0.56 | 0.01–1.11 | 0.047 | 0.23 | −0.39–0.86 | 0.47 |
| Adjusted | 0.56 | 0.02–1.10 | 0.044 | |||
| AAA volume (mL) | ||||||
| Unadjusted | 0.65 | −1.02–2.31 | 0.45 | 1.71 | −0.09–3.51 | 0.06 |
| Adjusted | 0.65 | −0.98–2.27 | 0.43 | 1.71 | −0.05–3.46 | 0.06 |
| AAA Thrombus volume (mL) | ||||||
| Unadjusted | 2.58 | 0.32–4.84 | 0.025 | 3.45 | 1.02–5.88 | 0.005 |
| Adjusted | 2.58 | 0.41–4.75 | 0.020 | 3.45 | 0.55–6.35 | 0.020 |
| Aortic lumen volume (mL) | ||||||
| Unadjusted | −1.93 | −3.42–−0.44 | 0.011 | −1.74 | −3.41–−0.08 | 0.040 |
| Adjusted | −1.93 | −3.32–−0.54 | 0.007 | −1.74 | −3.32–−0.16 | 0.030 |
| Models | AAA Diameter (mm) | AAA Volume (mL) | AAA Thrombus Volume (mL) | Aortic Lumen Volume (mL) | TAT (µg/L) * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SHR | 95% CI | p | SHR | 95% CI | p | SHR | 95% CI | p | SHR | 95% CI | p | SHR | 95% CI | p | |
| Unadjusted | 1.35 | 1.11–1.64 | 0.003 | 1.03 | 1.02–1.04 | <0.001 | 1.02 | 1.00–1.03 | 0.015 | 1.03 | 1.00–1.06 | 0.023 | 1.34 | 1.08–1.67 | 0.007 |
| Model 1 | 1.45 | 1.20–1.75 | <0.001 | 1.04 | 1.02–1.05 | <0.001 | 1.02 | 1.01–1.04 | 0.002 | 1.03 | 1.00–1.06 | 0.036 | 1.36 | 1.05–1.76 | 0.019 |
| Model 2 | 1.53 | 1.26–1.86 | <0.001 | 1.04 | 1.02–1.05 | <0.001 | 1.02 | 1.01–1.04 | 0.003 | 1.04 | 1.01–1.07 | 0.012 | 1.33 | 1.03–1.72 | 0.029 |
| Model 3 | 1.45 | 1.20–1.75 | <0.001 | 1.04 | 1.03–1.05 | <0.001 | 1.02 | 1.00–1.04 | 0.016 | 1.03 | 1.00–1.06 | 0.024 | 1.38 | 1.07–1.79 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Alonso, S.; Martinez-Aguilar, E.; Ravassa, S.; Orbe, J.; Paramo, J.A.; Fernandez-Alonso, L.; Roncal, C. Hemostatic Biomarkers and Volumetry Help to Identify High-Risk Abdominal Aortic Aneurysms. Life 2022, 12, 823. https://doi.org/10.3390/life12060823
Fernandez-Alonso S, Martinez-Aguilar E, Ravassa S, Orbe J, Paramo JA, Fernandez-Alonso L, Roncal C. Hemostatic Biomarkers and Volumetry Help to Identify High-Risk Abdominal Aortic Aneurysms. Life. 2022; 12(6):823. https://doi.org/10.3390/life12060823
Chicago/Turabian StyleFernandez-Alonso, Sebastian, Esther Martinez-Aguilar, Susana Ravassa, Josune Orbe, Jose A. Paramo, Leopoldo Fernandez-Alonso, and Carmen Roncal. 2022. "Hemostatic Biomarkers and Volumetry Help to Identify High-Risk Abdominal Aortic Aneurysms" Life 12, no. 6: 823. https://doi.org/10.3390/life12060823
APA StyleFernandez-Alonso, S., Martinez-Aguilar, E., Ravassa, S., Orbe, J., Paramo, J. A., Fernandez-Alonso, L., & Roncal, C. (2022). Hemostatic Biomarkers and Volumetry Help to Identify High-Risk Abdominal Aortic Aneurysms. Life, 12(6), 823. https://doi.org/10.3390/life12060823






