Molecular and Biochemical Mechanisms of Elicitors in Pest Resistance
Abstract
:1. Introduction
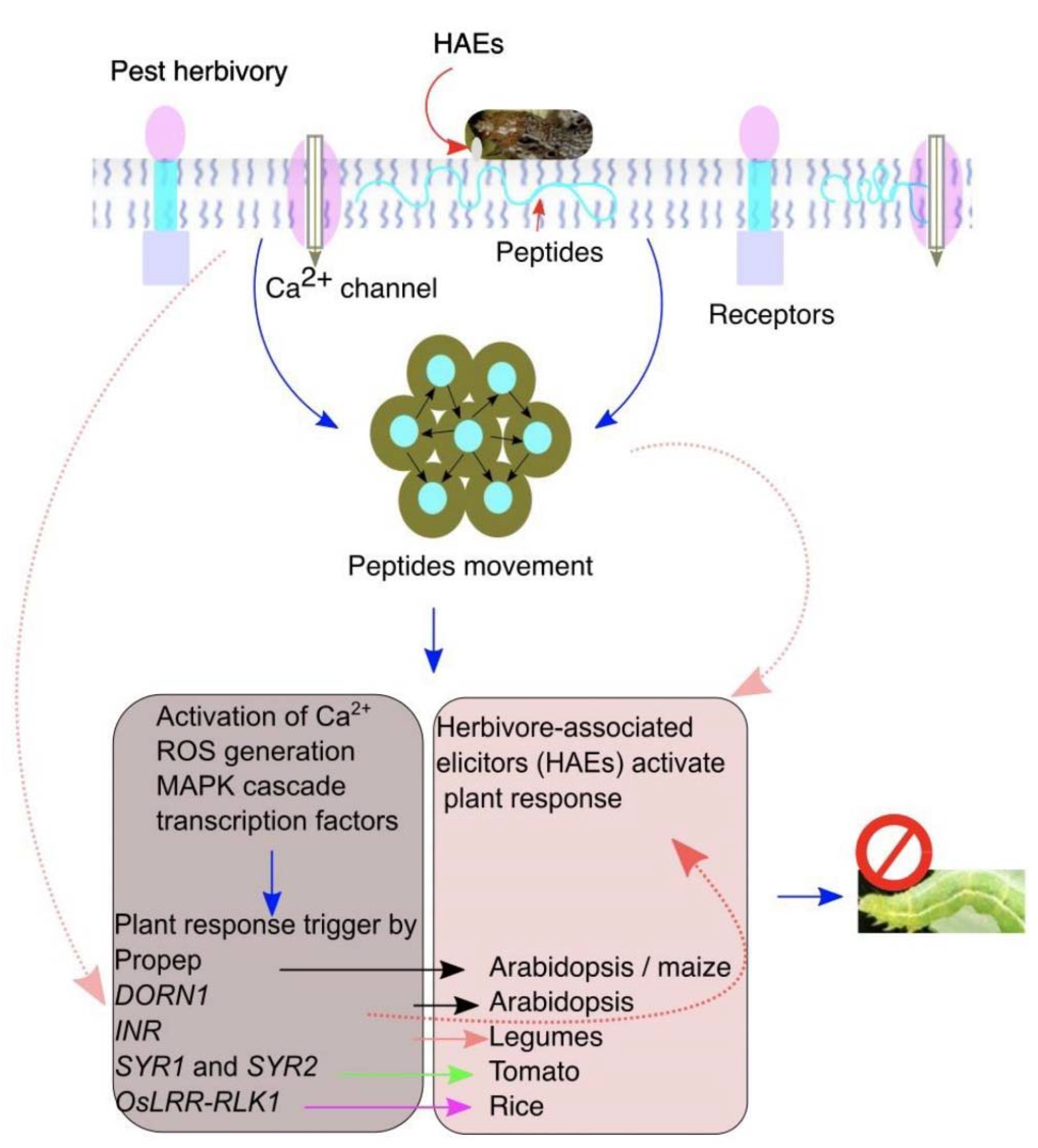
2. Plant Receptors Perceive Insect Herbivory
3. Detection of Herbivory and Encounter Mechanism
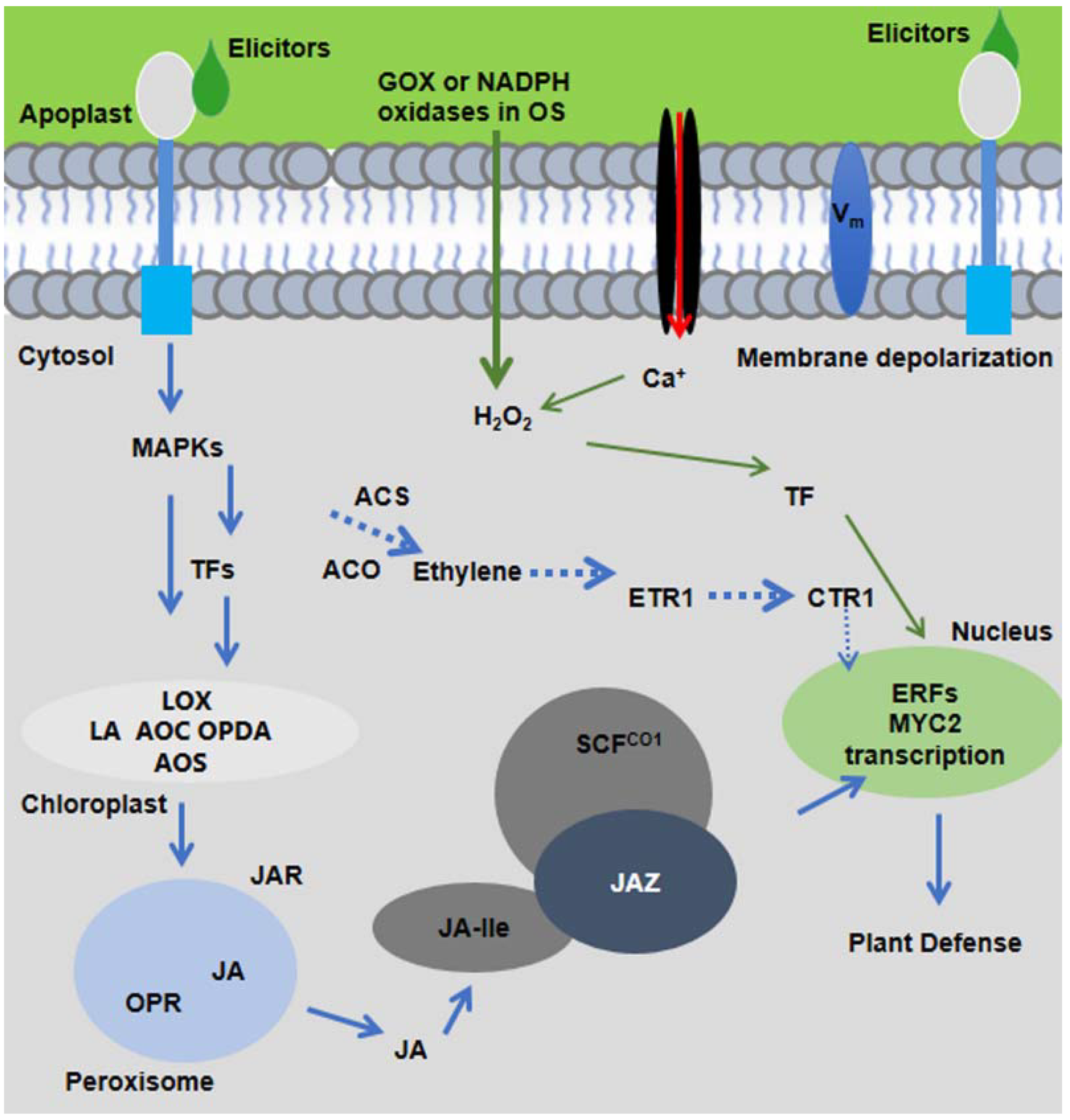
4. Plant and Insect Origin Elicitors
5. Elicitors of Plants’ Intracellular Products
6. Peptide Elicitors
7. Elicitors in OS of Insects
8. Elicitors in OS of Non-Lepidopteran Insects
9. Plant-Induced Responses
10. Plant Defense against Gall-Inducing Insects
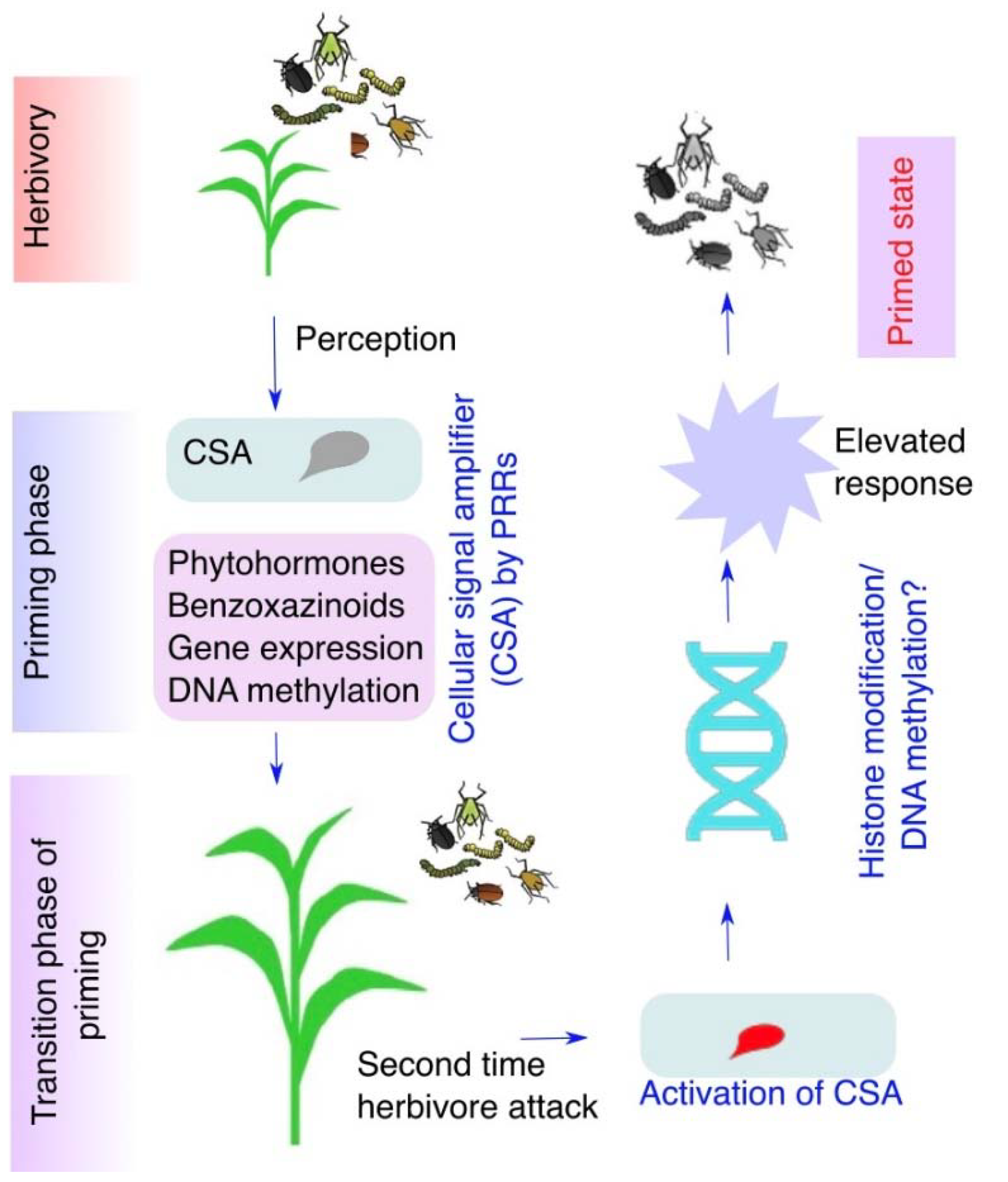
11. Regulation of Plant Responses at Primed Stage
12. Conclusions
12.1. Concluding Remarks
- Although the mechanism of induced responses is important to understand for better protection of plants, recently, the debate on how plants perceive HAEs to activate downstream-induced defenses has received great attention. However, investigations are required to explore the receptors that perceive insect herbivory.
- Plant receptors perceive elicitors from endo- and exogenous danger signals that are both plant and insect-derived to activate the long- and short-term downstream defenses.
- Upon the perception of herbivory, plants can respond by using exquisite defense strategies. As the perception is strong, plant responses are more robust against caterpillars. The potential of plants to recognize and distinguish between mechanical damage and the kind of insect herbivory indicate the capability of perception of the chemical cues present in the OS of attacker herbivores and feeding on specific host plants.
- Plant responses to insect herbivory are very specific according to the HAEs, and the specificity of plants largely depend on the perception of the nature of elicitors.
- As plants respond stronger and faster to repeated herbivore attacks, it would be interesting to know whether, how, and to what extent plant receptors are involved in the induction of long-term responses in plants.
12.2. Outstanding Questions
- To date, molecular signaling and biosynthesis mechanism of HAEs, such as FACs, caeliferins, egg deposition, and frass, to elicit the defense responses have not been extensively studied. It would be noteworthy to investigate the molecular signal transduction mechanism and biosynthesis of HAEs in plants and insects. Genome editing (e.g., knockout lines) and comparative transcriptomic approaches could be used for functional characterization.
- Indirect defenses are major shareholders in the repellence of herbivores. Genetic and functional characterization are required for the demonstration of genetic control of indirect responses and whether receptors perceiving insect herbivory could also function to emit volatiles to attract natural enemies to fend off insect herbivory.
- Insect infestation triggers short- and long-term plant responses. Defense is costly. There is a need to investigate how plant-defined long-term defenses are sustainable. Studies designed on the hypothesis of trade-off mechanisms could explain long-term and short-term sustainable defense responses.
- The molecular mechanism of induction of defense priming is still at immature stage. According to the specificity of plant responses to insect herbivory, it would be interesting to identify the HAEs that elicit plant responses and prime for enhanced resistance.
- Plants are constantly facing threats to their survival. To what extent plants manage resources for growth and defense by employing the receptors of pathogens and insects requires further research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.Q.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.I. Making Sense of the Way Plants Sense Herbivores. Trends Plant Sci. 2021, 26, 288–298. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaventure, G.; VanDoorn, A.; Baldwin, I.T. Herbivore-associated elicitors: FAC signaling and metabolism. Trends Plant Sci. 2011, 16, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Reymond, P. Receptor kinases in plant responses to herbivory. Curr. Opin. Biotechnol. 2021, 70, 143–150. [Google Scholar] [CrossRef]
- Kallure, G.S.; Kumari, A.; Shinde, B.A.; Giri, A.P. Characterized constituents of insect herbivore oral secretions and their influence on the regulation of plant defenses. Phytochemistry 2022, 193, 113008. [Google Scholar] [CrossRef] [PubMed]
- Felton, G.W.; Tumlinson, J.H. Plant-insect dialogs: Complex interactions at the plant-insect interface. Curr. Opin. Plant Biol. 2008, 11, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Mattiacci, L.; Dicke, M.; Maartin, A.P. β-Glucosidase- an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 2036–2040. [Google Scholar] [CrossRef] [Green Version]
- Schmelz, E.A.; Carroll, M.J.; LeClere, S.; Phipps, S.M.; Meredith, J.; Chourey, P.S.; Alborn, H.T.; Teal, P.E. Fragments of ATP synthase mediate plant perception of insect attack. Proc. Natl. Acad. Sci. USA 2006, 103, 8894–8899. [Google Scholar] [CrossRef] [Green Version]
- Alborn, H.T.; Turlings, T.C.J.; Jones, T.H.; Stenhagen, G.; Loughrin, J.H.; Tumlinson, J.H. An elicitor of plant volatiles from beet armyworm oral secretion. Science 1997, 276, 945–949. [Google Scholar] [CrossRef]
- Acevedo, F.E.; Rivera-Vega, L.J.; Chung, S.H.; Ray, S.; Felton, G.W. Cues from chewing insects—The intersection of DAMPs, HAMPs, MAMPs and effectors. Curr. Opin. Plant Biol. 2015, 26, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A. Impacts of insect oral secretions on defoliation-induced plant defense. Curr. Opin. Insect Sci. 2015, 9, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-Y.; Mao, Y.-B. Research advances in plant-insect molecular interaction. F1000Research 2020, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zebelo, S.A.; Maffei, M.E. Role of early signalling events in plant-insect interactions. J. Exp. Bot. 2015, 66, 435–448. [Google Scholar] [CrossRef] [Green Version]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Qi, J.; Malook, S.u.; Shen, G.; Gao, L.; Zhang, C.; Li, J.; Zhang, J.; Wang, L.; Wu, J. Current understanding of maize and rice defense against insect herbivores. Plant Divers. 2018, 40, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.Q.; Zhou, W.W.; Pottinger, S.; Baldwin, I.T. Herbivore associated elicitor-induced defences are highly specific among closely related Nicotiana species. BMC Plant Biol. 2015, 15, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truitt, C.L.; Wei, H.X.; Pare, P.W. A plasma membrane protein from Zea mays binds with the herbivore elicitor volicitin. Plant Cell 2004, 16, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Q.; Wu, H.; Chen, H.; Liu, Y.L.; He, J.; Kang, H.Y.; Sun, Z.G.; Pan, G.; Wang, Q.; Hu, J.L.; et al. A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat. Biotechnol. 2015, 33, 301–305. [Google Scholar] [CrossRef]
- Hu, L.F.; Ye, M.; Kuai, P.; Ye, M.F.; Erb, M.; Lou, Y.G. OsLRR-RLK1, an early responsive leucine-rich repeat receptor-like kinase, initiates rice defense responses against a chewing herbivore. New Phytol. 2018, 219, 1097–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilardoni, P.A.; Hettenhausen, C.; Baldwin, I.T.; Bonaventure, G. Nicotiana attenuata LECTIN RECEPTOR KINASE1 Suppresses the Insect-Mediated Inhibition of Induced Defense Responses during Manduca sexta Herbivory. Plant Cell 2011, 23, 3512–3532. [Google Scholar] [CrossRef] [Green Version]
- Barbero, F.; Guglielmotto, M.; Capuzzo, A.; Maffei, M.E. Extracellular Self-DNA (esDNA), but Not Heterologous Plant or Insect DNA (etDNA), Induces Plasma Membrane Depolarization and Calcium Signaling in Lima Bean (Phaseolus lunatus) and Maize (Zea mays). Int. J. Mol. Sci. 2016, 17, 1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huffaker, A.; Pearce, G.; Veyrat, N.; Erb, M.; Turlings, T.C.J.; Sartor, R.; Shen, Z.X.; Briggs, S.P.; Vaughan, M.M.; Alborn, H.T.; et al. Plant elicitor peptides are conserved signals regulating direct and indirect antiherbivore defense. Proc. Natl. Acad. Sci. USA 2013, 110, 5707–5712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, Y.; Huffaker, A.; Bryan, A.C.; Tax, F.E.; Ryan, C.A. PEPR2 Is a Second Receptor for the Pep1 and Pep2 Peptides and Contributes to Defense Responses in Arabidopsis. Plant Cell 2010, 22, 508–522. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Tanaka, K.; Yangrong, C.; Yue, Q.; Qiu, J.; Liang, Y.; Yeol Lee, S.; Stacey, G. Identification of a Plant Receptor for Extracellular ATP. Science 2014, 343, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Einig, E.; Almeida-Trapp, M.; Albert, M.; Fliegmann, J.; Mithofer, A.; Kalbacher, H.; Felix, G. The systemin receptor SYR1 enhances resistance of tomato against herbivorous insects. Nat. Plants 2018, 4, 152–156. [Google Scholar] [CrossRef]
- Hopke, J.; Donatha, J.; Blechertb, S.; Boland, W. Herbivore-induced volatiles—The emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a b-glucosidase and jasmonic acid. FEBS Lett. 1994, 352, 146–150. [Google Scholar] [CrossRef] [Green Version]
- Alborn, H.T.; Hansen, T.V.; Jones, T.H.; Bennett, D.C.; Tumlinson, J.H.; Schmelz, E.A.; Teal, P.E.A. Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc. Natl. Acad. Sci. USA 2007, 104, 12976–12981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schafer, M.; Fischer, C.; Meldau, S.; Seebald, E.; Oelmuller, R.; Baldwin, I.T. Lipase Activity in Insect Oral Secretions Mediates Defense Responses in Arabidopsis. Plant Physiol. 2011, 156, 1520–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.J.; Wielsch, N.; Hafke, J.B.; Svatos, A.; Mithofer, A.; Boland, W. A porin-like protein from oral secretions of Spodoptera littoralis larvae induces defense-related early events in plant leaves. Insect Biochem. Mol. Biol. 2013, 43, 849–858. [Google Scholar] [CrossRef]
- Bricchi, I.; Occhipinti, A.; Bertea, C.M.; Zebelo, S.A.; Brillada, C.; Verrillo, F.; De Castro, C.; Molinaro, A.; Faulkner, C.; Maule, A.J.; et al. Separation of early and late responses to herbivory in Arabidopsis by changing plasmodesmal function. Plant J. 2013, 73, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Doss, R.P.; Oliver, J.E.; Proebsting, W.M.; Potter, S.W.; Kuy, S.R.; Clement, S.L.; Williamson, R.T.; Carney, J.R.; DeVilbiss, E.D. Bruchins: Insect-derived plant regulators that stimulate neoplasm formation. Proc. Natl. Acad. Sci. USA 2000, 97, 6218–6223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musser, R.O.; Hum-Musser, S.M.; Eichenseer, H.; Peiffer, M.; Ervin, G.; Murphy, J.B.; Felton, G.W. Caterpillar saliva beats plant defences. Nature 2002, 416, 599–600. [Google Scholar] [CrossRef]
- Diezel, C.; von Dahl, C.C.; Gaquerel, E.; Baldwin, I.T. Different Lepidopteran Elicitors Account for Cross-Talk in Herbivory-Induced Phytohormone Signaling. Plant Physiol. 2009, 150, 1576–1586. [Google Scholar] [CrossRef] [Green Version]
- Shangguan, X.X.; Zhang, J.; Liu, B.F.; Zhao, Y.; Wang, H.Y.; Wang, Z.Z.; Guo, J.P.; Rao, W.W.; Jing, S.L.; Guan, W.; et al. A Mucin-Like Protein of Planthopper Is Required for Feeding and Induces Immunity Response in Plants. Plant Physiol. 2018, 176, 552–565. [Google Scholar] [CrossRef] [Green Version]
- Doares, S.H.; Syrovets, T.; Weiler, E.W.; Ryan, C.A. Oligogalacturonides and Chitosan Activate Plant Defensive Genes through the Octadecanoid Pathway. Proc. Natl. Acad. Sci. USA 1995, 92, 4095–4098. [Google Scholar] [CrossRef] [Green Version]
- Gouhier-Darimont, C.; Schmiesing, A.; Bonnet, C.; Lassueur, S.; Reymond, P. Signalling of Arabidopsis thaliana response to Pieris brassicae eggs shares similarities with PAMP-triggered immunity. J. Exp. Bot. 2013, 64, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Mantelin, S.; Peng, H.C.; Li, B.B.; Atamian, H.S.; Takken, F.L.W.; Kaloshian, I. The receptor-like kinase SlSERK1 is required for Mi-1-mediated resistance to potato aphids in tomato. Plant J. 2011, 67, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Krol, E.; Mentzel, T.; Chinchilla, D.; Boller, T.; Felix, G.; Kemmerling, B.; Postel, S.; Arents, M.; Jeworutzki, E.; Al-Rasheid, K.A.; et al. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J. Biol. Chem. 2010, 285, 13471–13479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gust, A.A.; Pruitt, R.; Nurnberger, T. Sensing Danger: Key to Activating Plant Immunity. Trends Plant Sci. 2017, 22, 779–791. [Google Scholar] [CrossRef]
- Steinbrenner, A.D.; Munoz-Amatriain, M.; Chaparro, A.F.; Aguilar-Venegas, J.M.; Lo, S.; Okuda, S.; Glauser, G.; Dongiovanni, J.; Shi, D.; Hall, M.; et al. A receptor-like protein mediates plant immune responses to herbivore-associated molecular patterns. Proc. Natl. Acad. Sci. USA 2020, 117, 31510–31518. [Google Scholar] [CrossRef]
- Snoeck, S.; Guayazán-Palacios, N.; Steinbrenner, A.D. Molecular tug-of-war: Plant immune recognition of herbivory. Plant Cell 2022, 34, 1497–1513. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Engelberth, J.; Alborn, H.T.; Tumlinson, J.H.; Teal, P.E.A. Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc. Natl. Acad. Sci. USA 2009, 106, 653–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanDoorn, A.; Kallenbach, M.; Borquez, A.A.; Baldwin, I.T.; Bonaventure, G. Rapid modification of the insect elicitor N-linolenoyl-glutamate via a lipoxygenase-mediated mechanism on Nicotiana attenuata leaves. BMC Plant Biol. 2010, 10, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilker, M.; Fatouros, N.E. Plant responses to insect egg deposition. Annu. Rev. Entomol. 2015, 60, 493–515. [Google Scholar] [CrossRef]
- Little, D.; Gouhier-Darimont, C.; Bruessow, F.; Reymond, P. Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol. 2007, 143, 784–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, S.; Gaffor, I.; Acevedo, F.E.; Helms, A.; Chuang, W.P.; Tooker, J.; Felton, G.W.; Luthe, D.S. Maize Plants Recognize Herbivore-Associated Cues from Caterpillar Frass. J. Chem. Ecol. 2015, 41, 781–792. [Google Scholar] [CrossRef]
- Ray, S.; Alves, P.C.; Ahmad, I.; Gaffoor, I.; Acevedo, F.E.; Peiffer, M.; Jin, S.; Han, Y.; Shakeel, S.; Felton, G.W.; et al. Turnabout Is Fair Play: Herbivory-Induced Plant Chitinases Excreted in Fall Armyworm Frass Suppress Herbivore Defenses in Maize. Plant Physiol. 2016, 171, 694–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, S.; Basu, S.; Rivera-Vega, L.J.; Acevedo, F.E.; Louis, J.; Felton, G.W.; Luthe, D.S. Lessons from the Far End: Caterpillar FRASS-Induced Defenses in Maize, Rice, Cabbage, and Tomato. J. Chem. Ecol. 2016, 42, 1130–1141. [Google Scholar] [CrossRef]
- Basu, S.; Varsani, S.; Louis, J. Altering Plant Defenses: Herbivore-Associated Molecular Patterns and Effector Arsenal of Chewing Herbivores. Mol. Plant Microbe Interact. 2018, 31, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lange, E.S.; Laplanche, D.; Guo, H.J.; Xu, W.; Vlimant, M.; Erb, M.; Ton, J.; Turlings, T.C.J. Spodoptera frugiperda Caterpillars Suppress Herbivore-Induced Volatile Emissions in Maize. J. Chem. Ecol. 2020, 46, 344–360. [Google Scholar] [CrossRef]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Baldwin, I.T. Herbivory-induced signalling in plants: Perception and action. Plant Cell Environ. 2009, 32, 1161–1174. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Bos, J.I.B. Effector proteins that modulate plant–insect interactions. Curr. Opin. Plant Biol. 2011, 14, 422–428. [Google Scholar] [CrossRef]
- Gaquerel, E.; Weinhold, A.; Baldwin, I.T. Molecular Interactions between the Specialist Herbivore Manduca sexta (Lepidoptera, Sphigidae) and Its Natural Host Nicotiana attenuata. VIII. An Unbiased GCxGC-ToFMS Analysis of the Plant’s Elicited Volatile Emissions. Plant Physiol. 2009, 149, 1408–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, P.A.; Bos, J.I. Toward understanding the role of aphid effectors in plant infestation. Mol. Plant Microbe Interact. 2013, 26, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Tian, D.; Peiffer, M.; Shoemaker, E.; Tooker, J.; Haubruge, E.; Francis, F.; Luthe, D.S.; Felton, G.W. Salivary glucose oxidase from caterpillars mediates the induction of rapid and delayed-induced defenses in the tomato plant. PLoS ONE 2012, 7, e36168. [Google Scholar] [CrossRef] [Green Version]
- Louis, J.; Shah, J. Arabidopsis thaliana–Myzus persicae interaction: Shaping the understanding of plant defense against phloem-feeding aphids. Front. Plant Sci. 2013, 4, 213. [Google Scholar] [CrossRef] [Green Version]
- Consales, F.; Schweizer, F.; Erb, M.; Gouhier-Darimont, C.; Bodenhausen, N.; Bruessow, F.; Sobhy, I.; Reymond, P. Insect oral secretions suppress wound-induced responses in Arabidopsis. J. Exp. Bot. 2011, 63, 727–737. [Google Scholar] [CrossRef] [Green Version]
- Jeter, C.R.; Tang, W.Q.; Henaff, E.; Butterfield, T.; Roux, S.J. Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 2004, 16, 2652–2664. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Peiffer, M.; Luthe, D.S.; Felton, G.W. ATP Hydrolyzing Salivary Enzymes of Caterpillars Suppress Plant Defenses. PLoS ONE 2012, 7, e41947. [Google Scholar] [CrossRef] [Green Version]
- Vadassery, J.; Reichelt, M.; Mithofer, A. Direct Proof of Ingested Food Regurgitation by Spodoptera littoralis Caterpillars during Feeding on Arabidopsis. J. Chem. Ecol. 2012, 38, 865–872. [Google Scholar] [CrossRef]
- Maffei, M.E.; Mithofer, A.; Boland, W. Before gene expression: Early events in plant-insect interaction. Trends Plant Sci. 2007, 12, 310–316. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Pearce, G.; Ryan, C.A. The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc. Natl. Acad. Sci. USA 2006, 103, 10104–10109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klauser, D.; Desurmont, G.A.; Glauser, G.; Vallat, A.; Flury, P.; Boller, T.; Turlings, T.C.J.; Bartels, S. The Arabidopsis Pep-PEPR system is induced by herbivore feeding and contributes to JA-mediated plant defence against herbivory. J. Exp. Bot. 2015, 66, 5327–5336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenhardt, D.C.; Refi-Hind, S.; Stratmann, J.W.; Lincoln, D.E. Systemin and jasmonic acid regulate constitutive and herbivore-induced systemic volatile emissions in tomato, Solanum lycopersicum. Phytochemistry 2010, 71, 2024–2037. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, J. Indirect Defense Responses to Herbivory in Grasses. Plant Physiol. 2009, 149, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Zhang, X.; Liu, J.; Tao, K.; Li, C.; Xiao, S.; Zhang, W.; Li, J.-F. Plant elicitor peptide signalling confers rice resistance to piercing-sucking insect herbivores and pathogens. Plant Biotechnol. J. 2022, 20, 991–1005. [Google Scholar] [CrossRef]
- Giri, A.P.; Wunsche, H.; Mitra, S.; Zavala, J.A.; Muck, A.; Svatos, A.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant’s proteome. Plant Physiol. 2006, 142, 1621–1641. [Google Scholar] [CrossRef] [Green Version]
- Halitschke, R. Molecular Interactions between the Specialist Herbivore Manduca sexta (Lepidoptera, Sphingidae) and Its Natural Host Nicotiana attenuata. III. Fatty Acid-Amino Acid Conjugates in Herbivore Oral Secretions Are Necessary and Sufficient for Herbivore-Specific Plant Responses. Plant Physiol. 2001, 125, 711–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meldau, S.; Wu, J.; Baldwin, I.T. Silencing two herbivory-activated MAP kinases, SIPK and WIPK, does not increase Nicotiana attenuata’s susceptibility to herbivores in the glasshouse and in nature. New Phytol. 2009, 181, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Skibbe, M.; Qu, N.; Galis, I.; Baldwin, I.T. Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. Plant Cell 2008, 20, 1984–2000. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Hettenhausen, C.; Meldau, S.; Baldwin, I.T. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 2007, 19, 1096–1122. [Google Scholar] [CrossRef] [Green Version]
- Pohnert, G.; Jung, V.; Haukioja, E.; Lempa, K.; Boland, W. New fatty acid amides from regurgitant of lepidopteran (Noctuidae, Geometridae) caterpillars. Tetrahedron 1999, 55, 11275–11280. [Google Scholar] [CrossRef]
- Bede, J.C.; Musser, R.O.; Felton, G.W.; Korth, K.L. Caterpillar herbivory and salivary enzymes decrease transcript levels of Medicago truncatula genes encoding early enzymes in terpenoid biosynthesis. Plant Mol. Biol. 2006, 60, 519–531. [Google Scholar] [CrossRef]
- Pare, P.W.; Alborn, H.T.; Tumlinson, J.H. Concerted biosynthesis of an insect elicitor of plant volatiles. Proc. Natl. Acad. Sci. USA 1998, 95, 13971–13975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshinaga, N.; Aboshi, T.; Abe, H.; Nishida, R.; Alborn, H.T.; Tumlinson, J.H.; Mori, N. Active role of fatty acid amino acid conjugates in nitrogen metabolism in Spodoptera litura larvae. Proc. Natl. Acad. Sci. USA 2008, 105, 18058–18063. [Google Scholar] [CrossRef] [Green Version]
- Schmelz, E.A.; Huffaker, A.; Carroll, M.J.; Alborn, H.T.; Ali, J.G.; Teal, P.E.A. An Amino Acid Substitution Inhibits Specialist Herbivore Production of an Antagonist Effector and Recovers Insect-Induced Plant Defenses. Plant Physiol. 2012, 160, 1468–1478. [Google Scholar] [CrossRef] [Green Version]
- Yoshinaga, N.; Aboshi, T.; Ishikawa, C.; Fukui, M.; Shimoda, M.; Nishida, R.; Lait, C.G.; Tumlinson, J.H.; Mori, N. Fatty acid amides, previously identified in caterpillars, found in the cricket Teleogryllus taiwanemma and fruit fly Drosophila melanogaster larvae. J. Chem. Ecol. 2007, 33, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Meiners, T. How do plants “notice” attack by herbivorous arthropods? Biol. Rev. 2010, 85, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.; Bossi, S.; Spiteller, D.; Mithofer, A.; Boland, W. Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol. 2004, 134, 1752–1762. [Google Scholar] [CrossRef] [Green Version]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance calcium-based plant defense signaling. Sci. Adv. 2018, 361, 1112–1115. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Wunsche, H.; Baldwin, I.T. Narboh D, a respiratory burst oxidase homolog in Nicotiana attenuata, is required for late defense responses after herbivore attack. J. Integr. Plant Biol. 2013, 55, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, J. The essential role of jasmonic acid in plant-herbivore interactions—Using the wild tobacco Nicotiana attenuata as a model. J. Genet. Genom. 2013, 40, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Ushima, R.; Sugimoto, R.; Tamaoki, D.; Karahara, I.; Hanba, Y.; Wakasugi, T.; Tsuchida, T. A new galling insect model enhances photosynthetic activity in an obligate holoparasitic plant. Sci. Rep. 2021, 11, 13013. [Google Scholar] [CrossRef]
- Price, P.W.; Fernandes, G.W.; Waring, G.L. Adaptive Nature of Insect Galls. Environ. Entomol. 1987, 16, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Espírito-Santo, M. How Many Species of Gall-Inducing Insects Are There on Earth, and Where Are They? Ann. Entomol. Soc. Am. 2009, 100, 95–99. [Google Scholar] [CrossRef]
- Giron, D.; Huguet, E.; Stone, G.N.; Body, M. Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. J. Insect Physiol. 2016, 84, 70–89. [Google Scholar] [CrossRef]
- Stone, G.N.; Schönrogge, K. The adaptive significance of insect gall morphology. Trends Ecol. Evol. 2003, 18, 512–522. [Google Scholar] [CrossRef]
- Takeda, S.; Hirano, T.; Ohshima, I.; Sato, M.H. Recent Progress Regarding the Molecular Aspects of Insect Gall Formation. Int. J. Mol. Sci. 2021, 22, 9424. [Google Scholar] [CrossRef] [PubMed]
- Zagorchev, L.; Atanasova, A.; Albanova, I.; Traianova, A.; Mladenov, P.; Kouzmanova, M.; Goltsev, V.; Kalaji, H.M.; Teofanova, D. Functional Characterization of the Photosynthetic Machinery in Smicronix Galls on the Parasitic Plant Cuscuta campestris by JIP-Test. Cells 2021, 10, 1399. [Google Scholar] [CrossRef]
- Cambier, S.; Ginis, O.; Moreau, S.J.M.; Gayral, P.; Hearn, J.; Stone, G.N.; Giron, D.; Huguet, E.; Drezen, J.-M. Gall Wasp Transcriptomes Unravel Potential Effectors Involved in Molecular Dialogues with Oak and Rose. Front. Physiol. 2019, 10, 926. [Google Scholar] [CrossRef]
- Eitle, M.W.; Carolan, J.C.; Griesser, M.; Forneck, A. The salivary gland proteome of root-galling grape phylloxera (Daktulosphaira vitifoliae Fitch) feeding on Vitis spp. PLoS ONE 2019, 14, e0225881. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Cui, K.; Lu, Q.; Wang, C.; Wu, H.; Yang, Z.; Ding, W.; Shao, S.; Wang, H.; et al. Molecular mechanisms of tannin accumulation in Rhus galls and genes involved in plant-insect interactions. Sci. Rep. 2018, 8, 9841. [Google Scholar] [CrossRef]
- Wang, H.; Cui, K.; Shao, S.; Liu, J.; Chen, H.; Wang, C.; Wu, H.; Yang, Z.; Lu, Q.; King-Jones, K.; et al. Molecular response of gall induction by aphid Schlechtendalia chinensis (Bell) attack on Rhus chinensis Mill. J. Plant Interact. 2017, 12, 465–479. [Google Scholar] [CrossRef] [Green Version]
- Hirano, T.; Kimura, S.; Sakamoto, T.; Okamoto, A.; Nakayama, T.; Matsuura, T.; Ikeda, Y.; Takeda, S.; Suzuki, Y.; Ohshima, I.; et al. Reprogramming of the Developmental Program of Rhus javanica During Initial Stage of Gall Induction by Schlechtendalia chinensis. Front. Plant Sci. 2020, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shi, F.; Chen, Y.; Wang, M.; Zhao, Y.; Geng, G. Transcriptome Analysis of Chinese Chestnut (Castanea mollissima Blume) in Response to Dryocosmus kuriphilus Yasumatsu Infestation. Int. J. Mol. Sci. 2019, 20, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.B.; Jin, L.P.; Wei, D.; Huang, Z.H. Study on the differential gene expression of elm leaves fed on by Tetraneura akinire Sasaki. Genes Genom. 2019, 41, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Galis, I.; Gaquerel, E.; Pandey, S.P.; Baldwin, I.T. Molecular mechanisms underlying plant memory in JA-mediated defence responses. Plant Cell Environ. 2009, 32, 617–627. [Google Scholar] [CrossRef]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef] [Green Version]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; De Moraes, C.M. Plant Defense Priming against Herbivores: Getting Ready for a Different Battle1. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erb, M.; Veyrat, N.; Robert, C.A.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015, 6, 6273–6283. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Glauser, G.; Lou, Y.; Erb, M.; Hu, L. Molecular Dissection of Early Defense Signaling Underlying Volatile-Mediated Defense Regulation and Herbivore Resistance in Rice. Plant Cell 2019, 31, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Sugimoto, K.; Ramadan, A.; Arimura, G. Memory of plant communications for priming anti-herbivore responses. Sci. Rep. 2013, 3, 1872–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malook, S.U.; Xu, Y.; Qi, J.; Li, J.; Wang, L.; Wu, J. Mythimna separata herbivory primes maize resistance in systemic leaves. J. Exp. Bot. 2021, 72, 3792–3805. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Gordon-Weeks, R.; Flors, V.; Camanes, G.; Turlings, T.C.; Ton, J. Belowground ABA boosts aboveground production of DIMBOA and primes induction of chlorogenic acid in maize. Plant Signal. Behav. 2009, 4, 636–638. [Google Scholar] [CrossRef]
- Bandoly, M.; Hilker, M.; Steppuhn, A. Oviposition by Spodoptera exigua on Nicotiana attenuata primes induced plant defence against larval herbivory. Plant J. 2015, 83, 661–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmann, S.; De Vos, M.; Casteel, C.L.; Tian, D.L.; Halitschke, R.; Sun, J.Y.; Agrawal, A.A.; Felton, G.W.; Jander, G. Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol. 2012, 158, 854–863. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.H.; Shan, W.X.; Ayliffe, M.A.; Wang, M.B. Epigenetic Mechanisms: An Emerging Player in Plant-Microbe Interactions. Mol. Plant Microbe Interact. 2016, 29, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Elicitors | Receptors | Source of Elicitors | Host Plant | References |
|---|---|---|---|---|
| DNA | n.d. | These elicitors are of plant source | Bean, maize | [25] |
| Pep | Pep receptor (PEPR) | Maize | [26,27] | |
| ATP | ATP receptors (DORN1/P2K1) | Arabidopsis | [28] | |
| Systemin | Systemin receptor (SYR1) | Tomato | [29] | |
| FACs (volicitin) | Unknown membrane proteins | Spodoptera exigua | Maize | [10] |
| β-Glucosidase | n.d. | Pieris brassicae | Maize | [30] |
| Caeliferins | n.d. | Schistocerca americana | Maize | [31] |
| Inceptin | Inceptin receptor (INR) | Spodoptera frugiperda | Maize | [9] |
| Lipase | n.d. | Schistocerca gregaria | Arabidopsis | [32] |
| Porin-like proteins | n.d. | Spodoptera littoralis | Arabidopsis | [33] |
| β-Galactofuranose polysaccharide | HAK/PBL27 | Spodoptera spp. | Arabidopsis | [34] |
| Bruchins | n.d. | Bruchus pisorum, Nilaparvata lugens | Cowpea, pea | [35] |
| Glucose oxidase | n.d. | Helicoverpa zea, Spodoptera exigua, Helicoverpa armigera | Nicotiana | [36,37] |
| Mucin-like protein | n.d. | Callosobruchus maculatus | Rice | [38] |
| Oligouronides | n.d. | Produced by breakdown of plant cell walls by insect feeding | Tomato | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malook, S.u.; Maqbool, S.; Hafeez, M.; Karunarathna, S.C.; Suwannarach, N. Molecular and Biochemical Mechanisms of Elicitors in Pest Resistance. Life 2022, 12, 844. https://doi.org/10.3390/life12060844
Malook Su, Maqbool S, Hafeez M, Karunarathna SC, Suwannarach N. Molecular and Biochemical Mechanisms of Elicitors in Pest Resistance. Life. 2022; 12(6):844. https://doi.org/10.3390/life12060844
Chicago/Turabian StyleMalook, Saif ul, Saiqa Maqbool, Muhammad Hafeez, Samantha Chandranath Karunarathna, and Nakarin Suwannarach. 2022. "Molecular and Biochemical Mechanisms of Elicitors in Pest Resistance" Life 12, no. 6: 844. https://doi.org/10.3390/life12060844
APA StyleMalook, S. u., Maqbool, S., Hafeez, M., Karunarathna, S. C., & Suwannarach, N. (2022). Molecular and Biochemical Mechanisms of Elicitors in Pest Resistance. Life, 12(6), 844. https://doi.org/10.3390/life12060844








